The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
Abstract
C42H27MnN5O9·2H2O, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.20 × 0.15 × 0.10 mm |
| Wavelength: | Ga Kα radiation (1.34138 Å) |
| μ: | 2.45 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX-II, φ and ω |
| θmax, completeness: | 57.0°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 24,998, 7320, 0.034 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 6995 |
| N(param)refined: | 540 |
| Programs: | Bruker [1], Shelx [2, 3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.27754 (14) | 1.06304 (15) | 0.36388 (12) | 0.0185 (3) |
| H1 | 0.194091 | 1.081205 | 0.366117 | 0.022* |
| C2 | 0.31347 (15) | 1.15417 (16) | 0.28889 (12) | 0.0208 (3) |
| H2 | 0.256168 | 1.233232 | 0.240454 | 0.025* |
| C3 | 0.43511 (15) | 1.12688 (16) | 0.28644 (13) | 0.0232 (3) |
| H3 | 0.462595 | 1.187341 | 0.235675 | 0.028* |
| C4 | 0.51709 (14) | 1.01067 (16) | 0.35851 (12) | 0.0201 (3) |
| H4 | 0.600826 | 0.990896 | 0.357942 | 0.024* |
| C5 | 0.47404 (13) | 0.92419 (14) | 0.43127 (11) | 0.0149 (3) |
| C6 | 0.55378 (14) | 0.79673 (15) | 0.51235 (11) | 0.0156 (3) |
| C7 | 0.67991 (14) | 0.74805 (15) | 0.51795 (12) | 0.0174 (3) |
| H7 | 0.720245 | 0.796917 | 0.469915 | 0.021* |
| C8 | 0.74594 (14) | 0.62557 (15) | 0.59588 (12) | 0.0182 (3) |
| C9 | 0.68217 (14) | 0.55818 (15) | 0.66497 (12) | 0.0188 (3) |
| H9 | 0.724679 | 0.474829 | 0.718189 | 0.023* |
| C10 | 0.55665 (14) | 0.61323 (15) | 0.65570 (11) | 0.0167 (3) |
| C11 | 0.48122 (14) | 0.54860 (15) | 0.72610 (12) | 0.0167 (3) |
| C12 | 0.53062 (15) | 0.42921 (16) | 0.81052 (12) | 0.0214 (3) |
| H12 | 0.615364 | 0.385732 | 0.826379 | 0.026* |
| C13 | 0.45404 (16) | 0.37469 (16) | 0.87116 (13) | 0.0244 (3) |
| H13 | 0.485864 | 0.293401 | 0.929144 | 0.029* |
| C14 | 0.33089 (16) | 0.43998 (16) | 0.84626 (12) | 0.0220 (3) |
| H14 | 0.276937 | 0.404191 | 0.886238 | 0.026* |
| C15 | 0.28823 (15) | 0.55902 (16) | 0.76143 (12) | 0.0191 (3) |
| H15 | 0.203692 | 0.604299 | 0.744486 | 0.023* |
| C16 | 0.88009 (14) | 0.56570 (15) | 0.60686 (12) | 0.0188 (3) |
| C17 | 0.94391 (14) | 0.63969 (15) | 0.58409 (12) | 0.0200 (3) |
| H17 | 0.901187 | 0.731573 | 0.557279 | 0.024* |
| C18 | 1.06926 (14) | 0.58050 (15) | 0.60017 (12) | 0.0189 (3) |
| H18 | 1.111675 | 0.631341 | 0.586412 | 0.023* |
| C19 | 1.13188 (14) | 0.44607 (15) | 0.63662 (12) | 0.0179 (3) |
| C20 | 1.07064 (14) | 0.37115 (15) | 0.65493 (13) | 0.0200 (3) |
| H20 | 1.114317 | 0.279875 | 0.676443 | 0.024* |
| C21 | 0.94552 (14) | 0.43086 (16) | 0.64150 (13) | 0.0210 (3) |
| H21 | 0.903397 | 0.379550 | 0.656011 | 0.025* |
| C22 | 1.32833 (14) | 0.26314 (15) | 0.73166 (12) | 0.0173 (3) |
| C23 | 1.29628 (15) | 0.17456 (16) | 0.81579 (13) | 0.0221 (3) |
| H23 | 1.213986 | 0.188906 | 0.828598 | 0.027* |
| C24 | 1.39074 (16) | 0.06429 (17) | 0.87995 (13) | 0.0270 (4) |
| H24 | 1.372821 | 0.000531 | 0.937408 | 0.032* |
| C25 | 1.51253 (16) | 0.04430 (18) | 0.86218 (13) | 0.0273 (4) |
| H25 | 1.574780 | −0.032564 | 0.907811 | 0.033* |
| C26 | 1.54343 (15) | 0.13401 (16) | 0.77984 (13) | 0.0227 (3) |
| H26 | 1.625541 | 0.120970 | 0.768693 | 0.027* |
| C27 | 1.44938 (14) | 0.24466 (15) | 0.71358 (12) | 0.0175 (3) |
| C28 | 1.34159 (14) | 0.42881 (15) | 0.59467 (12) | 0.0182 (3) |
| H28 | 1.318222 | 0.509705 | 0.536059 | 0.022* |
| C29 | 0.17876 (14) | 0.97187 (15) | 0.70556 (12) | 0.0170 (3) |
| C30 | 0.18701 (14) | 0.85198 (15) | 0.80137 (11) | 0.0154 (3) |
| C31 | 0.30275 (14) | 0.76766 (16) | 0.84478 (12) | 0.0209 (3) |
| H31 | 0.368630 | 0.787308 | 0.812064 | 0.025* |
| C32 | 0.32578 (15) | 0.65464 (16) | 0.93515 (13) | 0.0212 (3) |
| H32 | 0.405990 | 0.599157 | 0.963995 | 0.025* |
| C33 | 0.23167 (15) | 0.62444 (15) | 0.98186 (12) | 0.0188 (3) |
| C34 | 0.11092 (14) | 0.70866 (15) | 0.94217 (11) | 0.0166 (3) |
| C35 | 0.08566 (14) | 0.82631 (14) | 0.85069 (11) | 0.0148 (3) |
| C36 | 0.01533 (15) | 0.67568 (16) | 0.99367 (12) | 0.0210 (3) |
| C37 | −0.10299 (16) | 0.75960 (18) | 0.95996 (13) | 0.0265 (4) |
| H37 | −0.166925 | 0.738129 | 0.995851 | 0.032* |
| C38 | −0.12917 (15) | 0.87635 (17) | 0.87299 (13) | 0.0243 (3) |
| H38 | −0.211584 | 0.934920 | 0.851856 | 0.029* |
| C39 | −0.03855 (14) | 0.90941 (15) | 0.81655 (12) | 0.0172 (3) |
| C40 | −0.08264 (13) | 1.03079 (15) | 0.71613 (12) | 0.0170 (3) |
| C41 | 0.04162 (17) | 0.55059 (17) | 1.08325 (13) | 0.0276 (4) |
| C42 | 0.25848 (16) | 0.50011 (16) | 1.07230 (13) | 0.0246 (4) |
| Mn1 | 0.29453 (2) | 0.80915 (2) | 0.56406 (2) | 0.01468 (8) |
| N1 | 0.35552 (11) | 0.95020 (12) | 0.43352 (10) | 0.0158 (3) |
| N2 | 0.49429 (11) | 0.73061 (12) | 0.58049 (10) | 0.0152 (3) |
| N3 | 0.36087 (12) | 0.61288 (13) | 0.70241 (10) | 0.0166 (3) |
| N4 | 1.26014 (11) | 0.38371 (12) | 0.65354 (10) | 0.0169 (3) |
| N5 | 1.45487 (12) | 0.34989 (13) | 0.62675 (10) | 0.0191 (3) |
| O1W | 0.31244 (10) | 0.71768 (10) | 0.45505 (8) | 0.0180 (2) |
| H1WA | 0.264634 | 0.775209 | 0.400678 | 0.027* |
| H1WB | 0.385146 | 0.696325 | 0.430482 | 0.027* |
| O1 | 0.23290 (10) | 0.95302 (11) | 0.62611 (8) | 0.0198 (2) |
| O2W | 0.11053 (10) | 0.83413 (10) | 0.56663 (8) | 0.0181 (2) |
| H2WA | 0.064860 | 0.886830 | 0.596045 | 0.027* |
| H2WB | 0.081048 | 0.873871 | 0.503208 | 0.027* |
| O2 | 0.12934 (12) | 1.07665 (11) | 0.71403 (9) | 0.0257 (3) |
| O3 | −0.03961 (10) | 1.01641 (11) | 0.63370 (8) | 0.0212 (2) |
| O3W | 0.08445 (16) | 1.07305 (19) | 0.91338 (12) | 0.0515 (4) |
| H3WA | 0.099073 | 1.068205 | 0.853217 | 0.077* |
| H3WB | 0.005729 | 1.113478 | 0.913978 | 0.077* |
| O4 | −0.16537 (10) | 1.13257 (11) | 0.72126 (9) | 0.0236 (3) |
| O4W | −0.1811 (2) | 1.1763 (2) | 0.90384 (18) | 0.0769 (7) |
| H4WA | −0.167306 | 1.171801 | 0.843562 | 0.115* |
| H4WB | −0.220129 | 1.129273 | 0.936976 | 0.115* |
| O5 | 0.35812 (13) | 0.42007 (12) | 1.10932 (10) | 0.0351 (3) |
| O6 | 0.16235 (12) | 0.47052 (12) | 1.11754 (10) | 0.0316 (3) |
| O7 | −0.03107 (14) | 0.51016 (14) | 1.12796 (11) | 0.0411 (3) |
1 Source of material
The reagents were purchased from standard commercial sources and used without further purification. A mixture of MnCO3 (0.011 g, 0.10 mmol), 1,4,5,8-naphthalenetetracarboxylic acid (contains monoanhydride) (0.030 g, 0.10 mmol), and 2262-phimphtpy (0.043 g, 0.10 mmol) was dispersed in mixed solvent of H2O (4 mL) and C2H5OH (4 mL) solutions in a 16 mL Teflon-lined stainless steel autoclave, which was heated for 3 d at 413 K under autogenous pressure and slowly cooled to room temperature. Yellow block crystals were obtained.
2 Experimental details
The structure was solved with the Shelxt-2018 program. All H-atoms from C atoms were positioned with idealized geometry and refined isotropically (Uiso(H) = 1.2Ueq(C)) using a riding model with C–H = 0.93 and 0.97 Å.
3 Comment
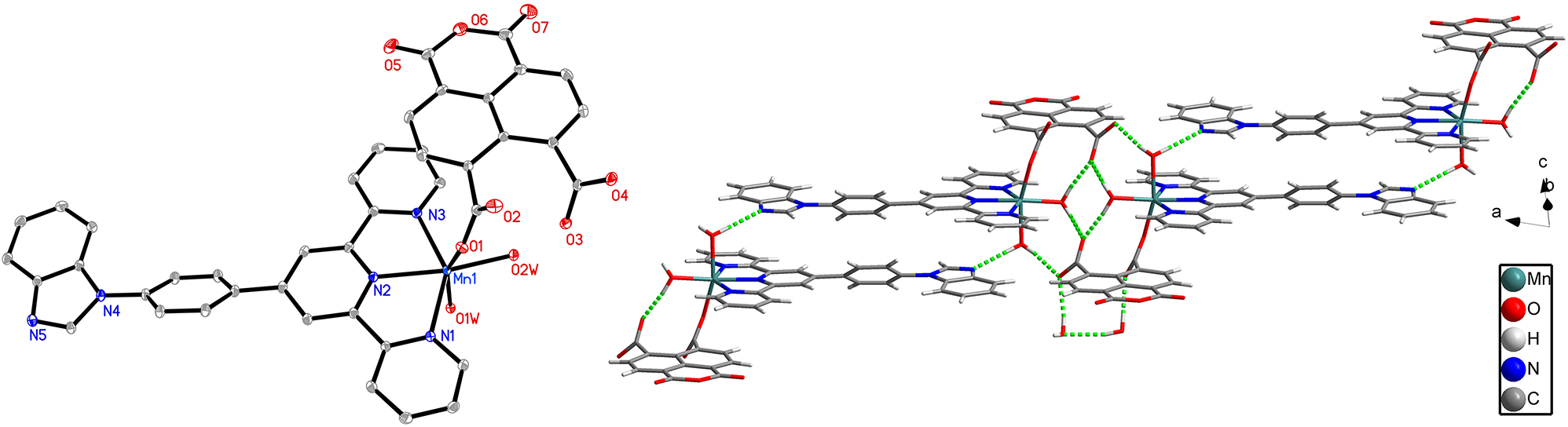
2,2′:6′,2″-Terpyridine (tpy) as a building unit with its large π conjugation, has received great interest in the realm of metal-organic frameworks (MOFs) [5], [6], [7], [8], [9], [10], [11], [12]. By using a grafting strategy [13], 4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″ terpyridine(2262-phimphtpy) has been designed and synthesized with a combination of benzimidazole and terpyridine functional groups. A discrete neutral mononuclear Mn(II) complex was obtained successfully along with naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride (ntdda2−) [14, 15] and its structure has been determined. To the best of our knowledge, it is the first report of the first Mn(II) complex coordinated with the ntdda2− anion.
The Ortep diagram is presented in the left part of the figure. The asymmetric unit contains one Mn(II) ion, one phimphtpy ligand, and one ntdda2− anion. As shown in the left part of the figure, Mn1 is coordinated by three pyridine nitrogen atoms (Mn1–N, 2.2185(13)–2.2779(13) Å) from one phimphtpy ligand, one oxygen atom from one ntdda2− anion and two oxygen atoms from two coordinated water molecules showing a distorted octahedral geometry with N1, N2, N3 and O2W in the equatorial plane and O1 and O1W in axial positions. Mn(II) to O/N distances and bond angles are within the normal ranges and similar to that of previously reported complexes [16, 17].
The supramolecular association of the molecules in the title complex is essentially based on hydrogen bonds between the water molecules as donors and the diverse oxygen atoms from water molecules and ntdda2− anion and nitrogen atoms from benzimidazole as acceptors. One-dimensional (1D) chains are formed through seven kinds of hydrogen bonds with the distances of H atoms and acceptors between 1.80 Å and 2.09 Å as shown in the right part of the figure. The 3D structure is obtained through these 1D chains which are linked by weak hydrogen bond O4W–H4WB···O3W with the distance of H atom and acceptor 2.55 Å and six kinds of π–π interactions between aromatic benzene, pyridine and imidazole rings with Cg–Cg (the aromatic rings center was defined as Cg) distances between 3.44 Å and 3.90 Å.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the 2022 Innovation and Entrepreneurship Training Program for Hengyang Normal University students (No. cxcy2022033), and the Innovation Platform Open Fund Project of Hunan Provincial Education Department of China (No. 20K016).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Chen, X. L., Shang, L., Huang, M. P., Tong, Y. Q., Zhang, J. N., Xue, W. N. Two complexes based on terpyridine/benzotricarboxylic acid ligands: synthesis, structures and properties. Chin. J. Inorg. Chem. 2021, 37, 340–350.Search in Google Scholar
6. Gomez, G. E., Ridenour, J. A., Byrne, N. M., Sheychenko, A. P., Cahill, C. L. Novel heterometallic uranyl-transition metal materials: structure, topology, and solid state photoluminescence properties. Inorg. Chem. 2019, 58, 7243–7254; https://doi.org/10.1021/acs.inorgchem.9b00255.Search in Google Scholar PubMed
7. Zhang, H. M., Yang, J., Liu, Y. Y., Kang, D. W., Ma, J. F. A family of coordination polymers assembled with a flexible hexacarboxylate ligand and auxiliary N-donor ligands: syntheses, structures, and physical properties. CrystEngComm 2015, 17, 3181–3196; https://doi.org/10.1039/c5ce00181a.Search in Google Scholar
8. Li, L., Zhao, Y., Wang, X. G., Song, W. C., Huang, Z. G., Zhao, X. J., Yang, E. C. The first 2,6-di(1,6-naphthyridin-2-yl)pyridine-based redox photochromic coordination polymer platform with selective vapochromism for trolamine. Inorg. Chem. Front. 2021, 8, 4044–4051; https://doi.org/10.1039/d1qi00683e.Search in Google Scholar
9. Huang, Z. Q., Chen, J. Q., Zhao, S. M., Qiu, Z. F., Zhao, Y., Sun, W. Y. Supramolecular assemblies of Zn(II)complexes with a D-pi-A ligand for sensing specific organic molecules. CrystEngComm 2022, 24, 3612–3620; https://doi.org/10.1039/d2ce00452f.Search in Google Scholar
10. Wang, Z., Zhu, C. Y., Wei, Z. W., Fan, Y. N., Pan, M. Breathing-ignited long persistent luminescence in a resilient metal-organic framework. Chem. Mater. 2020, 32, 841–848; https://doi.org/10.1021/acs.chemmater.9b04440.Search in Google Scholar
11. Fu, W. W., Ye, S. Q., Liu, Y., Chen, M. S., Kuang, D. Z. Syntheses, crystal structures and luminescence properties of two copper(II) complexes with terpyridine and dicarboxylate ligands. Transit. Met. Chem. 2015, 40, 227–233; https://doi.org/10.1007/s11243-015-9910-9.Search in Google Scholar
12. Yang, J., Hu, R. X., Zhang, M. B. Construction of monomers and chains assembled by 3d/4f metals and 4′-(4-carboxyphenyl)-2,2′:6′,2″-terpyridine. J. Solid State Chem. 2012, 196, 398–403; https://doi.org/10.1016/j.jssc.2012.07.002.Search in Google Scholar
13. Webb, W. R., Potter, M. E., Stewart, D. J., Elliott, S. J., Sazio, P. J. A., Zhang, Z. X., Luo, H. K., Teng, J. H., Zhang, L. L., Ivaldi, C., Miletto, I., Gianotti, E., Raja, R. The significance of metal coordination in imidazole-functionalized metal-organic frameworks for carbon dioxide utilization. Chem. Eur. J. 2020, 26, 13606–13610; https://doi.org/10.1002/chem.202001561.Search in Google Scholar PubMed
14. Sun, Y. Q., Deng, S., Ge, S. Z., Liu, Q., Chen, Y. P. A novel 2D dipyrazol-bridged Cadmium(II) complex based on tetranuclear Cd4O4 clusters: synthesis, structure and luminescence. J. Clust. Sci. 2013, 24, 605–617; https://doi.org/10.1007/s10876-012-0527-2.Search in Google Scholar
15. Xu, Y., Yuan, D., Wu, B., Jiang, F., Zhou, Y., Hong, M. Two photoluminescent coordination polymers based on naphthalene-1,4,5,8-tetracarboxylic acid 4,5-anhydride. Inorg. Chem. Commun. 2005, 8, 651–655; https://doi.org/10.1016/j.inoche.2005.04.010.Search in Google Scholar
16. Qiao, Y., Wei, B., Wang, L. Y., Li, X. Y., Che, G. B., Liu, C. B., Zhang, X. J. Hydrothermal syntheses and crystal structures of two complexes constructed from 3,5-bis((4′-carboxylbenzyl)oxy)benzoic acid and 4′-(4-Pyridyl)-2,2′:6′,2″-terpyridine mixed ligands. Chin. J. Inorg. Chem. 2016, 32, 1261–1266.Search in Google Scholar
17. Ren, Y. X., Chai, H. M., Hou, X. Y., Wang, J. J., Fu, F. Crystal structures and fluorescence properties of two 2D Mn-II/Cd-II trimellitic complexes containing terpyridine. J. Mol. Struct. 2015, 1101, 28–32; https://doi.org/10.1016/j.molstruc.2015.08.021.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of (N-([1,1′:4′,1″-terphenyl]-4,4′-diethyl)-2-(bis(pyridin-2-ylmethyl)amino)acetamide-κ4N,N,N″, O)tri(nitrato-kO, O′) samarium(III) - methanol - acetonitrile (1/1/1), C40H39SmN8O14
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-chloro-4-methyl phenolate-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV), C27H27Cl2N3O6Ti
- N′-[(1E)-(4–Fluorophenyl)methylidene]adamantane-1-carbohydrazide, C18H21FN2O
- Crystal structure of 4-bromo-3-nitro-1H-pyrazole-5-carboxylic acid monohydrate, C4H2N3BrO4·H2O
- Crystal structure of dipyridine-k1N-tris(2,2,6,6-tetramethyl-5-oxohept-3-en-3-olato-k2O,O′)dysprosium(III), DyC43H67O6N2
- Crystal structure of cyclo[tetraiodido-bis{μ2-1-[(benzotriazol-1-yl)methyl]-1-H-1,3-(2-isopropyl-imidazol)-k2N:N}dicadmiun(II)], C26H30N10Cd2I4
- The crystal structure of tert-butyl (E)-3-(2-(benzylideneamino)phenyl)-1H-indole-1-carboxylate, C26H24N2O2
- The crystal structure of 4-(3-carboxy-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinolin-7-yl)-2-methylpiperazin-1-ium 2,5-dihydroxybenzoate methanol solvate, C27H32FN3O9
- Crystal structure of (μ2-1-(4,4′-bipyridine-κ2N:N′)-bis[diaqua-(4-iodopyridine-2,6-dicarboxylato-κ3O,N,O′)–cobalt(II)], C24H20Co2I2N4O12
- The crystal structure of dimethyl 4,4′-(10,20-diphenylporphyrin-5,15-diyl)dibenzoate dichloromethane solvate, C49H36N4O4Cl2
- (E)-2-((E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene)hydrazine-1-carbothioamide C14H23N3S1
- The crystal structure of [1-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinolin-2(1H)-one], C16H12F3NO
- Crystal structure of (E)-2-amino-N′-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)benzohydrazide – dimethylformamide – water (1/1/2), C15H16N4O3·C3H7NO·2H2O
- Crystal structure of 3-(4-bromophenyl)-5-methyl-1H-pyrazole, C10H9BrN2
- Crystal structure of 1,10-phenanthrolinium bromide dihydrate, C12H9N2Br
- Crystal structure of N-(4′-chloro-[1,1′-biphenyl]-2-yl)formamide, C13H10ClNO
- The crystal structure of nitroterephthalic acid, C8H5NO6
- Crystal structure of (2-((4-bromo-2,6-dichlorophenyl)amino)phenyl) (morpholino)methanone, C17H15BrCl2N2O2
- Crystal structure of tetraaqua-bis(ethanol-κO)-tetrakis(μ2-trifluoroacetate-κ2O:O′)-bis(trifluoroacetate-κ2O)digadolinium(III) Gd2C16H20O18F18
- The crystal structure of dimethyl 4,4′-[10,20-bis(2,6-difluorophenyl)porphyrin-5,15-diyl]dibenzoate chloroform solvate, C50H32Cl6F4N4O4
- The crystal structure of N,N′-((nitroazanediyl)bis(methylene))diacetamide, C6H12O4N4
- The crystal structure of [bis(2,2′-bipyridine-6-carboxylato-κ3N,N,O)magnesium(II)]dihydrate, C22H18N4O6Mg
- Crystal structure of poly[diaqua-(bis(μ2-1,4-bis(imidazol-1-ylmethyl)benzene)-κ2N,N′] cobalt(II)-tetraqua-bis(1,4-bis(imidazol-1-ylmethyl)benzene)-κ1N)-cobalt(II) di(2,5-thiophenedicarboxylate) dihydrate, C68H76Co2N16O16S2
- Crystal structure of poly[chlorido-μ2-chlorido-(μ2-1-[(2-ethyl-4-methyl-1H-imidazol-1-yl)methyl]-1H-benzotriazole-κN:N’)cadmium(II)], C13H15CdN5Cl2
- The crystal structure of (4-hydroxybenzenesulfonate)-k1O-6,6′-((1E,1′E)- (ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene)) bis(2-methoxyphenol)-κ2N,N,μ2O,O,κ2O, O)-(methanol)-cobalt(II) sodium(I), C25H27CoN2NaO9S
- Crystal structure of (1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)amino)piperidin-1-yl)methanone, C17H18F6N6O
- Crystal structure of bis{[(cyclohexylimino)(phenylimino)-l5-(methyl)diethylazane-κ2N:N′]-(ethyl)-zinc(II)]}, C38H62N6Zn2
- Crystal structure of 2-[(4-bromobenzyl)thio]-5-(5-bromothiophen-2-yl)-1,3,4-oxadiazole, C13H8Br2N2OS2
- Crystal structure of 10-methoxy-7,11b,12,13-tetrahydro-6H-pyrazino [2′,3′:5,6]pyrazino[2,1-a]isoquinoline, C15H16N4O
- The crystal structure of 1-propyl-2-nitro-imidazole oxide, C6H9N3O3
- The crystal structure of 3-nitrobenzene-1,2-dicarboxylic acid–2-ethoxybenzamide (1/1), C17H16N2O8
- The structure of RUB-1, (C8H16N)6[B6Si48O108], a boron containing levyne-type zeolite, occluding N-methyl-quinuclidinium in the cage-like pores
- The crystal structure of diaqua-(naphthalene-4,5-dicarboxylate-1,8-dicarboxylic anhydride-κ1O)-(4′-(4-(1H-benzimidazolyl-1-yl)phenyl)-2,2′:6′,2″-terpyridine-κ3N,N′,N″)–manganese(II) dihydrate, C42H27MnN5O9·2H2O
- Crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene))bis (3-(3-bromopropoxy)phenol), C20H22Br2N2O4
- The crystal structure of 3-(2-hydroxyphenyl)-4-phenyl-6-(p-tolyl)-2H-pyran-2-one, C24H18O3
- Crystal structure of bis(μ2-2-(1,5-dimethyl–3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato-κ4O:O,N,O′)-(nitrato-κ2O,O′)dicobalt(II), C36H32Co2N8O4
- Synthesis and crystal structure of (3E,5S,10S,13S,14S,17Z)-17-ethylidene-10,13-dimethylhexadecahydro-3H-cyclopenta[α] phenanthren-3-one O-(4-fluorobenzoyl) oxime, C28H36FNO2
- The crystal structure of 4-aminiumbiphenyl benzenesulfonate, C18H17NO3S
- Synthesis and crystal structure of 1-(7-hydroxy-3-(4-hydroxy-3-nitrophenyl)-4-oxo-4H-chromen-8-yl)-N,N-dimethylmethanaminiumnitrate, C18H17N3O9
- Crystal structure of N-(Ar)-N′-(Ar′)-formamidine, C14H12Br2N2O
- The crystal structure of 4-(2,4-dichlorophenyl)-2-(4-fluorophenyl)-5-methyl-1H-imidazole, C16H11Cl2FN2
- Crystal structure of 1-(4–chlorophenyl)-4-benzoyl-3-methyl-1H-pyrazol-5-ol, C17H13ClN2O2
- The crystal structure of 5-amino-1-methyl-4-nitroimidazole, C4H6O2N4
- Crystal structure of 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene-N,N′-bis(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-1,3,2-diazaborol-2-yl)-l2-germenediamine, C63H94B2GeN8
- The crystal structure of (bromido, chlorido)-tricarbonyl-(5,5′-dimethyl-2,2′-bipyridine)-rhenium(I), C15H12Br0.2Cl0.8N2O3Re1
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-bis(propan-2-yl)-1H-pyrazol-4-amine, C26H36N6
- The crystal structure of poly[2-(4-carboxypyridin-3-yl)terephthalpoly[diaqua-(μ4-2-(6-carboxylatopyridin-3-yl)terephthalato-κ5O,N:O′:O″,O‴)]) cadmium(II)] dihydrate, C28H20Cd3N2O16
- Crystal structure of [tetraaqua-bis((3-carboxy-5-(pyridin-4-yl)benzoate-κ1N)cobalt(II)] tetrahydrate, C26H32CoN2O16
- Crystal structure of bis(μ2-azido-κ2N:N)-tetrakis(azido-κ1N)-tetrakis(1,10-phenanthroline-κ2N,N′)dibismuth(III), C48H32N26Bi2
- Crystal structure of (Z)-N-(4-(4-(4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)butoxy)phenyl)acetamide, C30H31NO8
- Crystal structure of poly[diaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-bis(μ2-5-carboxybenzene-1,3-dicarboxylato-O,O′:O″)-aqua-di-zinc dihydrate solvate], C27H28N4O16Zn2
- Crystal structure of 2-(3,5,5-trimethylcyclohex-2-en-1-ylidene)malononitrile, C12H14N2
- Crystal structure of chlorido-(5-nitro-2-phenylpyridine-κ2N,C)-[(methylsulfinyl)methane-κ1S]platinum(II), C13H13ClN2O3PtS
- The crystal structure of the co-crystal 1,4-dioxane–4,6-bis(nitroimino)-1,3,5-triazinan-2-one(2/1), C11H19N7O9
- Crystal structure of [N(E),N′(E)]-N,N′-(1,4-phenylenedimethylidyne)bis-3,5-dimethyl-1H-pyrazol-4-amine di-methanol solvate, C18H20N6·2(CH3OH)
- Crystal structure of catena-poly[bis(μ2-azido-k2N:N′)-(nitrato-K2N:N′)-bis(1,10-phenanthroline-K2N:N′)samarium(III)], C24H16N11O3Sm
- Crystal structure of (Z)-2-(4-((5-bromopentyl)oxy)benzylidene)-4,5,6-trimethoxybenzofuran-3(2H)-one, C23H25BrO6
- Crystal structure of bis(3,5-dimethyl-1H-pyrazol-4-ammonium) tetrafluoroterephthate, 2[C5H10N3][C8F4O4]
- Crystal structure of 2-amino-4-(2-fluoro-4-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O
- Crystal structure of 6-(pyridin-3-yl)-1,3,5-triazine-2,4-diamine-sebacic acid (2/1), C13H17N6O2

