Abstract
C24H19N3O, monoclinic, P21/c (no. 14), a = 10.4783(5) Å, b = 11.7171(5) Å, c = 14.9194(6) Å, β = 93.991(2)°, Z = 4, V = 1827.29(14) Å3, Rgt(F) = 0.0521, wR ref (F2) = 0.1508, T = 150(2) K.
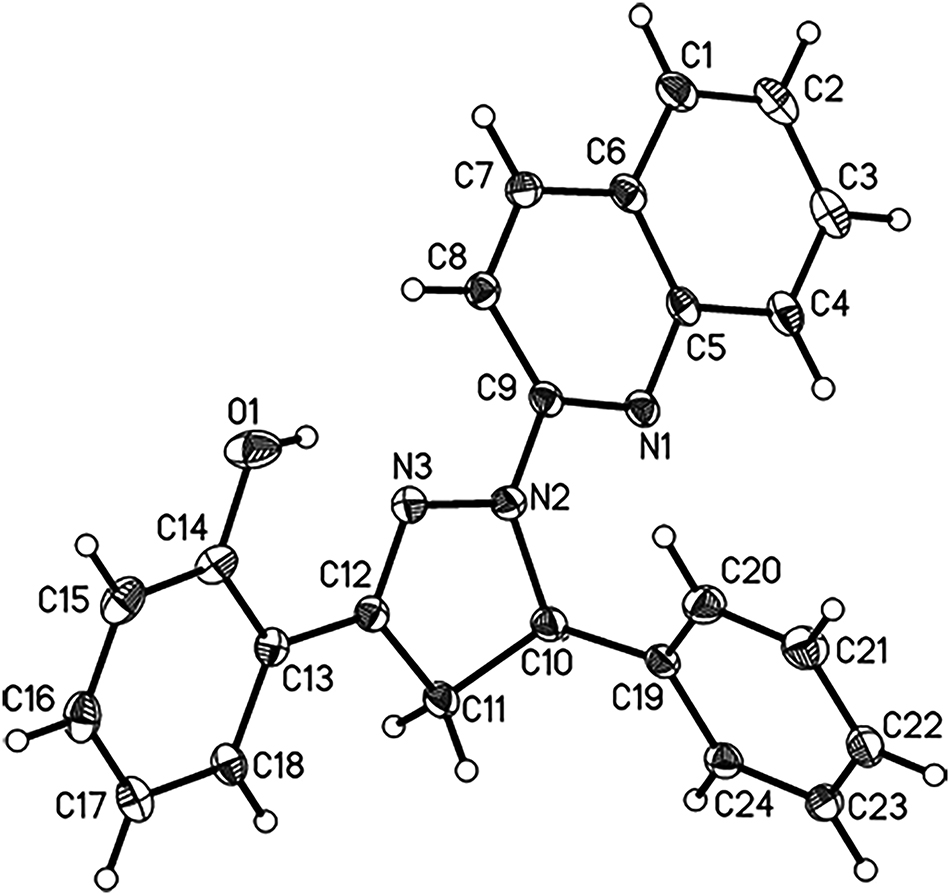
The asymmetric unit of the molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.21 × 0.21 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX II, ω |
| θmax, completeness: | 27.5°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 16,737, 4165, 0.058 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3391 |
| N(param)refined: | 254 |
| Programs: | Bruker [1], SHELX [2], [3], [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.03398 (15) | −0.07204 (13) | 0.67616 (9) | 0.0363 (3) |
| H1 | 0.062716 | −0.148579 | 0.683588 | 0.044* |
| C2 | −0.06060 (15) | −0.03185 (15) | 0.72672 (10) | 0.0413 (4) |
| H2 | −0.096828 | −0.080328 | 0.769236 | 0.050* |
| C3 | −0.10401 (15) | 0.08103 (15) | 0.71570 (10) | 0.0404 (4) |
| H3 | −0.170155 | 0.108159 | 0.750592 | 0.049* |
| C4 | −0.05199 (14) | 0.15283 (14) | 0.65503 (9) | 0.0340 (3) |
| H4 | −0.082184 | 0.229043 | 0.648630 | 0.041* |
| C5 | 0.04618 (13) | 0.11395 (12) | 0.60210 (8) | 0.0274 (3) |
| C6 | 0.08931 (13) | −0.00042 (12) | 0.61288 (8) | 0.0287 (3) |
| C7 | 0.18571 (13) | −0.03735 (11) | 0.55713 (9) | 0.0294 (3) |
| H7 | 0.216026 | −0.113715 | 0.561185 | 0.035* |
| C8 | 0.23475 (13) | 0.03607 (11) | 0.49802 (9) | 0.0272 (3) |
| H8 | 0.299106 | 0.012112 | 0.460326 | 0.033* |
| C9 | 0.18704 (12) | 0.15010 (11) | 0.49406 (8) | 0.0248 (3) |
| C10 | 0.17755 (13) | 0.33883 (11) | 0.41141 (8) | 0.0273 (3) |
| H10 | 0.082264 | 0.331514 | 0.405260 | 0.033* |
| C11 | 0.22931 (13) | 0.35856 (12) | 0.31828 (9) | 0.0303 (3) |
| H11A | 0.162265 | 0.344883 | 0.269386 | 0.036* |
| H11B | 0.263078 | 0.436977 | 0.312805 | 0.036* |
| C12 | 0.33452 (12) | 0.27128 (11) | 0.31697 (8) | 0.0248 (3) |
| C13 | 0.42898 (12) | 0.26678 (11) | 0.25005 (9) | 0.0261 (3) |
| C14 | 0.53215 (13) | 0.18893 (12) | 0.25635 (10) | 0.0329 (3) |
| C15 | 0.62124 (14) | 0.18941 (13) | 0.19190 (12) | 0.0409 (4) |
| H15 | 0.691157 | 0.137646 | 0.196868 | 0.049* |
| C16 | 0.60912 (14) | 0.26466 (13) | 0.12044 (11) | 0.0386 (4) |
| H16 | 0.670182 | 0.263616 | 0.076270 | 0.046* |
| C17 | 0.50872 (14) | 0.34143 (13) | 0.11281 (10) | 0.0350 (3) |
| H17 | 0.500725 | 0.392986 | 0.063596 | 0.042* |
| C18 | 0.41994 (13) | 0.34274 (12) | 0.17732 (9) | 0.0299 (3) |
| H18 | 0.351585 | 0.396124 | 0.172185 | 0.036* |
| C19 | 0.21608 (12) | 0.43379 (11) | 0.47688 (8) | 0.0259 (3) |
| C20 | 0.32214 (15) | 0.42667 (13) | 0.53781 (10) | 0.0370 (3) |
| H20 | 0.370847 | 0.358272 | 0.542364 | 0.044* |
| C21 | 0.35727 (16) | 0.51964 (14) | 0.59228 (11) | 0.0426 (4) |
| H21 | 0.430532 | 0.514630 | 0.633336 | 0.051* |
| C22 | 0.28623 (15) | 0.61915 (13) | 0.58699 (10) | 0.0367 (3) |
| H22 | 0.310240 | 0.682138 | 0.624555 | 0.044* |
| C23 | 0.17973 (14) | 0.62663 (12) | 0.52661 (10) | 0.0330 (3) |
| H23 | 0.130452 | 0.694733 | 0.522873 | 0.040* |
| C24 | 0.14526 (13) | 0.53458 (11) | 0.47169 (9) | 0.0287 (3) |
| H24 | 0.072643 | 0.540280 | 0.430111 | 0.034* |
| N1 | 0.09585 (10) | 0.18889 (9) | 0.54289 (7) | 0.0268 (3) |
| N2 | 0.23597 (11) | 0.22764 (9) | 0.43640 (7) | 0.0279 (3) |
| N3 | 0.33205 (10) | 0.19781 (9) | 0.38185 (7) | 0.0263 (3) |
| O1 | 0.54848 (11) | 0.11343 (10) | 0.32507 (9) | 0.0489 (3) |
| H1A | 0.486348 | 0.117934 | 0.357564 | 0.073* |
1 Source of materials
All chemicals were of reagent grade and used without further purification. The title compound was prepared following a literature procedure [5].
The intermediate is synthesized through aldol condensation reaction. 2-Hydroxy acetophenone (5.25 mmol) and benzaldehyde (5 mmol) were added to a 100 mL round-bottom flask along with 20 mL ethanol (EtOH). The mixture was stirred at room temperature for 10 min. An aqueous solution (2 mL) of NaOH (5 mmol) was then added drop wise. Subsequently, the mixture was agitated and allowed to stand at room temperature for additional 10 h. After completion, the reaction mixture was poured into ice cold water and neutralized with diluted HCl. Finally, the precipitated solid was filtered, washed with excess of cold water, dried and then recrystallized from ethanol to obtain the 2′-hydroxychalcone as yellow crystals. Yield: ca. 86 %.
The second step of synthetic procedure is as follows: A mixture of 2′-hydroxychalcone (10 mmol) and 2-hydrazinoquinoline (10 mmol) was dissolved in ethanol (30 ml) containing sodium hydroxide (30 mmol) and then refluxed for 4 h on an oil-bath. Completion of the reaction was confirmed by TLC. Five milliliter H2O were added and the reaction mixture was further stirred for 5 min. The above mixture was neutralized with diluted HCl. The orange yellow precipitates were isolated by filtration and recrystallized from ethanol. Yield: ca. 81 %. Suitable crystals of the title compound were grown from the mixed solution of ethanol and DMF by slow evaporation at room temperature.
2 Experimental details
The H atoms were added using riding models. Their Uiso values were set to 1.2Ueq of the parent atoms.
3 Comment
The construction of 1,3,5-trisubstituted pyrazolines has attracted great attention in recent years due to their wide applications in pharmaceuticals, fluorescent probes and electroluminescent materials [6], [7], [8], [9], [10]. In view of the structural diversity and important significance of 1,3,5-trisubstituted pyrazolines, research involving single crystal structures is of great significance to reveal the structure-activity relationship. The title compound 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol bearing both pyrazoline and quinoline moieties belongs to an important kind of 1, 3, 5-trisubstituted pyrazoline compounds.
The title compound crystallizes in the monoclinic space group P21/c with one molecule in the asymmetric unit (see the Figure). Bond lengths and angles are all in the normal range. A pyrazoline group as the five-membered core ring is formed by addition cyclization reaction of chalcone and hydrazine. The bond length of C12–N3 (1.2970 Å) indicates the existence of C=N in the pyrazoline ring, which is typical for similar compounds [11], [12], [13]. The pyrazoline ring is close to being planar with the largest deviation from the mean plane being 0.0883° for atom C10. In addition to the pyrazoline ring, it is substituted by a quinoline group, and a phenyl group and a phenol-2-yl group. The pyrazoline ring makes dihedral angles of 9.9° and 19.2° with the phenol and quinoline rings, respectively. Due to the steric hindrance effect, the benzene ring is almost perpendicular to the attached pyrazoline ring, showing a dihedral angle of approximately 103.9°. Phenolic hydroxyl group forms an intramolecular hydrogen bond to the pyrazoline N3 atom [O1⋯N3: 2.6656(16) Å]. π⋯π interactions are observed for parallel quinoline rings with the centroid-centroid distance between the two pyridine moieties of 3.66 Å.
Funding source: Zhongjing Scholars Research Funding of Henan University of Chinese Medicine
Award Identifier / Grant number: 00104311–2023–50
-
Research ethics: This study did not involve any animal experiments.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was supported by the Zhongjing Scholars Research Funding of Henan University of Chinese Medicine (00104311–2023–50).
-
Data availability: All relevent data are within the paper.
References
1. Siemens SAINT, Program for Data Extraction and Reduction; Siemens Analytical X-Ray Instruments: Madison, WI, 1994–1996.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL (version 5.0), Reference Manual; Siemens Industrial Automation, Analytical Instruments: Madison, WI, 1995.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Khan, S. S., Hasan, A. Synthesis of some new bioactive 1–N-substituted 3,5-diaryl-2-pyrazolined. Heterocycl. Commun. 2007, 13, 131–138.10.1515/HC.2007.13.2-3.131Search in Google Scholar
6. Ansari, M. I., Khan, S. A. Synthesis and antimicrobial activity of some novel quinoline-pyrazoline-based coumarinyl thiazole derivatives. Med. Chem. Res. 2017, 26, 1481–1496; https://doi.org/10.1007/s00044-017-1855-4.Search in Google Scholar
7. Insuasty, B., Chamizo, L., Munoz, J., Tigreros, A., Quiroga, J., Abonia, R., Nogueras, M., Cobo, J. Synthesis of 1-substituted 3-aryl-5-aryl (hetaryl)-2-pyrazolines and study of their antitumor activity. Arch. Pharm. 2012, 345, 275–286; https://doi.org/10.1002/ardp.201100170.Search in Google Scholar PubMed
8. Lokeshwari, D. M., Achutha, D. K., Srinivasan, B., Shivalingegowda, N., Krishnappagowda, L. N., Kariyappa, A. K. Synthesis of novel 2-pyrazoline analogues with potent anti-inflammatory effect mediated by inhibition of phospholipase A2: crystallographic, in silico docking and QSAR analysis. Bioorg. Med. Chem. Lett. 2017, 27, 3806–3811; https://doi.org/10.1016/j.bmcl.2017.06.063.Search in Google Scholar PubMed
9. Gayathri, K., Velmurugan, K., Nandhakumar, R., Malathi, M., Mathusalini, S., Mohan, P. S., Shankar, R. New pyrazolo-quinoline scaffold as a reversible colorimetric fluorescent probe for selective detection of Zn2+ ions and its imaging in live cells. J. Photochem. Photobiol. A 2017, 341, 136–145; https://doi.org/10.1016/j.jphotochem.2017.03.035.Search in Google Scholar
10. Ajantha, J., Varathan, E., Bharti, V., Subramanian, V., Easwaramoorthi, S., Chand, S. Photophysical and charge transport properties of pyrazolines. RSC Adv. 2016, 6, 786–795; https://doi.org/10.1039/c5ra19520a.Search in Google Scholar
11. Najib, M. H. B., Tan, A.-L., Young, D. J., Ng, S. W., Tiekink, E. R. T. 3,5–Diphenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-5-ol. Acta Crystallogr. 2012, E68, o2310–o2311; https://doi.org/10.1107/s1600536812029340.Search in Google Scholar
12. Goh, J. H., Fun, H. K., Adhikarib, A., Kalluraya, B. 5–Bromo-2-[5-(4- nitrophenyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl]pyrimidine. Acta Crystallogr. 2009, E65, o3134–o3135; https://doi.org/10.1107/s1600536809048600.Search in Google Scholar
13. Abdullah, A. A., Abdel-Wahab, B. F., Hegazy, A. S., Kariuki, B. M., El-Hiti, G. A. The crystal structure of 2-(3-(4-bromophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)- 8H-indeno[1,2-d]thiazole, C25H17BrFN3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 897–899; https://doi.org/10.1515/ncrs-2020-0088.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of bis(dimethylammonium) poly[(μ4-1,1′-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato))-κ6O1, N2:O2:O3:O1′,N2′]nickel (II)], C22H26N6NiO8
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-((pyridin-4-ylmethyl)amino)benzoato-κ2N:O)cobalt(II)] – 4,4′-bipyridine – water (1/2/2)
- Crystal structure of (2S,3S,4S,5S, Z)-2,3,5,6-tetrakis(benzyloxy)-4-hydroxyhexanal oxime, C34H37NO6
- The crystal structure of hexakis(3-thiophenecarboxylato-κ2O,O″)-bis(1,10-phenanthroline-κ2N,N′) trimanganese(II), C54H34N4O12S6Mn3
- Crystal structure of catena-poly[(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(4-bromobenzoate-κ2O:O′)-(μ-2-bromobenzoate-κ2O,O′)nickel(II)] – water (2/1), C30H21Br2N2NiO4.5
- The crystal structure of poly[(μ3-1,3-phenylenedioxydiacetate-κ5O,O,O′,O″,O‴)-bis(4′-(4-(1H-imidazol-1-yl)phenyl)-4,2′:6′,4″-terpyridine-kN) cadmium(II)], C58H42CdN10O6
- The crystal structure of 5-chloro-6′-methyl-3-(4-(methylsulfonyl)phenyl)-[2,3′-bipyridin]-1′-ium 4-methylbenzenesulfonate
- Crystal structure of poly[(μ-benzoato)-(μ-cis-4–hydroxy-D-proline)lithium], C12H14LiNO5
- The crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-copper(II)] monohydrate, C7H6NO6ICu
- The crystal structure of catena-[diaqua-(4-acetylphenoxyacetato-κ2O,O)-bis(4-acetylphenoxyacetato-κ3O,O:O)-dihydrate-lanthanum(III)]–4,4′-bipyridine (2/1), C35H35NO14La
- The crystal structure of catena-poly[(4-iodopyridine-2,6-dicarboxylato-κ4 O,N,O′,O′′)(4-imidazol-1-yl-pyridine-κN)copper(II)], C15H9N4O4ICu
- Crystal structure of polybis(μ 4-3,5-dicarboxylatopyrazol-1-yl)-bis(N,N-dimethylformamide)tri-copper(II)–acetonitrile (1/2), C20H22Cu3N8O10
- Crystal structure of poly[(μ2-5-hydroxy-isophthalato-κ4O,O′:O″,O‴)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)cadmium(II)], C21H24CdN4O5
- The crystal structure of poly[bis(μ2-1,4-bi(1-imidazolyl)benzene-κ2N:N′)bis(μ2-4,4′-methylenebis(3-hydroxy-2-naphthoate)-κ2O:O′)cobalt(II)], C35H24CoN4O6
- The crystal structure of a cobalt-vanadium-oxido hydrate
- The crystal structure of catena-poly[(μ 2-2H-1,2,3-triazole-4,5-dicarboxylato-κ 2 O, O′)-(μ 2-1,3-bis((1H-imidazol-1-yl)methyl)benzene-κ 2 N,N′) zinc(II)], C18H15N7O4Zn
- Crystal structure of poly[diaqua-(bis(m2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′-manganese] dichloride, C28H32MnN8O2Cl2
- The crystal structure of 9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino [3,2-a]isoquinolin-7-ium (E)-3-(4-nitrophenyl)acrylate pentahydrate, C29H34N2O13
- Crystal structure of poly[(μ6-ammoniotris(methylene))tris(hydrogen phosphonato)cadmium(II)], C3H10CdNO9P3
- Crystal structure of Zn2[(1,1′-(hexane-1,6-diyl)bis(3-(pyridin-3-yl)urea))·(H2O)2·(DMF)2·(SO4)2], C24H50N8O18S2Zn2
- The crystal structure of 2-anilino-1,4-naphthoquinone, C10H11NO2
- Crystal structure of (E)-2-(2-(4-(diethylamino)styryl)-1-ethyl-1,4-dihydroquinolin-4-yl) malononitrile, C26H26N4
- Crystal structure of ethyl 2-((2,6-dichloro-4-(cyanomethyl)phenyl) amino)benzoate, C17H14Cl2N2O2
- Synthesis and crystal structure of 2-(3-oxo-3-phenylpropyl)isoindoline-1,3-dione, C17H13NO3
- The crystal structure of bis(acetonitrile-κ1N)tetrakis(μ2-2,6-difluorobenzoato-κ2O:O′)rhodium(II) (Rh–Rh), C32H18F8O8N2Rh2
- The crystal structure of a new polymorph of 6-hydroxy-2-naphthoic acid, C11H8O3
- The crystal structure of [(8-carboxymethoxy-quinoline-2-carboxylate-κ4N,O,O,O)-2,2′-bipyridine-κ2N-copper(II)] tetrahydrate, C22H23N3O9Cu
- The crystal structure of ethyl 4-hydroxy-2-(4-methoxyphenyl)-5-oxo-1-(2-oxo-2H-chromen-6-yl)-2,5-dihydro-1H-pyrrole-3-carboxylate, C23H19NO7
- Crystal structure of 7-hydroxy-3,4-dihydronaphthalen-1(2H)-one, C10H10O2
- Crystal structure of bis(tetrapropylammonium) dodecacarbonyltetratelluridotetraferrate(2-), (Pr4N)2[Fe4Te4(CO)12]
- The crystal structure of poly[bis(μ2−3−aminopyridine−4−carboxylato−κ2N:O)Zinc(II)], [Zn(C6H5N2O2)2] n
- The crystal structure of methyl 5-nitro-2-(tosyloxy)benzoate, C15H13NO7S
- The crystal structure of 18-crown-6 ― tetraaqua-dichlorido-di-μ2-chloridodicopper(II) (2/1), C12H32O10Cu2Cl4
- Crystal structure of 6,6a,7,8,9,10-hexahydro-5H-pyrazino [2,3-e]pyrido[1,2-a]pyrazine, C10H14N4
- Crystal structure of catena-poly-{diaqua-bis[μ-(((4-chlorophenyl)sulfonyl)glycinato-κO)](μ2-4, 4′-bipyridine-κ2N:N′)cobalt(II)} dihydrate, C26H30Cl2CoN4O12S2
- Crystal structure of bis{N′-[1,3-diphenylprop-2-en-1-ylidene]-N-phenylcarbamohydrazonothioato}zinc(II), C44H36N6S2Zn
- Crystal structure of tetraaqua-bis(((4-chlorophenyl)sulfonyl)glycinato-κO)cobalt(II) dihydrate, C16H26Cl2CoN2O14S2
- Crystal structure of 2-(5-phenyl-1-(quinolin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol, C24H19N3O
- Crystal structure of 2-((2-fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-methoxy-3,4-dihydronaphthalen-1((2H))-one, C19H16F4O3
- Crystal structure of 2-amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of (2-phenylimino methylquinoline-κ 2 N,N′)-bis(1–phenylpyrazole-κ 2 C,N)-iridium(III) hexafluorophosphate, C34H26F6IrN6P
- Crystal structure of (3-hydroxy-4-methoxyphenyl)(pyrrolidin-1-yl)methanone, C12H15NO3
- The crystal structure of bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] C6H18O2S2NaInBr6
- Crystal structure of N-cyclopropyl-3-hydroxy-4-methoxybenzamide, C11H13NO3
- The crystal structure of (bis(benzimidazol-2-yl-methyl)amine-κ3N,N′,N″ )-(dihydrogen L-malate-κ2O,O ′)copper(II) perchlorate dihydrate, CuC20H24ClN5O12
- Crystal structure of (1E,1′E)-4,4′-(9,9-diethyl-9H-fluorene-2,7-diyl)dibenzaldehyde dioxime, C31H28N2O2
- Crystal structure of diethyl 1,9-bis(4-fluorophenyl)-4,6-diphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta [b]pyridine-3,7(2H)-dicarboxylate, C40H36F2N2O4
- Crystal structure of bis(benzene-1 carboxylato-O 3,5-carboxyl-κ1O)-[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) ─ benzene-1,3,5-tricarboxylic acid ─ water (1/2/4), C52H66N4NiO28
- Crystal structure of 1,4-dibromo-2,5-bis(2-methoxyethoxy)benzene-1,4-diol, C12H16Br2O4
- Crystal structure of dicarbonyl[N,N′-(1,2-dimethyl-1,2-ethanediylidene)bis[2,6-bis(1-methylethyl)benzenamine]-N,N′]nickel(0), C30H40N2NiO2
- Crystal structure of 1,4-dibromo-2,5-bis(prop-2-yn-1-yloxy)benzene, C12H8Br2O2
- Crystal structure of O-(3-(benzo[d]thiazol-2-yl)naphthalen-2-yl) O-phenyl carbonothioate, C24H15NO2S2
- The crystal structure of (E)-4-fluoro-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
- Crystal structure of (E)-1-(benzo[d]thiazol-2-yl)-N-(4,5-dihydropyren-2-yl)methanimine, C24H16N2S
- Crystal structure of 3-((4-bromophenyl)thio)-1H-indole, C14H10BrNS
- Synthesis and crystal structure of 1-((7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)methyl)piperidin-1-ium-4-carboxylate monohydrate, C22H22N2O9
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α]phenanthren-3-one O-(methacryloyl) oxime, C50H74N2O4
- Crystal structure of the hydrogen storage active phase La12Mg46LiMn
- The crystal structure of the salt: 4-((1,3-dioxoisoindolin-2-yl)carbamoyl)pyridine-1-ium 2-carboxybenzoate, C14H10N3O3·C8H5O4
- Crystal structure of (2-(2-pyridine)-benzimidazole-κ2 N,N′)-bis(1-phenylpyrazole-κ2 C,N)iridium(III) hexafluorophosphate, C30H22F6IrN7P
- Crystal structure of dichlorido-bis[2-(2,4-difluorophenyl)pyridine-κ1N]platinum(II), C22H14Cl2F4N2Pt
- Crystal structure of (5R,8R,9R,10R,12R,13R,14R, 17S,17Z)-2-((3-fluoropyridin-4-yl)methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C36H52FNO3

