Abstract
C23H23Co0.5N4O4, triclinic,
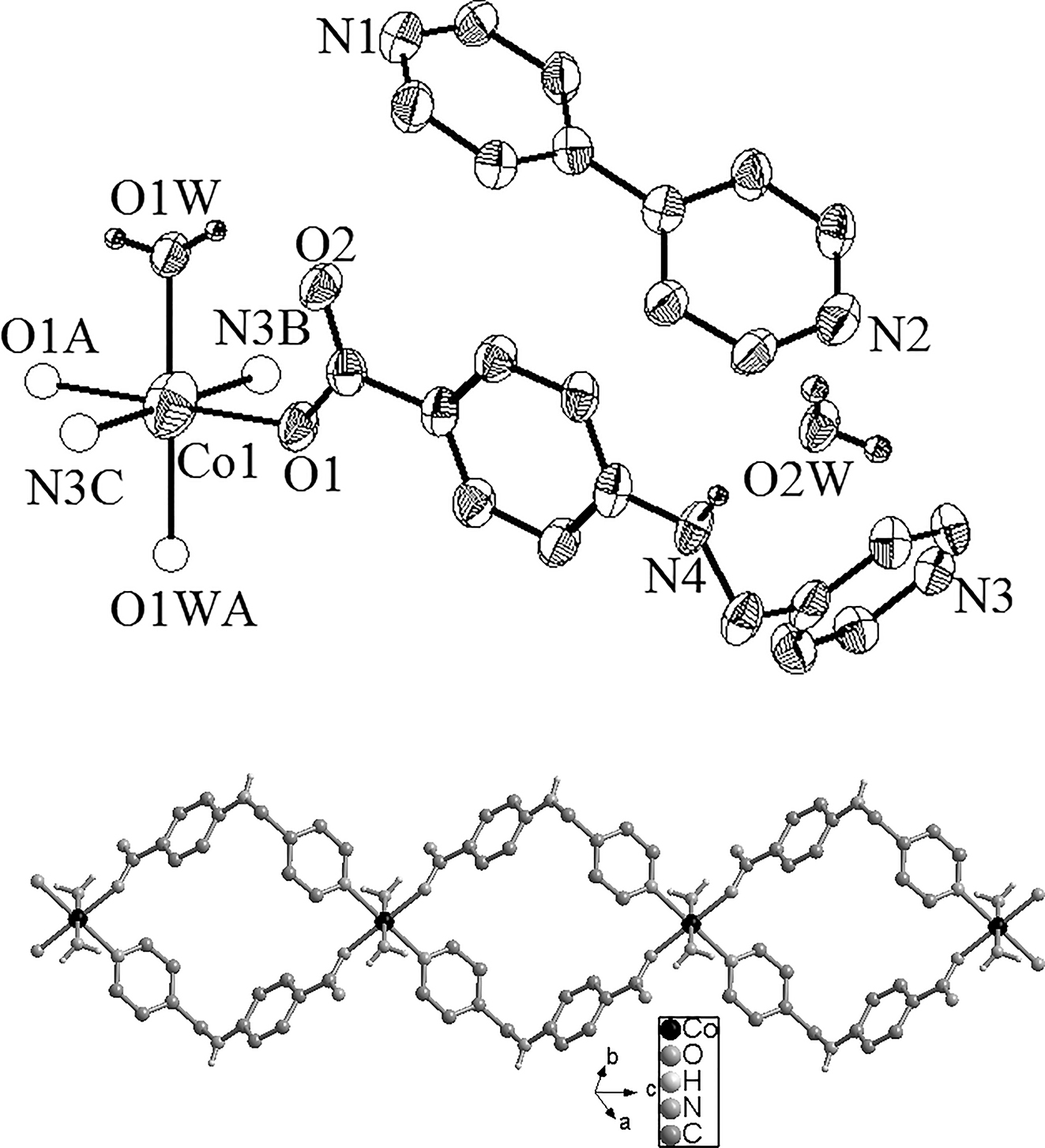
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Pink block |
| Size: | 0.33 × 0.22 × 0.21 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.46 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 25.5°, 98% |
| N(hkl)measured, N(hkl)unique, Rint: | 11,718, 3987, 0.030 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3510 |
| N(param)refined: | 289 |
| Programs: | Olex2 [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Co1 | 0.5000 | 0.5000 | 0.5000 | 0.02797 (18) |
| O1 | 0.36593 (18) | 0.56658 (17) | 0.67157 (14) | 0.0374 (4) |
| O1W | 0.54267 (17) | 0.69171 (16) | 0.44558 (14) | 0.0365 (4) |
| H1WA | 0.5218 | 0.7129 | 0.3824 | 0.055* |
| H1WB | 0.5050 | 0.7556 | 0.4953 | 0.055* |
| O2 | 0.33858 (19) | 0.78536 (17) | 0.65731 (15) | 0.0423 (4) |
| N3 | 0.3076 (2) | 0.5370 (2) | 1.43896 (17) | 0.0341 (5) |
| N4 | −0.0366 (2) | 0.7196 (2) | 1.21399 (17) | 0.0409 (5) |
| H4 | −0.0866 | 0.7966 | 1.2391 | 0.049* |
| C1 | 0.3162 (2) | 0.6810 (2) | 0.7168 (2) | 0.0297 (5) |
| C2 | 0.2281 (2) | 0.6902 (2) | 0.84817 (19) | 0.0299 (5) |
| C3 | 0.2095 (3) | 0.5786 (2) | 0.9179 (2) | 0.0375 (6) |
| H3 | 0.2542 | 0.4965 | 0.8818 | 0.045* |
| C4 | 0.1265 (3) | 0.5848 (3) | 1.0397 (2) | 0.0394 (6) |
| H4A | 0.1174 | 0.5076 | 1.0842 | 0.047* |
| C5 | 0.0564 (2) | 0.7066 (2) | 1.0960 (2) | 0.0319 (5) |
| C6 | 0.0790 (3) | 0.8202 (3) | 1.0266 (2) | 0.0371 (6) |
| H6 | 0.0371 | 0.9025 | 1.0629 | 0.044* |
| C7 | 0.1625 (2) | 0.8125 (2) | 0.9048 (2) | 0.0333 (5) |
| H7 | 0.1750 | 0.8894 | 0.8602 | 0.040* |
| C8 | −0.0563 (3) | 0.6125 (3) | 1.2990 (2) | 0.0413 (6) |
| H8A | −0.0604 | 0.5343 | 1.2595 | 0.050* |
| H8B | −0.1513 | 0.6355 | 1.3646 | 0.050* |
| C9 | 0.0699 (2) | 0.5822 (3) | 1.3493 (2) | 0.0332 (5) |
| C10 | 0.1700 (3) | 0.4660 (3) | 1.3284 (2) | 0.0417 (6) |
| H10 | 0.1597 | 0.3995 | 1.2838 | 0.050* |
| C11 | 0.2865 (3) | 0.4471 (3) | 1.3735 (2) | 0.0428 (6) |
| H11 | 0.3535 | 0.3675 | 1.3572 | 0.051* |
| C12 | 0.2088 (3) | 0.6496 (3) | 1.4606 (2) | 0.0422 (6) |
| H12 | 0.2193 | 0.7134 | 1.5078 | 0.051* |
| C13 | 0.0927 (3) | 0.6763 (3) | 1.4170 (2) | 0.0428 (6) |
| H13 | 0.0289 | 0.7576 | 1.4327 | 0.051* |
| N1 | 0.7693 (3) | 0.9349 (3) | 0.5992 (2) | 0.0593 (7) |
| N2 | 0.5241 (3) | 0.7615 (3) | 1.2177 (2) | 0.0528 (6) |
| C14 | 0.4614 (4) | 0.7275 (4) | 1.1437 (3) | 0.0705 (10) |
| H14 | 0.3825 | 0.6808 | 1.1756 | 0.085* |
| C15 | 0.5044 (4) | 0.7561 (4) | 1.0233 (3) | 0.0628 (9) |
| H15 | 0.4562 | 0.7280 | 0.9761 | 0.075* |
| C16 | 0.6206 (3) | 0.8275 (3) | 0.9722 (2) | 0.0391 (6) |
| C17 | 0.6863 (3) | 0.8631 (3) | 1.0487 (2) | 0.0544 (8) |
| H17 | 0.7648 | 0.9106 | 1.0199 | 0.065* |
| C18 | 0.6352 (3) | 0.8281 (3) | 1.1692 (3) | 0.0542 (8) |
| H18 | 0.6822 | 0.8530 | 1.2187 | 0.065* |
| C19 | 0.6722 (3) | 0.8638 (3) | 0.8434 (2) | 0.0377 (6) |
| C20 | 0.8158 (3) | 0.8900 (3) | 0.7836 (3) | 0.0579 (8) |
| H20 | 0.8835 | 0.8836 | 0.8242 | 0.069* |
| C21 | 0.8588 (4) | 0.9256 (4) | 0.6640 (3) | 0.0671 (9) |
| H21 | 0.9554 | 0.9441 | 0.6267 | 0.081* |
| C22 | 0.6332 (3) | 0.9090 (3) | 0.6555 (3) | 0.0535 (7) |
| H22 | 0.5687 | 0.9148 | 0.6120 | 0.064* |
| C23 | 0.5798 (3) | 0.8736 (3) | 0.7758 (2) | 0.0451 (7) |
| H23 | 0.4823 | 0.8566 | 0.8108 | 0.054* |
| O2W | −0.1821 (3) | 0.9664 (2) | 1.34689 (17) | 0.0612 (6) |
| H2WA | −0.2295 | 1.0421 | 1.3381 | 0.092* |
| H2WB | −0.1949 | 0.9537 | 1.4210 | 0.092* |
1 Source of material
All chemicals for synthesis were of reagent grade and used as received without further purification. The mixtures of 4-[(4-pyridinylmethyl)amino] benzoic acid (0.05 mmol, 12.0 mg), 4,4′-bipyridine (0.1 mmol, 16.2 mg), Co(Ac)2–4H2O (0.1 mmol, 25.1 mg), NaOH (0.1 mmol, 4.0 mg) and H2O (6 mL) was placed in a 23 mL Teflon-lined autoclave at 393 K for 4 days, and then slowly cooled down to room temperature for crystallization. Pink block crystals of the title compound were obtained. The yield was 62 % (based on Co). For C23H23Co0.5N4O4 anal. calcd., % C, 61.54 H, 5.16 N, 12.48 found, % C, 61.45 H, 5.38 N, 12.38.
2 Experimental details
Using Olex2 [1], the structure was solved with the ShelXT [2] structure solution program and refined with the ShelXL [3] refinement package. All hydrogen atoms were added to their calculated positions and refined using a riding model. The Uiso of the H-atoms were constrained to 1.2 times Ueq of their bonding carbon atoms and 1.5 times Ueq of their bonding oxygen atoms for the hydrogen atoms in water molecules.
3 Comment
Coordination polymers (CPs) represent a high-speed growing area in coordination and supramolecular chemistry [4], [5], [6], and especially for Co(II) compounds [7, 8]. The design and synthesis of coordination polymers via self-assembly of metal ions and organic ligands depend on the selection of metal centers and the organic ligands with controlled chemical and physical properties by the Schiff-base ligands [9, 10], because this class of ligand with O,N-bifunctional group has multiple coordination points and various coordination modes. The HL {HL = 4-[(4-pyridinylmethyl)amino]benzoic acid} starting material has one carboxylic group and one nitrogen atom at the terminal positions and one secondary amine group between the phenyl groups, which can supply more varied coordinating patterns (monodentate, bridging, chelating) to construct coordination frameworks. However, HL carboxylic acid ligands have not been extensively exploited, except for some limited cases [11], [12], [13].
The crystal structure of the compound shows a one-dimensional chain structure. The asymmetric unit contains one half crystallographically Co(II) cation, one completely deprotonated L− anion and one Bpy molecule and one coordinating water molecule and one crystallization water molecule as shown in the upper part of the figure (A: 1 − x, 1 − y, 1 − z; B: 1 − x, 1 − y, 2 − z; C: x, y, −1 + z). In this complex, each Co(II) cation is six-coordinated with a distorted octahedral geometry, [CoN2O4] by two N atoms from two symmetry-related Bpy ligand, two oxygen atoms from two symmetry-related L− anions, two oxygen atoms from two coordination water molecules. The Co–O bond lengths are 2.068(16), and 2.113(18) Å for Co1–O1, Co1–O1W, and one Co–N bond is 2.183(18) Å for Co1–N3, respectively. Two adjacent cobalt(II) are connected together by two L− anions adopting monodentate coordination mode to form an infinite one-dimensional chain along the c axis with the Co⋯Co distance of 11.8710(6) Å (lower part of the structure). There are massive H-bonding interactions in the compound via the participation of coordination waters and carboxylate O atoms (O(1W)–H(1WB)⋯O(2): 2.689(2) Å), between crystallization waters and carboxylate O atoms (O(2W)–H(2WA)⋯O(2): 2.732(3) Å), between coordination waters and N atoms of Bpy molecules (O(1W)–H(1WA)⋯N(2): 2.817(3) Å), between crystallization waters and N atoms of Bpy molecules (O(2W)–H(2WB)⋯N(1): 2.873(3) Å), between crystallization waters and amino N atoms of L− (N(4)–H(4)⋯O(2W): 2.923(3) Å). The free Bpy molecules as parallel pendent arms are hung on one side of the infinite chain through the hydrogen bonds. At last a three-dimensional supramolecular structure was gained through the H-bonding interactions.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Zhao, Y., Zeng, H., Zhu, X. W., Lu, W., Li, D. Metal-organic frameworks as photoluminescent biosensing platforms: mechanisms and applications. Chem. Soc. Rev. 2021, 50, 4484–4513; https://doi.org/10.1039/d0cs00955e.Search in Google Scholar PubMed
5. Ju, F. Y., Li, Y. P., Liu, G. Z. A helix-based cobalt(II) coordination polymer with mixed ligands of homophthalic acid and 2,5-bis(4′-pyridyl)-1,3,4-oxadiazole: synthesis, structure and magnetic property. Inorg. Nano-Met. Chem. 2022, 52, 991–995; https://doi.org/10.1080/24701556.2021.2025399.Search in Google Scholar
6. Li, S. L., Deng, L. X., Wu, G., Zhang, Y. T., Pan, X. E., Li, M. H., Li, S. Preparation of a new metal-organic framework/porous anodic alumina composite membrane, structural characterization, and CO2 adsorption. Russ. J. Gen. Chem. 2022, 92, 1574–1577; https://doi.org/10.1134/s1070363222080266.Search in Google Scholar
7. Li, G. L., Yin, W. D., Liu, Q. L., Gong, X. R., Zhao, Y. J., Liu, G. Z. N-Donor-induced two Co(II) coordination polymers derived from the flexible citraconic acid as photocatalysts for the decomposition of organic dyes. Z. Anorg. Allg. Chem. 2022, 648, 89–96; https://doi.org/10.1002/zaac.202100037.Search in Google Scholar
8. Wang, Y. F., Feng, Y. X., Wang, L. Y. Syntheses, crystal structures, and thermal analysis of two Co(II)-coordination polymers constructed from the semirigid tetracarboxylic acid and dipyridine ligand. Russ. J. Inorg. Chem. 2022, 67, 449–455; https://doi.org/10.1134/s0036023622040210.Search in Google Scholar
9. Chen, M. S., Hua, Q., Bai, Z. S., Okamura, T. A., Su, Z., Sun, W. Y., Ueyama, N. Syntheses and characterization of inorganic-organic hybrids with 4-(isonicotinamido)phthalate and some divalent metal centers. Polyhedron 2010, 29, 2454–2461; https://doi.org/10.1016/j.poly.2010.05.014.Search in Google Scholar
10. Madhab, C. D., Parimal, K. B. A porous coordination polymer exhibiting reversible single-crystal to single-crystal substitution reactions at Mn(II) centers by nitrile guest molecules. J. Am. Chem. Soc. 2009, 131, 10942–10949; https://doi.org/10.1021/ja9006035.Search in Google Scholar PubMed
11. Ying, S. M. Syntheses, crystal structures and characterizations of six coordination polymers from reduced Schiff base ligands. Inorg. Chim. Acta 2012, 387, 366–372; https://doi.org/10.1016/j.ica.2012.02.029.Search in Google Scholar
12. Yin, W. D., He, Y. Y., Shen, J., Li, G. L. Hydrothermal synthesis and crystal structure of catena-poly[bis(4-((pyridin-4-ylmethyl)amino)benzoato-κ3N:O,O′) zinc(II)-1,2-di(pyridin-4-yl)ethane water (1/1/1). Z. Kristallogr. N. Cryst. Struct. 2019, 234, 211–213.10.1515/ncrs-2018-0217Search in Google Scholar
13. Liu, G. Z., Li, Y. D. Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl) amino] benzoato-κ2N:O)cobalt(II)]-1,2bi(4-pyridyl)ethene-water (1/1/1), C50H50N8O8Co. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 719–721; https://doi.org/10.1515/ncrs-2022-0127.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

