Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
Abstract
C22H22N2O9, monoclinic, P21/c (no. 14), a = 7.7374(3) Å, b = 29.1082(11) Å, c = 8.7595(3) Å, β =
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.13 × 0.12 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.12 mm−1 |
| Diffractometer, scan mode: | ROD, Synergy Custom DW system, ω |
| θmax, completeness: | 29.3°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 14766, 4372, 0.028 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3383 |
| N(param)refined: | 318 |
| Programs: | CrysAlisPRO [1], Diamond [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.72325 (18) | 0.70755 (5) | 0.94430 (15) | 0.0302 (3) |
| H1 | 0.803283 | 0.721655 | 0.907218 | 0.045* |
| O2 | 0.41933 (19) | 0.58657 (5) | 0.37145 (15) | 0.0312 (3) |
| O3 | 0.40939 (19) | 0.57135 (5) | 0.83217 (15) | 0.0304 (3) |
| O4 | 0.0145 (2) | 0.39433 (5) | 0.22159 (16) | 0.0334 (4) |
| H4 | −0.011104 | 0.372950 | 0.279297 | 0.050* |
| O7 | −0.03272 (19) | 0.75635 (5) | 1.31442 (16) | 0.0327 (3) |
| O8 | −0.11739 (19) | 0.68461 (5) | 1.34302 (17) | 0.0363 (4) |
| N1 | 0.1660 (3) | 0.46773 (6) | 0.0737 (2) | 0.0410 (5) |
| N2 | 0.4231 (2) | 0.64722 (5) | 1.13785 (17) | 0.0226 (3) |
| H2 | 0.432622 | 0.632606 | 1.241837 | 0.027* |
| C1 | 0.6465 (2) | 0.67838 (7) | 0.8397 (2) | 0.0243 (4) |
| C2 | 0.6499 (2) | 0.68551 (7) | 0.6804 (2) | 0.0259 (4) |
| H2A | 0.702946 | 0.712403 | 0.645884 | 0.031* |
| C3 | 0.5784 (2) | 0.65445 (7) | 0.5765 (2) | 0.0254 (4) |
| H3 | 0.586891 | 0.659318 | 0.470431 | 0.031* |
| C4 | 0.4914 (2) | 0.61496 (6) | 0.6218 (2) | 0.0224 (4) |
| C5 | 0.4873 (2) | 0.60924 (6) | 0.7792 (2) | 0.0229 (4) |
| C6 | 0.5627 (2) | 0.63967 (6) | 0.8910 (2) | 0.0233 (4) |
| C7 | 0.4152 (2) | 0.58126 (6) | 0.5124 (2) | 0.0230 (4) |
| C8 | 0.3358 (2) | 0.54101 (6) | 0.5772 (2) | 0.0225 (4) |
| C9 | 0.3394 (3) | 0.53978 (7) | 0.7309 (2) | 0.0310 (5) |
| H9 | 0.289 (4) | 0.5194 (10) | 0.790 (3) | 0.055 (8)* |
| C10 | 0.2544 (2) | 0.50264 (6) | 0.4835 (2) | 0.0225 (4) |
| C11 | 0.2428 (3) | 0.50196 (7) | 0.3246 (2) | 0.0288 (4) |
| H11 | 0.290664 | 0.526699 | 0.272712 | 0.035* |
| C12 | 0.1626 (3) | 0.46591 (7) | 0.2390 (2) | 0.0291 (4) |
| C13 | 0.0898 (2) | 0.42847 (6) | 0.3068 (2) | 0.0259 (4) |
| C14 | 0.1054 (3) | 0.42864 (7) | 0.4681 (2) | 0.0273 (4) |
| H14 | 0.060505 | 0.403487 | 0.520180 | 0.033* |
| C15 | 0.1839 (3) | 0.46423 (7) | 0.5524 (2) | 0.0263 (4) |
| H15 | 0.192 (3) | 0.4620 (8) | 0.664 (3) | 0.037 (6)* |
| C16 | 0.5716 (2) | 0.62845 (7) | 1.0600 (2) | 0.0254 (4) |
| H16A | 0.574308 | 0.594636 | 1.072184 | 0.031* |
| H16B | 0.682111 | 0.640710 | 1.113133 | 0.031* |
| C17 | 0.2475 (2) | 0.63375 (7) | 1.0573 (2) | 0.0271 (4) |
| H17A | 0.243384 | 0.600068 | 1.041725 | 0.033* |
| H17B | 0.228892 | 0.648679 | 0.955154 | 0.033* |
| C18 | 0.1055 (3) | 0.64802 (7) | 1.1511 (2) | 0.0288 (4) |
| H18A | 0.118402 | 0.630865 | 1.249356 | 0.035* |
| H18B | −0.009066 | 0.640030 | 1.094522 | 0.035* |
| C19 | 0.1110 (2) | 0.69905 (7) | 1.1843 (2) | 0.0255 (4) |
| H19 | 0.086793 | 0.715777 | 1.084340 | 0.031* |
| C20 | 0.2929 (3) | 0.71344 (7) | 1.2601 (2) | 0.0277 (4) |
| H20A | 0.297033 | 0.747265 | 1.271701 | 0.033* |
| H20B | 0.314549 | 0.699651 | 1.363923 | 0.033* |
| C21 | 0.4343 (3) | 0.69811 (6) | 1.1647 (2) | 0.0258 (4) |
| H21A | 0.420425 | 0.714404 | 1.064811 | 0.031* |
| H21B | 0.550000 | 0.705980 | 1.219211 | 0.031* |
| C22 | −0.0265 (2) | 0.71368 (7) | 1.2880 (2) | 0.0267 (4) |
| O5a | 0.2909 (3) | 0.48698 (8) | 0.0261 (2) | 0.0538 (6) |
| O6a | 0.0492 (4) | 0.44931 (7) | −0.0124 (2) | 0.0544 (7) |
| O5Ab | 0.184 (4) | 0.5090 (7) | 0.019 (3) | 0.0538 (6) |
| O6Ab | 0.166 (5) | 0.4382 (7) | −0.017 (2) | 0.0544 (7) |
| O9 | −0.0796 (2) | 0.32321 (5) | 0.36681 (18) | 0.0378 (4) |
| H9A | −0.058854 | 0.300415 | 0.307328 | 0.057* |
| H9B | −0.019356 | 0.316840 | 0.454620 | 0.057* |
1 Source of materials
All reagents and chemicals were purchased from commercial sources and used without further purification. A mixture of 7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one (0.013 g), sodium nitrite (0.035 g), 6 mol/L nitric acid (1.0 mL) and water (10 mL) was sealed in a 20 mL vial and stood for 10 min. The mixture was sonicated for 1 min. The mixture was heated at 363 K for 24 h. The mixture was filtered and the residue was washed with water. The residue was dried at 353 K. 7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4H-chromen-4-one (0.015 g) was obtained. A mixture of nitro compound described before (0.015 g), piperidine-4-carboxylic acid (0.026 g), N,N-dimethyl formamide (1 mL), methanol (3 mL) and formaldehyde solution (20 μL, 37 %) was sealed in a 20 mL vial and sonicated for 10 min. Then the mixture was heated at 363 K for 24 h. The mixture evaporated for one day at room temperature. Red block shape crystals of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl) methyl)piperidin-1-ium-4-carboxylate monohydrate were obtained. 1H NMR (400 MHz, DMSO-d6) δ:8.50 (s, 1H, H9), 8.16 (d, J = 2.1 Hz, 1H, H11), 7.92 (d, J = 8.8 Hz, 1H, H3), 7.73 (dd, J = 8.7, 2.1 Hz, 1H, H15), 7.16 (d, J = 8.7 Hz, 1H, H14), 6.91 (d, J = 8.8 Hz, 1H, H2) 3.97 (s, 2H, H16A and H16B), 2.92 (m, 3H, H17B, H21B, H19), 2.32 (m, 2H, H17A, H21A), 1.87 (m, 2H, H18B, H20A), 1.59 (m, 2H, H18A, H20B). 13C NMR (100 MHz, DMSO-d6) δ:175.74 (C7), 174.47 (C22), 163.45 (C1), 155.16 (C5), 153.67 (C9), 152.30 (C13), 136.57 (C12), 135.43 (C15), 125.77 (C3), 125.26 (C10), 122.68 (C8), 121.20 (C11), 119.14 (C14), 116.05 (C4), 115.34 (C6), 108.37 (C2), 52.05 (C16), 51.76 (C17 and C21), 39.73 (C19), 27.75 (C18 and C20). IR spectra (potassium bromide pellet) were recorded on a Nicolet 6700. IR (v/cm−1): 3005, 2856, 2704, 1625, 1573, 1525, 1445, 1423, 1396, 1373, 1343, 1308, 1265, 1244, 1211, 1183, 1644, 1094, 1077, 1041, 962, 926, 823, 802, 785, 764, 717, 687, 640, 551, 495, 456, 428.
2 Experimental details
Carbon-bound H atoms with the exception of H15 and H9 were placed in calculated positions and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2 Ueq(C). The oxygen-bound, nitrogen-bound H atoms, H15 and H9 were located on a difference Fourier map. The O5 and O6 atoms were disordered.
3 Comment
Numerous publications describe the nitrated derivatives and Mannich base derivatives of flavonoids [5, 6]. Some derivatives of flavonoids possess important biological activities. Our previous investigations showed that daizein Mannich base derivatives of amino acid or aliphatic amine react with nitrating agent [7], [8], [9]. To extend this research, we investigated a new synthetic route. Nitro group was first introduced on the 3'-position of ring B of daidzein. Then we used the intermediate to react with piperidine-4-carboxylic acid by Mannich reaction and got the title compound. The presence of several groups in daizein derivatives expands the capability of studying their biological activity.
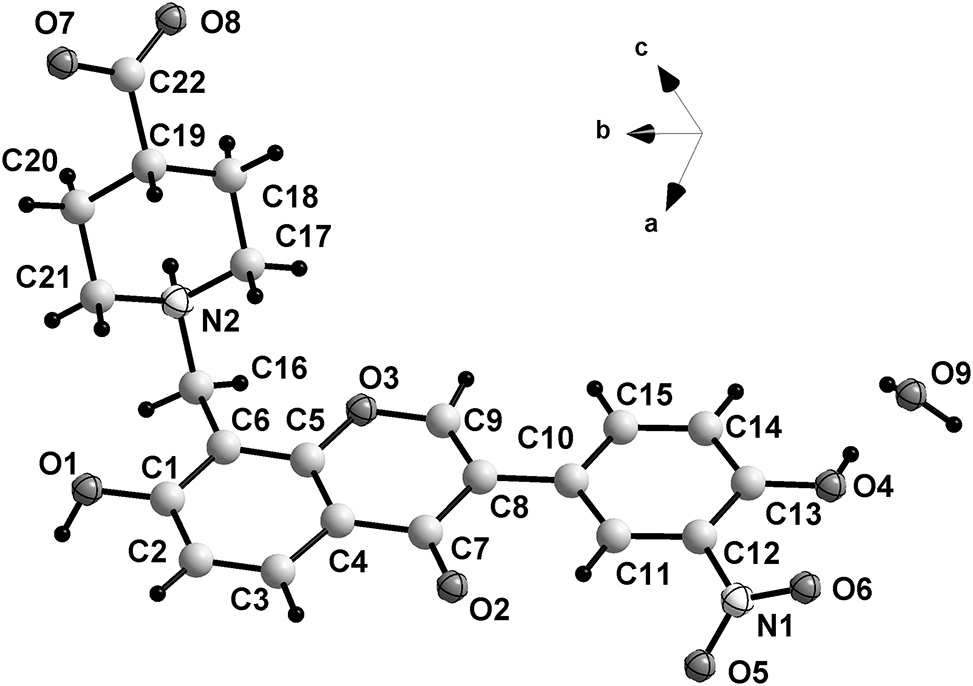
The title structure contains one zwitterion and one water molecule in the asymmetric unit (cf. the figure). The bond distances and bond angles are in normal ranges [7], [8], [9]. The dihedral angle between planar rings B (C10–C15) and C (C7–C9/O3/C5/C4) is
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financially supported by Guangxi Natural Science Foundation of China (No. 2020GXNSFBA297138) and 2021 High-level Talents Scientific Research Startup Fund of Hechi University (No. 2021GCC021).
-
Competing interests: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku. CrysAlisPRO; Rigaku Corporation: Yarnton, Oxfordshire, England, 2023.Search in Google Scholar
2. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 3.2; Crystal Impact: Bonn, Germany, 2012.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Lv, X.-H., Liu, H., Ren, Z.-L., Wang, W., Tang, F., Cao, H.-Q. Design, synthesis and biological evaluation of novel flavone Mannich base derivatives as potential antibacterial agents. Mol. Divers. 2019, 23, 299–306; https://doi.org/10.1007/s11030-018-9873-9.Search in Google Scholar PubMed
6. Tavera-Hernández, R., Jiménez-Estrada, M., Alvarado-Sansininea, J. J., Nieto-Camacho, A., López-Muñoz, H., Sánchez-Sánchez, L., Escobar, L. M. Synthesis of chrysin, quercetin and naringin nitroderivatives: antiproliferative, anti-inflammatory and antioxidant activity. Lett. Drug Des. Discov. 2021, 18, 795–805; https://doi.org/10.2174/1570180818666210122162313.Search in Google Scholar
7. Chen, H.-L., Yao, D.-M., Luo, Z.-P., Wang, Y.-P., Luo, L.-S. Synthesis and crystal structure of 3-(((7-hydroxy-3-(4-hydroxy-3,5-dinitrophenyl)-4-oxo-4H-chromen-8-yl)methyl) (nitroso)amino)propanoic acid, C19H14N4O11. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 1029–1031; https://doi.org/10.1515/ncrs-2022-0352.Search in Google Scholar
8. Chen, H.-L., Wang, Y.-P., Pan, L.-W., Wei, Z., Tang, L.-C. Synthesis and crystal structure of 5-(8-(((5-carboxypentyl)ammonio)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxy-3-nitrobenzenesulfonate monohydrate, C22H24N2O12S. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 217–219; https://doi.org/10.1515/ncrs-2022-0532.Search in Google Scholar
9. Chen, H.-L., Yao, D.-M., Luo, Z.-P., Wang, Y.-P., Tang, Y.-M. Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 949–951; https://doi.org/10.1515/ncrs-2023-0294.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3


