Abstract
C18H27N5O6, monoclinic, P21/n (no. 14), a = 11.6588(12) Å, b = 7.0645(6) Å, c = 25.700(3) Å, β = 94.899(3)°, V = 2109.0(4) Å3, Z = 4, Rgt(F) = 0.0630, wRref(F2) = 0.1632, T = 273 K.
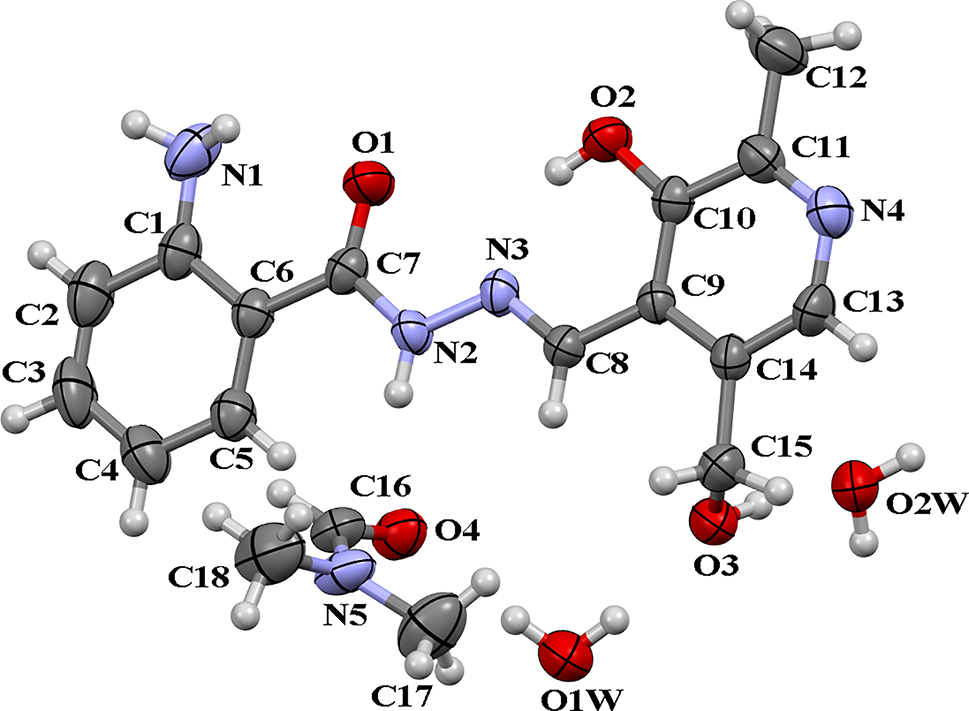
The crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.2 × 0.2 × 0.15 mm |
| Wavelength | Mo Kα radiation (0.71073 Å) |
| μ: | 0.1 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω-scans |
| θmax, completeness: | 26°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12424, 4070, 0.102 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 1844 |
| N(param)refined: | 274 |
| Programs: | OLEX2 [1], Bruker programs [2], SHELX [3], DIAMOND [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters(Å2)
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| O2 | 0.25686 (16) | 0.7385 (3) | 0.56082 (7) | 0.0499 (6) |
| H2 | 0.317899 | 0.744932 | 0.547259 | 0.075* |
| O3 | 0.58899 (16) | 0.7475 (3) | 0.71957 (8) | 0.0546 (6) |
| H3 | 0.554795 | 0.841077 | 0.729097 | 0.082* |
| O4 | 0.76673 (17) | 0.5615 (3) | 0.59111 (8) | 0.0552 (6) |
| O1 | 0.44549 (18) | 0.7995 (3) | 0.45887 (8) | 0.0591 (6) |
| N3 | 0.47432 (19) | 0.7123 (3) | 0.55708 (9) | 0.0387 (6) |
| N2 | 0.56592 (19) | 0.7175 (3) | 0.52767 (9) | 0.0410 (6) |
| H2A | 0.634301 | 0.691214 | 0.540902 | 0.049* |
| N4 | 0.1945 (2) | 0.6393 (4) | 0.69189 (10) | 0.0552 (7) |
| N5 | 0.8088 (2) | 0.2478 (4) | 0.59874 (11) | 0.0569 (7) |
| N1 | 0.5183 (3) | 0.8405 (4) | 0.36565 (10) | 0.0670 (8) |
| H1A | 0.520579 | 0.812343 | 0.333088 | 0.080* |
| H1B | 0.468888 | 0.767860 | 0.378926 | 0.080* |
| C8 | 0.4888 (2) | 0.6711 (4) | 0.60559 (11) | 0.0387 (7) |
| H8 | 0.562010 | 0.646382 | 0.621366 | 0.046* |
| C9 | 0.3894 (2) | 0.6637 (4) | 0.63563 (10) | 0.0350 (7) |
| C10 | 0.2779 (2) | 0.6954 (4) | 0.61193 (11) | 0.0398 (7) |
| C7 | 0.5450 (3) | 0.7663 (4) | 0.47624 (11) | 0.0400 (7) |
| C6 | 0.6441 (2) | 0.7765 (4) | 0.44447 (11) | 0.0410 (7) |
| C14 | 0.3998 (2) | 0.6215 (4) | 0.68922 (11) | 0.0402 (7) |
| C5 | 0.7569 (3) | 0.7514 (4) | 0.46639 (12) | 0.0478 (8) |
| H5 | 0.769695 | 0.728869 | 0.502064 | 0.057* |
| C11 | 0.1830 (2) | 0.6820 (4) | 0.64169 (13) | 0.0464 (8) |
| C1 | 0.6255 (3) | 0.8128 (4) | 0.39011 (12) | 0.0471 (8) |
| C16 | 0.7837 (3) | 0.4039 (5) | 0.57231 (13) | 0.0554 (9) |
| H16 | 0.778224 | 0.394951 | 0.536082 | 0.066* |
| C15 | 0.5149 (3) | 0.5877 (4) | 0.71894 (12) | 0.0511 (8) |
| H15A | 0.502894 | 0.553478 | 0.754617 | 0.061* |
| H15B | 0.552247 | 0.481755 | 0.703321 | 0.061* |
| C13 | 0.3015 (3) | 0.6112 (5) | 0.71469 (12) | 0.0545 (9) |
| H13 | 0.309135 | 0.582744 | 0.750155 | 0.065* |
| C4 | 0.8493 (3) | 0.7590 (4) | 0.43674 (15) | 0.0624 (9) |
| H4 | 0.923764 | 0.741537 | 0.451989 | 0.075* |
| C2 | 0.7218 (3) | 0.8189 (4) | 0.36098 (14) | 0.0642 (10) |
| H2B | 0.711275 | 0.840936 | 0.325219 | 0.077* |
| C3 | 0.8294 (3) | 0.7934 (5) | 0.38373 (16) | 0.0710(11) |
| H3A | 0.891478 | 0.798947 | 0.363330 | 0.085* |
| C12 | 0.0643 (3) | 0.7146 (5) | 0.61596 (14) | 0.0712 (10) |
| H12A | 0.040354 | 0.605789 | 0.595432 | 0.107* |
| H12B | 0.064725 | 0.823843 | 0.593771 | 0.107* |
| H12C | 0.011725 | 0.734930 | 0.642211 | 0.107* |
| C17 | 0.8150 (4) | 0.2473 (5) | 0.65483 (14) | 0.0864 (13) |
| H17A | 0.859477 | 0.353886 | 0.668085 | 0.130* |
| H17B | 0.850993 | 0.132417 | 0.667792 | 0.130* |
| H17C | 0.738740 | 0.255224 | 0.666090 | 0.130* |
| C18 | 0.8172 (3) | 0.0686 (5) | 0.57124(15) | 0.0847 (13) |
| H18A | 0.755625 | −0.013245 | 0.579521 | 0.127* |
| H18B | 0.889698 | 0.009787 | 0.581809 | 0.127* |
| H18C | 0.811873 | 0.091351 | 0.534289 | 0.127* |
| O2W | 0.50095 (18) | 1.0797 (3) | 0.75177 (8) | 0.0569 (6) |
| H2WA | 0.558553 | 1.132257 | 0.768455 | 0.085* |
| H2WB | 0.441112 | 1.098348 | 0.767793 | 0.085* |
| O1W | 0.81272 (18) | 0.7480 (4) | 0.69001 (10) | 0.0659 (6) |
| H1WA | 0.747355 | 0.746871 | 0.702423 | 0.099* |
| H1WB | 0.800454 | 0.698770 | 0.659905 | 0.099* |
1 Source of materials
A solution of 2-aminobenzohydrazide (151 mg, 1 mmol) in ethanol (25 ml) was added to a stirred ethanol solution (25 ml) of 3-hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde (167 mg, 1 mmol) in a three-necked flask. The reaction mixture was refluxed for 12 h, then the solution was cooled to room temperature. The yellow precipitated residue was removed from the solution by filtration and then washed with ethanol 3 times. The yellow solids (0.2 mmol) were dissolved in a solution mixture (45 ml, CH3OH/DMF, 8:1) within 7 days. Single crystals suitable for X-ray diffraction were obtained by slow evaporation at room temperature.
2 Experimental details
Crystal data, data collection and structure refinement details are summarized in Table 1. Using Olex2 [1], the structure was solved using Charge Flipping and refined with the ShelXL [3] refinement. All hydrogen atoms were positioned geometrically, with the d(C–H) = 0.97–0.99 Å, Uiso(H) = 1.2 times Ueq(C) and Uiso(H) = 1.5 times Ueq(O).
3 Comment
Acylhydrazones as a special kind of Schiff base have been widely investigated because their structures are similar to that of natural biological substances [5]. Acylhydrazones exhibit strong coordination abilities and flexible coordination modes due to the N, O donor atoms. It has also been found that acylhydrazones containing pyridine ring are easier to coordinate because of the additional N donor atom. In the crystal structure of the title compound, the asymmetric unit of the title compound consists of one formula unit of the title molecule, one DMF and two water molecules. The dihedral angle of the amino-phenyl ring and pyridine ring is 2.83°. The bond angles and bond lengths are in the normal ranges [6, 7]. There are intramolecular hydrogen bonds (O2–H2B⋯N3 and N1–H1B⋯O1) in the molecules. The hydrogen bonds between the target compound and water molecules extend the framework into a 2D structure.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Guizhou Normal University Qian Shi Xinmiao [2022]B04), the Provincial General Project of University Student Innovation and Entrepreneurship Fund, China (No. 202210663072).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
[1] Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
[2] Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Suche in Google Scholar
[3] Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
[4] Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0. Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
[5] Cui, P., Cai, M., Meng, Y., Yang, Y., Song, H., Liu, Y., Wang, Q. Design, synthesis and biological activities of echinopsine derivatives containing acylhydrazone moiety. Sci. Rep. 2022, 12, 2935–2944; https://doi.org/10.1038/s41598-022-06775-7.Suche in Google Scholar PubMed PubMed Central
[6] Park, H., Kim, J., Kwon, E., Kim, T. H. Crystal structure of flucetosulfuron. Acta Crystallogr. 2017, E73, 1439–1442; https://doi.org/10.1107/s2056989017012737.Suche in Google Scholar PubMed PubMed Central
[7] Al-Wahaibi, L. H., Blacque, O., Tiekink, E. R. T., El-Emam, A. A. Crystal structure of 4-chloro-N′-[(1E)-pyridin-3-ylmethylidene]benzohydrazide, C13H10ClN3O. Z. Kristallogr. - N. Cryst. Struct. 2023, 238, 517–520; https://doi.org/10.1515/ncrs-2023-0060.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2

