The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
Abstract
C25H27CoN2NaO9S, monoclinic, P21/c (no. 14), a = 12.8169(11) Å, b = 7.9965(7) Å, c = 25.597(2) Å, β = 91.530(2)°, V = 2622.5(4) Å3, Z = 4, Rgt(F) = 0.0611, wRref(F2) = 0.1596, T = 298 K.
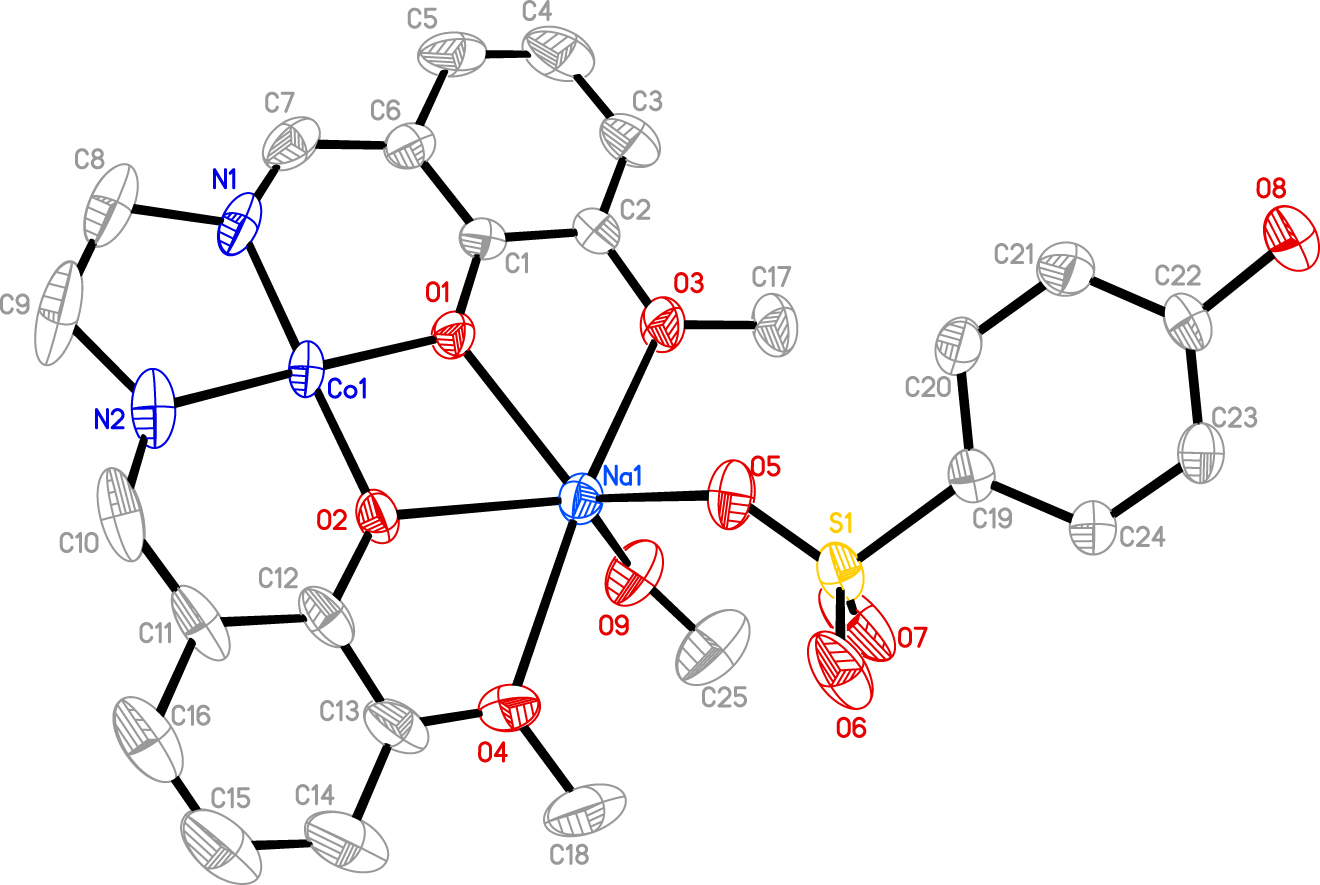
The crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange block |
| Size: | 0.39 × 0.26 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.81 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 25°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12200, 4625, 0.050 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3106 |
| N(param)refined: | 355 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| Co1 | 0.48163 (5) | 0.16901 (8) | 0.45260 (3) | 0.0435 (2) |
| S1 | 0.86373 (11) | 0.57559 (16) | 0.37347 (5) | 0.0533 (4) |
| Na1 | 0.72970 (14) | 0.2508 (3) | 0.41758 (7) | 0.0525 (5) |
| O1 | 0.5672 (2) | 0.1240 (4) | 0.39909 (13) | 0.0490 (9) |
| O2 | 0.5979 (3) | 0.2658 (4) | 0.48073 (12) | 0.0523 (9) |
| O3 | 0.7098 (3) | 0.1142 (5) | 0.33201 (14) | 0.0645 (11) |
| O4 | 0.7811 (3) | 0.3631 (6) | 0.50647 (16) | 0.0792 (12) |
| O5 | 0.7636 (4) | 0.5015 (6) | 0.37822 (18) | 0.1002 (16) |
| O6 | 0.8777 (4) | 0.7151 (5) | 0.40655 (15) | 0.0911 (15) |
| O7 | 0.9458 (4) | 0.4572 (6) | 0.38135 (15) | 0.1039 (18) |
| O8 | 0.8919 (3) | 0.8118 (6) | 0.15852 (15) | 0.0835 (13) |
| H8 | 0.9457 | 0.8647 | 0.1540 | 0.125* |
| O9 | 0.8703 (3) | 0.0519 (5) | 0.42447 (18) | 0.0824 (13) |
| H9 | 0.8555 | −0.0465 | 0.4191 | 0.124* |
| N1 | 0.3693 (3) | 0.0616 (6) | 0.4236 (2) | 0.0596 (13) |
| N2 | 0.3979 (4) | 0.2204 (6) | 0.5066 (2) | 0.0689 (15) |
| C1 | 0.5437 (4) | 0.0431 (6) | 0.35637 (19) | 0.0424 (11) |
| C2 | 0.6201 (4) | 0.0325 (6) | 0.31839 (19) | 0.0495 (13) |
| C3 | 0.6032 (6) | −0.0533 (8) | 0.2734 (2) | 0.0732 (18) |
| H3 | 0.6555 | −0.0594 | 0.2490 | 0.088* |
| C4 | 0.5100 (7) | −0.1311 (9) | 0.2636 (3) | 0.095 (2) |
| H4 | 0.4990 | −0.1906 | 0.2327 | 0.114* |
| C5 | 0.4347 (6) | −0.1215 (8) | 0.2985 (3) | 0.080 (2) |
| H5 | 0.3713 | −0.1743 | 0.2914 | 0.096* |
| C6 | 0.4489 (4) | −0.0337 (7) | 0.3455 (2) | 0.0536 (14) |
| C7 | 0.3669 (4) | −0.0220 (8) | 0.3808 (3) | 0.0672 (17) |
| H7 | 0.3057 | −0.0797 | 0.3724 | 0.081* |
| C8 | 0.2751 (5) | 0.0750 (12) | 0.4537 (4) | 0.113 (3) |
| H8A | 0.2231 | 0.1387 | 0.4340 | 0.135* |
| H8B | 0.2471 | −0.0359 | 0.4596 | 0.135* |
| C9 | 0.2946 (6) | 0.1532(12) | 0.5021 (4) | 0.134 (4) |
| H9A | 0.2848 | 0.0727 | 0.5299 | 0.161* |
| H9B | 0.2445 | 0.2428 | 0.5064 | 0.161* |
| C10 | 0.4248 (7) | 0.3031 (8) | 0.5473 (3) | 0.084 (2) |
| H10 | 0.3729 | 0.3244 | 0.5712 | 0.101* |
| C11 | 0.5249 (6) | 0.3653 (7) | 0.5599 (2) | 0.0723 (19) |
| C12 | 0.6080 (5) | 0.3395 (6) | 0.5261 (2) | 0.0585 (15) |
| C13 | 0.7070 (6) | 0.3927 (7) | 0.5420 (2) | 0.0726 (18) |
| C14 | 0.7230 (8) | 0.4697 (9) | 0.5898 (3) | 0.103 (3) |
| H14 | 0.7893 | 0.5039 | 0.6008 | 0.124* |
| C15 | 0.6378 (9) | 0.4950 (11) | 0.6210 (3) | 0.112 (3) |
| H15 | 0.6482 | 0.5478 | 0.6531 | 0.134* |
| C16 | 0.5415 (8) | 0.4467 (9) | 0.6070 (3) | 0.097 (2) |
| H16 | 0.4859 | 0.4677 | 0.6287 | 0.117* |
| C17 | 0.7917 (4) | 0.1146 (8) | 0.2969 (2) | 0.0762 (18) |
| H17A | 0.8137 | 0.0018 | 0.2906 | 0.114* |
| H17B | 0.8493 | 0.1776 | 0.3115 | 0.114* |
| H17C | 0.7685 | 0.1648 | 0.2646 | 0.114* |
| C18 | 0.8843 (6) | 0.4123 (11) | 0.5189 (3) | 0.115 (3) |
| H18A | 0.8866 | 0.5312 | 0.5242 | 0.173* |
| H18B | 0.9287 | 0.3827 | 0.4908 | 0.173* |
| H18C | 0.9079 | 0.3567 | 0.5503 | 0.173* |
| C19 | 0.8727 (4) | 0.6454 (5) | 0.30939(18) | 0.0424 (11) |
| C20 | 0.7944 (4) | 0.6157 (7) | 0.2730 (2) | 0.0549 (14) |
| H20 | 0.7350 | 0.5575 | 0.2825 | 0.066* |
| C21 | 0.8032 (4) | 0.6717 (7) | 0.2227 (2) | 0.0637 (15) |
| H21 | 0.7500 | 0.6509 | 0.1981 | 0.076* |
| C22 | 0.8901 (4) | 0.7581 (7) | 0.20851 (19) | 0.0530 (13) |
| C23 | 0.9675 (4) | 0.7911 (6) | 0.2448 (2) | 0.0509 (13) |
| H23 | 1.0260 | 0.8520 | 0.2355 | 0.061* |
| C24 | 0.9586 (4) | 0.7344 (6) | 0.29456 (19) | 0.0480 (12) |
| H24 | 1.0118 | 0.7564 | 0.3190 | 0.058* |
| C25 | 0.9771 (6) | 0.0700 (10) | 0.4267 (4) | 0.123 (3) |
| H25A | 0.9948 | 0.1756 | 0.4424 | 0.185* |
| H25B | 1.0036 | 0.0658 | 0.3920 | 0.185* |
| H25C | 1.0075 | −0.0188 | 0.4473 | 0.185* |
1 Source of materials
The Schiff base ligand named 6,6′-((1E,1′E)-(ethane-1,2-diylbis(azaneylylidene)) bis(methaneylylidene)) bis(2-methoxyphenol) was prepared according to the literature [3]. The Schiff base ligand (0.1 mmol, 32.8 mg) was dissolved in 10 mL of methanol. The CoCl2·6H2O (0.1 mmol, 23.7 mg) and 4-hydroxybenzenesulfonic acid sodium salt (0.2 mmol, 39.3 mg) were added to the above solution. This mixture was stirred for 3 h and then filtered, resulting in an orange coloured solution. Crystals of the title compound were obtained by slow evaporation within two weeks.
2 Experimental details
All hydrogen atomic positions were taken from a difference Fourier map. Hydrogen atoms were assigned with common isotropic displacement factors Uiso(H) = 1.2 times Ueq (C, phenyl ring and methylene carbon) and Uiso(H) = 1.5 times Ueq(C, methyl carbon). All the H atoms were refined as riding on their parent atom.
3 Comment
Recently, coordination compounds composed of sulfonic acid have received considerable attention due to their appealing properties and prospective uses in adsorption and separation processes [4], [5], [6], [7], [8]. Sulfonate ligands with acidic –SO3H groups possess robust coordination ability, leading to an enhanced range of potential geometric arrangements between O-donors and metal ions, resulting in the formation of numerous structures [9, 10]. As a part of our current research on the exploration of functional coordination complexes, we report a new coordination compound based on sulfonate ligands.
The asymmetric unit of the title structure contains one Schiff base ligand, one Co(II) ion, one Na(I) ion, one 4-hydroxybenzenesulfonate and one coordinated methanol molecule. The Co centre is four coordinated with two O atoms and two N atoms from the Schiff base ligands. The Na centre is six-coordinated with four O atoms from the Schiff base ligands, one O atom from the sulfonate and one O atom from coordinated methanol. The bond lengths for Co–O/N in title compound range from 1.811(4) to 1.819(5) Å, and the bond lengths for Na–O range from 2.291(5) to 2.517(4) Å. The bond lengths within these moieties are in the expected ranges [11], [12], [13].
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was funded by Liaocheng University Doctoral Foundation (318051514).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2004.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
3. Pasatoiu, T. D., Sutter, J. P., Madalan, A. M., Fellah, F. Z. C., Duhayon, G., Andruh, M. Preparation, crystal structures, and magnetic features for a series of dinuclear [NiIILnIII] Schiff–Base complexes: evidence for slow relaxation of the magnetization for the DyIII derivative. Inorg. Chem. 2011, 50, 5890–5898; https://doi.org/10.1021/ic2004276.Search in Google Scholar PubMed
4. Ahmed, I., Jhung, S. H. Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions. Chem. Eng. J. 2017, 310, 197–215; https://doi.org/10.1016/j.cej.2016.10.115.Search in Google Scholar
5. Xin, Q., Liu, T., Li, Z., Wang, S., Li, Y., Li, Z., Ouyang, J., Jiang, Z., Wu, H. Mixed matrix membranes composed of sulfonated poly (ether ether ketone) and a sulfonated metal–organic framework for gas separation. J. Membr. Sci. 2015, 488, 67–78; https://doi.org/10.1016/j.memsci.2015.03.060.Search in Google Scholar
6. Wang, Y., Ye, Q., Yu, M. H., Zhang, X. J., Deng, C. H. Sulfonic acid-based metal organic framework functionalized magnetic nanocomposite combined with gas chromatography-electron capture detector for extraction and determination of organochlorine. Chin. Chem. Lett. 2020, 31, 1843–1846; https://doi.org/10.1016/j.cclet.2020.02.054.Search in Google Scholar
7. Dong, X.-Y., Wang, R., Wang, J.-Z., Zang, S.-Q., Mak, T. C. W. Highly selective Fe3+ sensing and proton conduction in a water-stable sulfonate-carboxylate Tb-organic-framework. J. Mater. Chem. A 2015, 3, 641–647; https://doi.org/10.1039/c4ta04421e.Search in Google Scholar
8. Liu, S. S., Liu, Q. Q., Huang, S. Z., Zhang, C., Dong, X. Y., Zang, S. Q. Sulfonic and phosphonic porous solids as proton conductors. Coord. Chem. Rev. 2022, 451, 214241; https://doi.org/10.1016/j.ccr.2021.214241.Search in Google Scholar
9. Yoo, D. K., Lee, G., Mondol, M. M. H., Lee, H. J., Kim, C. M., Jhung, S. H. Preparation and applications of metal–organic frameworks composed of sulfonic acid. Coord. Chem. Rev. 2023, 474, 214868; https://doi.org/10.1016/j.ccr.2022.214868.Search in Google Scholar
10. Liu, R.-L., Wang, D.-Y., Shi, J.-R., Li, G. Proton conductive metal sulfonate frameworks. Coord. Chem. Rev. 2021, 431, 213747; https://doi.org/10.1016/j.ccr.2020.213747.Search in Google Scholar
11. Funes, A. V., Carrella, L., Rechkemmer, Y., Slageren, J. V., Rentschler, E., Alborés, P. Synthesis, structural characterization and magnetic behaviour of a family of [CoIII2 LnIII2] butterfly compounds. Dalton Trans. 2017, 46, 3400–3409; https://doi.org/10.1039/c6dt04713k.Search in Google Scholar PubMed
12. Deacy, A. C., Moreby, E., Phanopoulos, A., Williams, C. K. Co(III)/alkali-metal(I) heterodinuclear catalysts for the ring-opening copolymerization of CO2 and propylene oxide. J. Am. Chem. Soc. 2020, 142, 19150–19160; https://doi.org/10.1021/jacs.0c07980.Search in Google Scholar PubMed PubMed Central
13. Reath, A. H., Ziller, J. W., Tsay, C., Ryan, A. J., Yang, J. Y. Redox potential and electronic structure effects of proximal nonredox active cations in cobalt Schiff Base complexes. Inorg. Chem. 2017, 56, 3713–3718; https://doi.org/10.1021/acs.inorgchem.6b03098.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2

