Abstract
C22H14Cl2F4N2Pt, monoclinic, P21/n (no. 14), a = 16.858(9) Å, b = 14.876(8) Å, c = 17.125(9) Å, β = 98.552(6)°, V = 4247(4) Å3, Z = 8, R gt (F) = 0.0337, wR ref (F2) = 0.0778, T = 296 K.
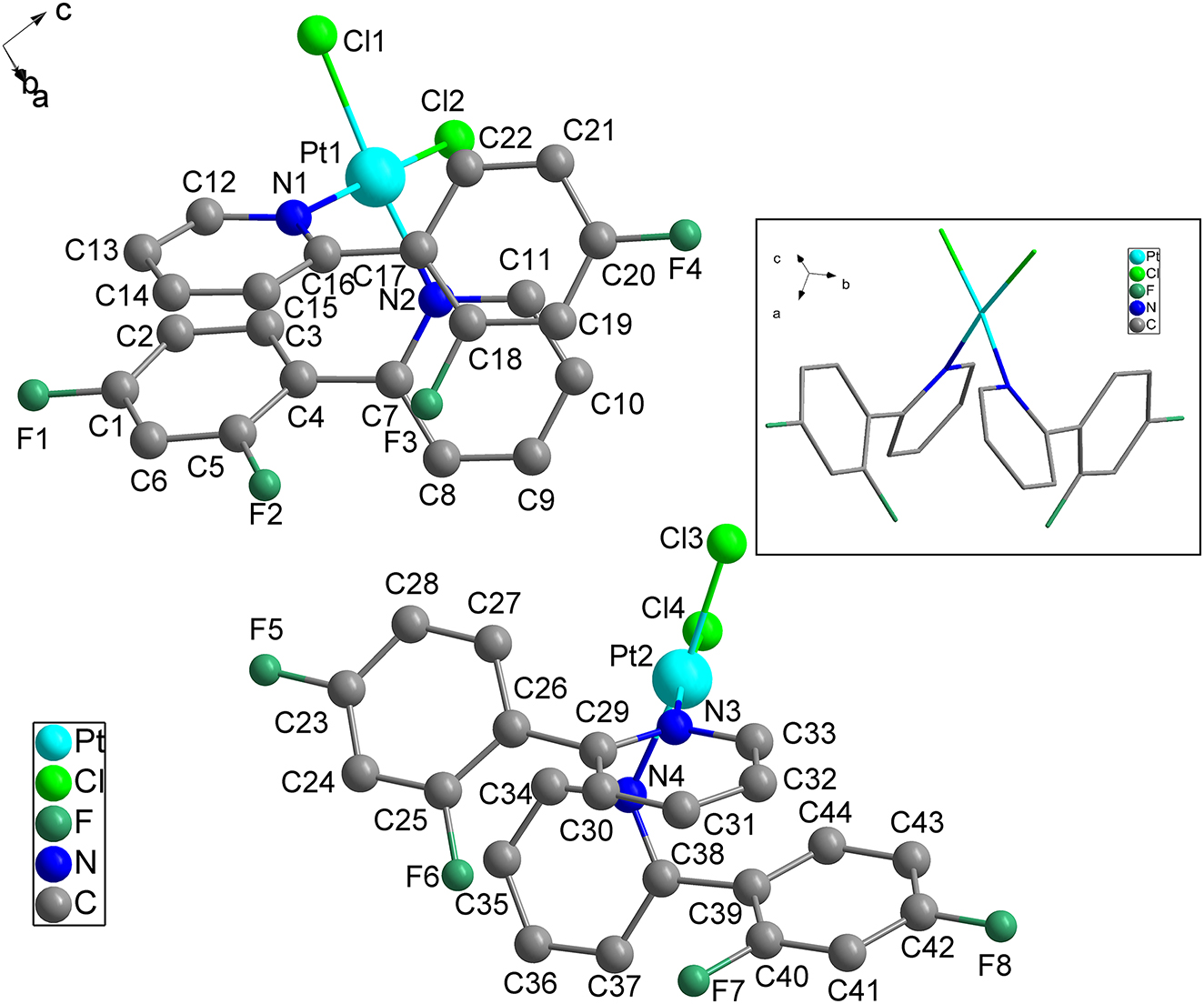
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow cube |
| Size: | 0.08 × 0.06 × 0.06 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 6.91 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 26.9°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 31,284, 8484, 0.060 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 6411 |
| N(param)refined: | 559 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Pt1 | 0.67725 (2) | 0.33224 (2) | 0.83343 (2) | 0.03549 (7) |
| Cl1 | 0.57814 (8) | 0.23191 (10) | 0.85281 (9) | 0.0560 (4) |
| Cl2 | 0.63300 (9) | 0.43984 (9) | 0.91278 (9) | 0.0539 (4) |
| F1 | 0.5717 (3) | 0.4418 (3) | 0.4792 (2) | 0.1147 (17) |
| F2 | 0.8289 (3) | 0.3955 (3) | 0.6100 (2) | 0.0968 (13) |
| F3 | 0.9427 (2) | 0.2695 (3) | 0.7658 (3) | 0.0915 (12) |
| F4 | 1.0004 (2) | 0.2177 (3) | 1.0365 (2) | 0.1103 (16) |
| N1 | 0.7098 (2) | 0.2458 (3) | 0.7512 (2) | 0.0379 (10) |
| N2 | 0.7733 (2) | 0.4132 (3) | 0.8248 (2) | 0.0397 (10) |
| C1 | 0.6242 (6) | 0.4419 (4) | 0.5466 (4) | 0.073 (2) |
| C2 | 0.5974 (4) | 0.4671 (4) | 0.6149 (4) | 0.0660 (19) |
| H2 | 0.543993 | 0.483036 | 0.614493 | 0.079* |
| C3 | 0.6506 (4) | 0.4685 (3) | 0.6848 (3) | 0.0502 (15) |
| H3 | 0.632793 | 0.485306 | 0.731553 | 0.060* |
| C4 | 0.7314 (3) | 0.4447 (3) | 0.6856 (3) | 0.0444 (13) |
| C5 | 0.7525 (4) | 0.4193 (4) | 0.6141 (4) | 0.0620 (17) |
| C6 | 0.7010 (5) | 0.4175 (4) | 0.5443 (4) | 0.074 (2) |
| H6 | 0.718091 | 0.400130 | 0.497387 | 0.089* |
| C7 | 0.7913 (3) | 0.4509 (3) | 0.7574 (3) | 0.0441 (13) |
| C8 | 0.8632 (4) | 0.4953 (4) | 0.7577 (4) | 0.0634 (18) |
| H8 | 0.875399 | 0.520853 | 0.711365 | 0.076* |
| C9 | 0.9169 (4) | 0.5021 (5) | 0.8264 (5) | 0.075 (2) |
| H9 | 0.965680 | 0.531174 | 0.826467 | 0.090* |
| C10 | 0.8979 (4) | 0.4658 (4) | 0.8942 (4) | 0.0655 (19) |
| H10 | 0.933861 | 0.469400 | 0.940843 | 0.079* |
| C11 | 0.8253 (3) | 0.4240 (4) | 0.8929 (3) | 0.0504 (15) |
| H11 | 0.811146 | 0.402460 | 0.939865 | 0.060* |
| C12 | 0.6570 (4) | 0.2347 (3) | 0.6850 (3) | 0.0502 (14) |
| H12 | 0.606050 | 0.259239 | 0.683155 | 0.060* |
| C13 | 0.6740 (4) | 0.1894 (4) | 0.6202 (4) | 0.0618 (18) |
| H13 | 0.635675 | 0.183519 | 0.575513 | 0.074* |
| C14 | 0.7491 (4) | 0.1526 (4) | 0.6227 (4) | 0.0616 (17) |
| H14 | 0.763348 | 0.122699 | 0.579135 | 0.074* |
| C15 | 0.8027 (4) | 0.1611 (4) | 0.6910 (3) | 0.0557 (16) |
| H15 | 0.853240 | 0.135063 | 0.693992 | 0.067* |
| C16 | 0.7832 (3) | 0.2075 (3) | 0.7552 (3) | 0.0410 (13) |
| C17 | 0.8397 (3) | 0.2128 (3) | 0.8295 (3) | 0.0419 (13) |
| C18 | 0.9180 (4) | 0.2402 (4) | 0.8335 (4) | 0.0568 (16) |
| C19 | 0.9728 (4) | 0.2453 (5) | 0.9016 (5) | 0.072 (2) |
| H19 | 1.024490 | 0.266918 | 0.901321 | 0.086* |
| C20 | 0.9475 (4) | 0.2173 (5) | 0.9687 (4) | 0.071 (2) |
| C21 | 0.8717 (4) | 0.1868 (4) | 0.9712 (4) | 0.0620 (17) |
| H21 | 0.856711 | 0.166911 | 1.018399 | 0.074* |
| C22 | 0.8170 (3) | 0.1859 (3) | 0.9014 (3) | 0.0490 (14) |
| H22 | 0.764576 | 0.167145 | 0.902669 | 0.059* |
| Pt2 | 1.18565 (2) | 0.53581 (2) | 0.83735 (2) | 0.03959 (7) |
| Cl3 | 1.16205 (10) | 0.43187 (11) | 0.93062 (9) | 0.0660 (4) |
| Cl4 | 1.09727 (9) | 0.63671 (11) | 0.87734 (10) | 0.0659 (4) |
| F5 | 0.9815 (2) | 0.4044 (3) | 0.5253 (2) | 0.0971 (14) |
| F6 | 1.2586 (2) | 0.4461 (3) | 0.5761 (2) | 0.0874 (12) |
| F7 | 1.4176 (2) | 0.5909 (3) | 0.7083 (3) | 0.1012 (14) |
| F8 | 1.5388 (3) | 0.6492 (3) | 0.9653 (3) | 0.1240 (18) |
| N3 | 1.2720 (2) | 0.4520 (3) | 0.8076 (2) | 0.0381 (10) |
| N4 | 1.1989 (2) | 0.6194 (3) | 0.7460 (2) | 0.0401 (10) |
| C23 | 1.0498 (4) | 0.4074 (4) | 0.5775 (4) | 0.0613 (17) |
| C24 | 1.1195 (4) | 0.4262 (4) | 0.5497 (3) | 0.0638 (18) |
| H24 | 1.120301 | 0.438370 | 0.496496 | 0.077* |
| C25 | 1.1887 (4) | 0.4266 (4) | 0.6035 (4) | 0.0525 (15) |
| C26 | 1.1912 (3) | 0.4099 (3) | 0.6833 (3) | 0.0391 (12) |
| C27 | 1.1175 (3) | 0.3914 (3) | 0.7080 (3) | 0.0411 (13) |
| H27 | 1.115796 | 0.379675 | 0.761045 | 0.049* |
| C28 | 1.0470 (3) | 0.3903 (4) | 0.6551 (4) | 0.0516 (15) |
| H28 | 0.998324 | 0.378025 | 0.672323 | 0.062* |
| C29 | 1.2681 (3) | 0.4081 (3) | 0.7379 (3) | 0.0398 (12) |
| C30 | 1.3329 (4) | 0.3611 (4) | 0.7202 (4) | 0.0575 (16) |
| H30 | 1.329079 | 0.330641 | 0.672401 | 0.069* |
| C31 | 1.4036 (4) | 0.3581 (4) | 0.7719 (5) | 0.0669 (19) |
| H31 | 1.448217 | 0.328131 | 0.758879 | 0.080* |
| C32 | 1.4060 (4) | 0.4005 (4) | 0.8427 (4) | 0.0673 (19) |
| H32 | 1.452513 | 0.398332 | 0.879370 | 0.081* |
| C33 | 1.3412 (4) | 0.4458 (4) | 0.8602 (4) | 0.0541 (16) |
| H33 | 1.343810 | 0.473470 | 0.909180 | 0.065* |
| C34 | 1.1327 (4) | 0.6311 (4) | 0.6910 (3) | 0.0551 (15) |
| H34 | 1.084344 | 0.605974 | 0.700082 | 0.066* |
| C35 | 1.1346 (4) | 0.6785 (4) | 0.6226 (4) | 0.0608 (18) |
| H35 | 1.088116 | 0.684974 | 0.586289 | 0.073* |
| C36 | 1.2049 (5) | 0.7163 (4) | 0.6079 (4) | 0.0671 (19) |
| H36 | 1.207090 | 0.747173 | 0.561072 | 0.080* |
| C37 | 1.2724 (4) | 0.7078 (4) | 0.6639 (3) | 0.0537 (15) |
| H37 | 1.320577 | 0.734071 | 0.655742 | 0.064* |
| C38 | 1.2677 (3) | 0.6593 (3) | 0.7330 (3) | 0.0429 (13) |
| C39 | 1.3386 (3) | 0.6534 (3) | 0.7965 (3) | 0.0435 (13) |
| C40 | 1.4110 (4) | 0.6219 (4) | 0.7810 (4) | 0.0608 (17) |
| C41 | 1.4788 (4) | 0.6180 (5) | 0.8371 (5) | 0.082 (2) |
| H41 | 1.526743 | 0.594307 | 0.825574 | 0.098* |
| C42 | 1.4719 (5) | 0.6508 (5) | 0.9107 (5) | 0.083 (2) |
| C43 | 1.4030 (4) | 0.6829 (4) | 0.9297 (4) | 0.0673 (19) |
| H43 | 1.400939 | 0.704430 | 0.980329 | 0.081* |
| C44 | 1.3344 (4) | 0.6838 (3) | 0.8730 (3) | 0.0512 (15) |
| H44 | 1.286063 | 0.704526 | 0.886069 | 0.061* |
1 Source of materials
2-(2,4-Difluorophenyl)pyridine (0.2294 g, 0.0012 mol) and K2PtCl4 (1.6602 g, 0.0004 mol) were suspended in 2-ethoxyethanol (90 mL). Under nitrogen atmosphere, the suspension was stirred at 80 °C for 10 h. 2-Ethoxyethanol was removed by rotary evaporator, dichloromethane (20 mL) was added and stirred at 20 °C for 10 h. The title crystals were obtained by slow evaporation from dichloromethane at room temperature.
2 Experimental details
Absorption corrections were used by using multi-scan program [1]. The structure was solved with Shelx [3, 4]. Hydrogen atoms were placed in their geometrically idealized positions. Hydrogen atoms were constrained to ride on their parent atoms.
3 Comment
Platinum-based drugs are widely used for chemotherapeutic eradication of cancer [5]. Cisplatin, carboplatin, and oxaliplatin have excellent performance in the treatment of lung cancer, ovarian cancer and colon cancer respectively [6], [7], [8]. Platinum(II) complexes can also play an important role in detection of highly toxic pollutant perchlorate in the environment [9]. In addition, platinum(II) complexes also have significant applications in catalysis and optics [10, 11].
The title compound is a planar quadrilateral configuration with platinum(II) as the central metal. The coordination sites of the compound were occupied by two nitrogens atoms and two chlorine atoms respectively. The atoms are derived from the bidentate ligand 2-(2,4-difluorophenyl)pyridine, the chlorine atoms are derived from K2PtCl4. The distances of Pt1–Cl1 bonds are 2.3009(16) Å, the distances of Pt1–Cl2 bonds are 2.2951(16) Å, the distances of Pt1–N1 bonds are 2.041(4) Å, the distances of Pt1–N2 bonds are 2.041(4) Å. The distances of Pt2–Cl3 bonds are 2.2999(17) Å, the distances of Pt2–Cl4 bonds are 2.2903(17) Å, the distances of Pt2–N3 bonds are 2.039(4) Å, the distances of Pt2–N4 bonds are 2.037(4) Å. In addition, the Cl2–Pt1–Cl1 angle is 93.44(6)°, the N1–Pt1–Cl1 angle is 88.08(12)°, the N1–Pt1–Cl2 angle is 172.86(12)°, the N1–Pt1–N2 angle is 91.36(16)°, the N2–Pt1–Cl1 angle is 173.79(12)°, the N2–Pt1–Cl2 angle is 87.87(12)°, the Cl4–Pt2–Cl3 angle is 92.61(7)°, the N3–Pt2–Cl3 angle is 88.06(12)°, the N3–Pt2–Cl4 angle is 175.07(12)°, the N4–Pt2–Cl3 angle is 173.76(12)°, the N4–Pt2–Cl4 angle is 88.84(12)°, the N4–Pt2–N3 angle is 91.01(16)°, the Cl2–Pt1–Cl1 angle is 93.44(6)°, the Cl2–Pt1–Cl1 angle is 93.44(6)°, the Cl2–Pt1–Cl1 angle is 93.44(6)°, the Cl2–Pt1–Cl1 angle is 93.44(6)°, the Cl2–Pt1–Cl1 angle is 93.44(6)°. All bond lengths and bond angles fall within the normal ranges [12, 13].
In the columns, several p⋯p interactions between adjacent aromatic rings are present. All the p⋯p interactions are in the range of 3.624(4)–3.969(4) Å [14]. Also the crystal packing is consolidated by weak C–F⋯p interactions. The C–F⋯p (centroid) distance in these motifs were in the range of 3.474(5)–3.719(5) Å [15].
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Isabel, U., Sheldrick, G. M. An introduction to experimental phasing of macromolecules illustrated by SHELX; new autotracing features. Acta Crystallogr. 2018, D74, 106–116.10.1107/S2059798317015121Search in Google Scholar PubMed PubMed Central
5. Chunyu, Z., Chao, X., Xueyun, G., Qingqiang, Y. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132; https://doi.org/10.7150/thno.69424.Search in Google Scholar PubMed PubMed Central
6. Jamie, E. C., Yu, S., Boris, S., Patrick, M. F. Preoperative and postoperative systemic therapy for operable non? Small-cell lung cancer. J. Clin. Oncol. 2023, 62, 546–555.10.1200/JCO.21.01589Search in Google Scholar PubMed PubMed Central
7. Chan, J. K., Brady, M. F., Penson, R. T., Huang, H., Birrer, M. J., Walker, J. L., DiSilvestro, P. A., Rubin, S. C., Martin, L. P., Davidson, S. A., Huh, W. K., O’Malley, D. M., Boente, M. P., Michael, H., Monk, B. J. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. New Engl. J. Med. 2016, 374, 738–748; https://doi.org/10.1056/nejmoa1505067.Search in Google Scholar PubMed PubMed Central
8. Cohen, R., Taieb, J., Fiskum, J., Yothers, G., Goldberg, R., Yoshino, T., Alberts, S., Allegra, C., de Gramont, A., Seitz, J.-F., O’Connell, M., Haller, D., Wolmark, N., Erlichman, C., Zaniboni, A., Lonardi, S., Kerr, R., Grothey, A., Sinicrope, F. A., André, T., Shi, Q. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: an ACCENT pooled analysis of 12 adjuvant trials. J. Clin. Oncol. 2021, 39, 642–651; https://doi.org/10.1200/jco.20.01600.Search in Google Scholar PubMed PubMed Central
9. Zhen, S., Yushu, L., Jiguang, L., Kun, L., Xincun, D. Ultrasensitive dual-mode visualization of perchlorate in water, soil and air boosted by close and stable Pt–Pt packing endowed low-energy absorption and emission. J. Mater. Chem. A 2022, 10, 8195–8207; https://doi.org/10.1039/d2ta00843b.Search in Google Scholar
10. Zhong, W., Xu, J., Yang, Y., Jiayou, Y., Mingxu, L., Cheng, H., Chunying, D. A platinum(II)-based molecular cage with aggregation-induced emission for enzymatic photocyclization of alkynylaniline. Angew. Chem. Int. Ed. 2023, 62, e202214577.10.1002/anie.202214577Search in Google Scholar PubMed
11. Zhe, F., Jie-Jie, L., Huijie, S., Dunru, Z. A water-tuned reversible spin transition with the largest hysteresis loop in 3D Hofmann frameworks pillared by flexible ligands. Inorg. Chem. Front. 2023, 10, 305–315; https://doi.org/10.1039/d2qi01873j.Search in Google Scholar
12. Tatsuto, K., Aika, H., Akira, O. Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) – acetone (1/1), C13H12Cl4N2PtO. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 423–424; https://doi.org/10.1515/ncrs-2019-0728.Search in Google Scholar
13. Xu, F.-F., Zeng, W., Sun, M.-J., Gong, Z.-L., Li, Z.-Q., Zhao, Y. S., Yao, J., Zhong, Y.-W. Organoplatinum(II) cruciform: a versatile building block to fabricate 2D microcrystals with full-color and white phosphorescence and anisotropic photon transport. Ang. Chem. Int. Ed. 2022, 61, e202116603; https://doi.org/10.1002/ange.202116603.Search in Google Scholar
14. Alyssa, K. A., Alexander, C. M., Lulio, A. S., Lee, R. A., Jeffery, A. B., Karah, E. K. Synthesis, structural characterization, and luminescence properties of heteroleptic bismuthorganic compounds. CrystEngComm 2021, 23, 8183–8197; https://doi.org/10.1039/d1ce01242h.Search in Google Scholar
15. Rybalova, T. V., Bagryanskaya, I. Y. CF⋯p, F⋯H, and F⋯F intermolecular interactions and F-aggregation: role in crystal engineering of fluoroorganic compounds. J. Struct. Chem. 2009, 50, 741–753; https://doi.org/10.1007/s10947-009-0113-0.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

