Abstract
C18H17NO3S, monoclinic, Cc (no. 9), a = 32.036(3) Å, b = 7.2154(7) Å, c = 7.4283(8) Å, β = 99.943(2)∘, V = 1691.3(3) Å3, Z = 4, R gt (F) = 0.0343, wR ref (F2) = 0.0821, T = 298 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.45 × 0.40 × 0.27 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.21 mm−1 |
| Diffractometer, scan mode: | Rigaku Saturn724+, ω |
| θmax, completeness: | 25.0°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 4107, 2059, 0.025 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 1758 |
| N(param)refined: | 209 |
| Programs: | Shelx [1, 2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | 0.20187 (9) | 0.2741 (3) | −0.0820 (4) | 0.0364 (7) |
| H1A | 0.192194 | 0.161180 | −0.114541 | 0.055* |
| H1B | 0.197584 | 0.348387 | −0.179004 | 0.055* |
| H1C | 0.188229 | 0.318595 | 0.003405 | 0.055* |
| O1 | 0.17069 (9) | 0.3925 (3) | 0.2256 (3) | 0.0473 (7) |
| O2 | 0.17113 (8) | 0.0663 (3) | 0.2803 (4) | 0.0472 (7) |
| O3 | 0.19201 (9) | 0.2756 (3) | 0.5321 (4) | 0.0466 (7) |
| S1 | 0.16581 (3) | 0.24753 (11) | 0.35653 (12) | 0.0334 (2) |
| C1 | 0.24689 (11) | 0.2643 (4) | −0.0098 (5) | 0.0350 (8) |
| C2 | 0.26330 (13) | 0.1106 (5) | 0.0853 (5) | 0.0475 (10) |
| H2 | 0.245987 | 0.009307 | 0.096756 | 0.057* |
| C3 | 0.30558 (13) | 0.1064 (5) | 0.1641 (6) | 0.0498 (11) |
| H3 | 0.316370 | 0.001708 | 0.229087 | 0.060* |
| C4 | 0.33229 (12) | 0.2539 (5) | 0.1489 (5) | 0.0408 (9) |
| C5 | 0.31494 (14) | 0.4068 (5) | 0.0491 (6) | 0.0568 (11) |
| H5 | 0.332297 | 0.507203 | 0.035156 | 0.068* |
| C6 | 0.27306 (13) | 0.4137 (5) | −0.0292 (6) | 0.0520 (10) |
| H6 | 0.262191 | 0.517689 | −0.095034 | 0.062* |
| C7 | 0.37750 (13) | 0.2506 (5) | 0.2406 (6) | 0.0464 (10) |
| C8 | 0.39104 (14) | 0.1487 (6) | 0.3966 (7) | 0.0636 (12) |
| H8 | 0.371423 | 0.077298 | 0.444642 | 0.076* |
| C9 | 0.43264 (16) | 0.1489 (7) | 0.4838 (8) | 0.0755 (14) |
| H9 | 0.440699 | 0.078418 | 0.588872 | 0.091* |
| C10 | 0.46204 (15) | 0.2524 (6) | 0.4159 (8) | 0.0709 (13) |
| H10 | 0.490133 | 0.253494 | 0.474651 | 0.085* |
| C11 | 0.44974 (15) | 0.3548 (7) | 0.2604 (7) | 0.0677 (13) |
| H11 | 0.469657 | 0.424959 | 0.213057 | 0.081* |
| C12 | 0.40837 (13) | 0.3545 (6) | 0.1743 (7) | 0.0597 (11) |
| H12 | 0.400628 | 0.425130 | 0.069073 | 0.072* |
| C13 | 0.11208 (12) | 0.2592 (4) | 0.3857 (5) | 0.0375 (8) |
| C14 | 0.09669 (13) | 0.1319 (5) | 0.4960 (6) | 0.0554(11) |
| H14 | 0.114542 | 0.041636 | 0.556587 | 0.067* |
| C15 | 0.05478 (15) | 0.1386 (7) | 0.5163 (7) | 0.0706 (14) |
| H15 | 0.044445 | 0.052781 | 0.591052 | 0.085* |
| C16 | 0.02814 (15) | 0.2710 (7) | 0.4272 (7) | 0.0670 (14) |
| H16 | −0.000190 | 0.274422 | 0.440381 | 0.080* |
| C17 | 0.04341 (16) | 0.3960 (7) | 0.3203 (7) | 0.0696 (13) |
| H17 | 0.025347 | 0.486226 | 0.261026 | 0.084* |
| C18 | 0.08524 (13) | 0.3932 (5) | 0.2966 (6) | 0.0555 (11) |
| H18 | 0.095228 | 0.480184 | 0.221971 | 0.067* |
1 Source of materials
In a representative experiment 4-aminobiphenyl (16.9 mg, 0.10 mmol) was dissolved in 10 mL of methanol, then benzenesulfonic acid (15.8 mg, 0.10 mmol) in 2 mL of methanol was added. The solution was stirred for 5 min, then filtered into a test tube and left standing at room temperature. After ca. 12 days colorless block crystals were collected.
2 Experimental details
Hydrogen atoms attached to the C atoms were placed in calculated positions with d(C–H) = 0.93 Å [1]. Positions of the active hydrogen atoms were located from the Fourier difference syntheses [2].
3 Comment
Salts have always been eye-catching aspects of crystal engineering CE that can offer an alternative but efficient approach for improving the physicochemical characteristics of a target compound, such as solubility, bioavailability, hygroscopicity, stability, density, elasticity, plasticity, and melting point [3]. Meanwhile, salts are also known as the paradise of material science for exploring novel phenomena and functionalities of optical [4], room-temperature phosphorescence [5], ferroelectricity [6], etc. Therefore, molecular salts have attracted considerable interest and found their potential application in various fields of pharmaceutical [7], energetic [8], and photovoltaics industries [9, 10].
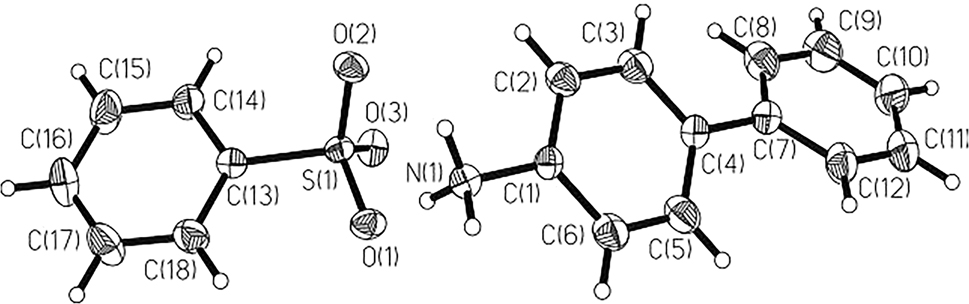
In the asymmetric unit there existed each of a 4-aminiumbiphenyl and benzenesulfonate (Figure 1), akin to diiso-propyl-ammonium benzene-sulfonate [11] and 2-aminiumbenzoic acid benzene-sulfonate [12].
The benzenesulfonic acid donates its sulfonic H to the NH2 of 4-aminobiphenyl. There is an ion pair with no solvent molecules. The bond lengths and angles within these moieties are in the expected ranges. At 4-aminiumbiphenyl the both phenyl twisted by 27.7° from each other. The C13–C18 plane intersected at 30.5/28.2° with both rings at the cation. In the title crystal structure all of the bond geometries are in the normal range. The S–O lengths are ranging from 1.438(2) to 1.455(2) Å, the S(1)–C(13) (1.774(3) Å) was akin to the filed one [13]. The benzenesulfonate is unambiguously tetrahedral, with three O and a aryl unit: O(3)–S(1)–O(2) (113.11(13)); O(3)–S(1)–O(1) (113.06(13)); O(2)–S(1)–O(1) (110.77(13)); O(3)–S(1)–C(13) (108.01(13)); O(2)–S(1)–C(13) (105.84(13)); and O(1)–S(1)–C(13) (105.45(13)°) are for tetrahedral environment.
One 4-aminiumbiphenyl was anchored to one benzenesulfonate by the N–H⋯O hydrogen bond of 2.775(3) Å from one H of the –NH3+ and one O at the –SO3−, and CH–O contact of 3.581 Å from the aryl CH ortho to the –NH3+ and the second O at the –SO3− to make a heteroadduct. The heteroadducts were linked by the N–H⋯O hydrogen bond of 2.781(3)–2.829(3) Å the –NH3+ and the –SO3−, N–H⋯S hydrogen bond of 3.644(2) Å with the –SO3− and CH–O contact of 3.233 Å from the aryl CH ortho to the –SO3− and the –SO3− to set up 1D chain in the c-axis. The chains were merged by the N(1)–H(1B)⋯O(1)#2 hydrogen bond and the O⋯O contact of 2.934 Å between the –SO3− to establish 2D sheet in the bc plane. The O⋯O contact was comparable with the document [14]. The sheet enclosed the R12(3), R22(8), R33(9), and R33(10) rings according to Bernstein [15].
Funding source: Zhejiang Province New Seedling Plan
Award Identifier / Grant number: 2022R412A037
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Zhejiang Province New Seedling Plan of China under Grant No. 2022R412A037, and Jiyang 533 project RC2022F01 for Shouwen Jin.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Samipillai, M., Rohani, S. The role of higher coformer stoichiometry ratio in pharmaceutical cocrystals for improving their solid-state properties: the cocrystals of progesterone and 4-hydroxybenzoic acid. J. Cryst. Growth 2019, 507, 270; https://doi.org/10.1016/j.jcrysgro.2018.10.050.Search in Google Scholar
4. Ganie, A. A., Ismail, T. M., Sajith, P. K., Dar, A. A. Validation of the supramolecular synthon preference through DFT and physicochemical property investigations of pyridyl salts of organo-sulfonates. New J. Chem. 2021, 45, 4780–4790; https://doi.org/10.1039/d0nj05485b.Search in Google Scholar
5. Zhou, B., Yan, D. P. Hydrogen-bonded two-component ionic crystals showing enhanced long-lived room-temperature phosphorescence via TADF-assisted foster resonance energy transfer. Adv. Funct. Mater. 2018, 29, 1807599; https://doi.org/10.1002/adfm.201807599.Search in Google Scholar
6. Akutagawa, T., Takeda, T., Hoshino, N. Dynamics of proton, ion, molecule, and crystal lattice in functional molecular assemblies. Chem. Commun. 2021, 57, 8378–8401; https://doi.org/10.1039/d1cc01586a.Search in Google Scholar PubMed
7. Batisai, E. Multicomponent crystals of anti-tuberculosis drugs: a mini-review. RSC Adv. 2020, 10, 37134; https://doi.org/10.1039/d0ra06478e.Search in Google Scholar PubMed PubMed Central
8. Liu, W., Liu, W. L., Pang, S. P. Structures and properties of energetic cations in energetic salts. RSC Adv. 2017, 7, 3617–3627; https://doi.org/10.1039/c6ra26032b.Search in Google Scholar
9. Bates, M., Richard, R. Organic salt photovoltaics. Sustain. Energy Fuels 2017, 1, 955–968; https://doi.org/10.1039/c7se00142h.Search in Google Scholar
10. Damayanti, J. D., Pratama, D. E., Lee, T. Green technology for salt formation: slurry reactive crystallization studies for papaverine HCl and 1:1 haloperidol-maleic acid salt. Cryst. Growth Des. 2019, 19, 2881–2891; https://doi.org/10.1021/acs.cgd.9b00098.Search in Google Scholar
11. Seye, D., Diop, C. A. K., Diop, L., Geiger, D. K. Diiso-propyl-ammonium benzene-sulfonate. IUCrData 2018, 3, x180876; https://doi.org/10.1107/s2414314618008763.Search in Google Scholar
12. Lu, Y., Xu, W. Q., Sun, H. L., Jin, J. Y., Liu, H., Jin, S. W., Wang, D. Q., Guo, M. Single-crystal and molecular structures of six hydrogen-bonding 3D supramolecular salts from 2-aminobenzoic acid, 3-aminobenzoic acid, 4-aminobenzoic acid, and acidic components. J. Mol. Struct. 2019, 1178, 639–654; https://doi.org/10.1016/j.molstruc.2018.10.080.Search in Google Scholar
13. Zhang, Y. T., Zhang, Y. Q., Ye, W., Li, Z. H., Jin, S. W., Guo, M., Bai, L. Q., Wang, D. Q. Eleven adducts constructed from 4-methylbenzo[d]thiazol-2-amine and organic acids via coupling of classical H-bonds and noncovalent interactions. J. Mol. Struct. 2021, 1241, 130614; https://doi.org/10.1016/j.molstruc.2021.130614.Search in Google Scholar
14. Zhang, Y. T., HuK, K., Chen, J. Y., Zhang, L. J., Xu, W. Q., Jin, S. W., Wang, D. Q. Eleven adducts from 4-methylbenzo[d]thiazol-2-amineand carboxylic acids via classical H-bonds and noncovalent associations. J. Mol. Struct. 2021, 1229, 129819; https://doi.org/10.1016/j.molstruc.2020.129819.Search in Google Scholar
15. Bernstein, J., Davis, R. E., Shimoni, L., Chang, N. L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem., Int. Ed. 1995, 34, 1555–1573; https://doi.org/10.1002/anie.199515551.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2

