Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
Abstract
C44H42Mn5N12O36, triclinic, P
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
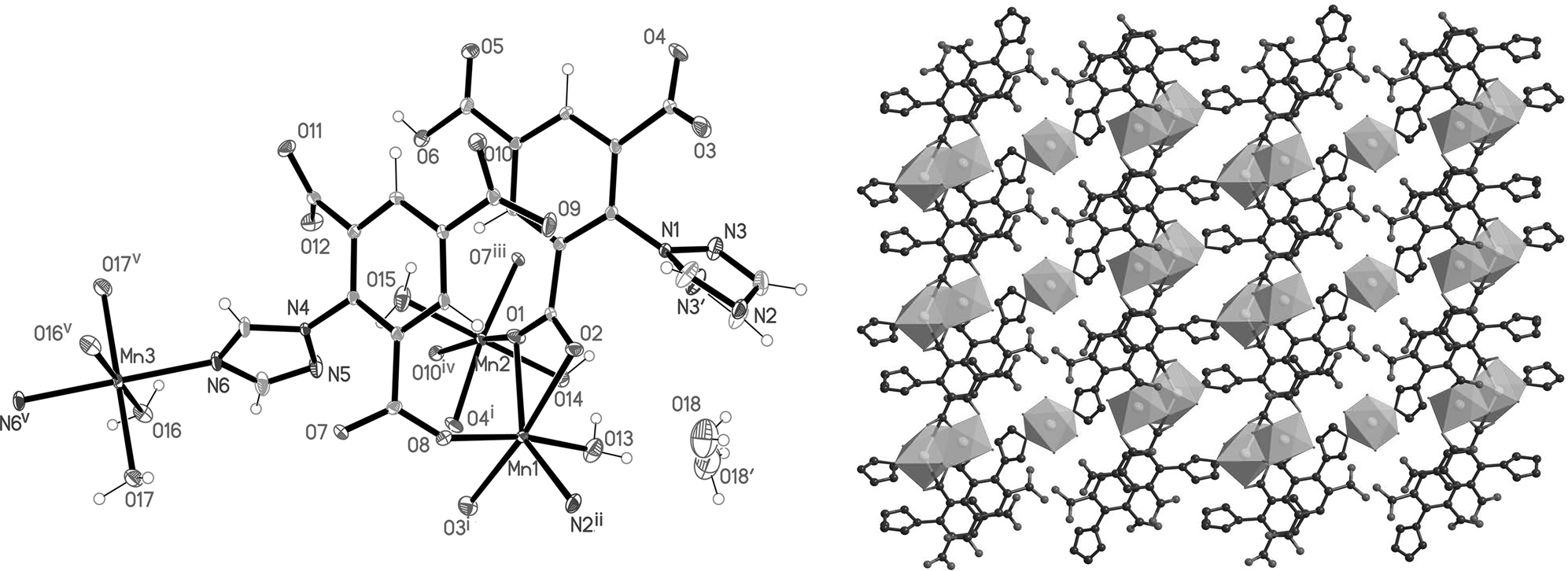
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.12 × 0.12 × 0.11 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 10.1 mm−1 |
| Diffractometer, scan mode: | Xcalibur, |
| θ max, completeness: | 71.1°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 9402, 5274, 0.052 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4024 |
| N(param)refined: | 469 |
| Programs: | CrysAlisPRO [1, 2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Mn1 | 0.45071 (10) | 0.28163 (8) | 0.39359 (3) | 0.01672 (18) |
| Mn2 | 0.09647 (10) | 0.37918 (8) | 0.27257 (3) | 0.01573 (18) |
| Mn3 | 1.000000 | 0.500000 | 0.000000 | 0.0165 (2) |
| N1 | 0.3360 (5) | −0.1919 (4) | 0.41308 (18) | 0.0167 (8) |
| N2 | 0.4059 (6) | −0.2316 (4) | 0.51512 (19) | 0.0237 (9) |
| N3a | 0.1741 (18) | −0.1842 (19) | 0.4510 (6) | 0.032 (3) |
| N3′b | 0.1746 (12) | −0.1000 (13) | 0.4530 (4) | 0.036 (2) |
| N4 | 0.7642 (5) | 0.2476 (4) | 0.14042 (17) | 0.0143 (7) |
| N5 | 0.6311 (6) | 0.3810 (4) | 0.1596 (2) | 0.0310 (10) |
| N6 | 0.8424 (5) | 0.4011 (4) | 0.0749 (2) | 0.0213 (8) |
| O1 | 0.3114 (5) | 0.1948 (3) | 0.32006 (17) | 0.0253 (7) |
| O2 | 0.4665 (5) | 0.0397 (4) | 0.39225 (16) | 0.0248 (7) |
| O3 | 0.4345 (6) | −0.4936 (4) | 0.38126 (18) | 0.0327 (9) |
| O4 | 0.2547 (6) | −0.5069 (4) | 0.30786 (19) | 0.0343 (9) |
| O5 | 0.2717 (6) | −0.2287 (4) | 0.10105 (18) | 0.0380 (10) |
| O6 | 0.3103 (6) | −0.0180 (4) | 0.10497 (16) | 0.0306 (8) |
| H6 | 0.296782 | −0.014717 | 0.065129 | 0.046* |
| O7 | 0.9507 (5) | 0.2483 (3) | 0.25107 (16) | 0.0218 (7) |
| O8 | 0.7386 (5) | 0.2312 (4) | 0.33643 (16) | 0.0252 (7) |
| O9 | 0.7816 (5) | −0.3036 (4) | 0.31846 (17) | 0.0324 (9) |
| O10 | 0.9299 (5) | −0.4168 (3) | 0.22121 (16) | 0.0227 (7) |
| O11 | 0.7504 (5) | −0.0354 (4) | 0.01712 (16) | 0.0267 (8) |
| O12 | 0.5428 (5) | 0.1776 (4) | 0.06150 (17) | 0.0300 (8) |
| O13 | 0.1755 (5) | 0.3567 (4) | 0.45747 (19) | 0.0405 (10) |
| H13B | 0.094071 | 0.340659 | 0.437700 | 0.061* |
| H13C | 0.187311 | 0.310359 | 0.494270 | 0.061* |
| O14 | −0.1019 (5) | 0.4346 (4) | 0.36933 (17) | 0.0252 (8) |
| H14A | −0.202822 | 0.507581 | 0.363504 | 0.038* |
| H14B | −0.130931 | 0.361752 | 0.381934 | 0.038* |
| O15 | 0.2838 (5) | 0.3252 (4) | 0.17842 (19) | 0.0398 (10) |
| H15C | 0.380998 | 0.347059 | 0.178917 | 0.048* |
| H15B | 0.315387 | 0.234429 | 0.171627 | 0.048* |
| O16 | 0.7442 (5) | 0.6988 (3) | 0.01266 (18) | 0.0269 (8) |
| H16A | 0.762504 | 0.764711 | −0.012586 | 0.040* |
| H16B | 0.650745 | 0.681982 | 0.001894 | 0.040* |
| O17 | 1.0954 (5) | 0.5777 (4) | 0.08392 (17) | 0.0311 (8) |
| H17A | 1.165719 | 0.623903 | 0.068315 | 0.047* |
| H17C | 1.158958 | 0.504873 | 0.106295 | 0.047* |
| O18c | 0.1901 (17) | 0.1530 (15) | 0.5542 (6) | 0.079 (3) |
| H18Cc | 0.084980 | 0.140562 | 0.558641 | 0.095* |
| H18Dc | 0.270661 | 0.083422 | 0.573491 | 0.095* |
| O18′d | 0.099 (3) | 0.242 (3) | 0.5697 (8) | 0.082 (5) |
| H18Gd | 0.139287 | 0.266968 | 0.602443 | 0.098* |
| H18Ed | −0.021893 | 0.266337 | 0.577582 | 0.098* |
| C1 | 0.3725 (6) | 0.0667 (5) | 0.3443 (2) | 0.0162 (9) |
| C2 | 0.3403 (6) | −0.0531 (5) | 0.3100 (2) | 0.0146 (8) |
| C3 | 0.3392 (6) | −0.1815 (5) | 0.3416 (2) | 0.0157 (9) |
| C4 | 0.3244 (6) | −0.2938 (5) | 0.3061 (2) | 0.0164 (9) |
| C5 | 0.3092 (6) | −0.2754 (5) | 0.2380 (2) | 0.0174 (9) |
| H5 | 0.299379 | −0.349246 | 0.213713 | 0.021* |
| C6 | 0.3085 (6) | −0.1486 (5) | 0.2056 (2) | 0.0145 (9) |
| C7 | 0.3258 (6) | −0.0385 (5) | 0.2416 (2) | 0.0156 (9) |
| H7 | 0.327674 | 0.045766 | 0.219666 | 0.019* |
| C8 | 0.4714 (8) | −0.2500 (6) | 0.4519 (3) | 0.0363 (13) |
| H8 | 0.599808 | −0.298748 | 0.435626 | 0.044* |
| C9a | 0.2164 (16) | −0.210 (2) | 0.5140 (7) | 0.034 (3) |
| H9a | 0.131097 | −0.213067 | 0.551319 | 0.041* |
| C9′b | 0.2247 (14) | −0.1296 (15) | 0.5129 (5) | 0.040 (3) |
| H9′b | 0.143744 | −0.084873 | 0.551349 | 0.048* |
| C10 | 0.3365 (7) | −0.4413 (5) | 0.3355 (2) | 0.0187 (9) |
| C11 | 0.2929 (6) | −0.1360 (5) | 0.1316 (2) | 0.0201 (10) |
| C12 | 0.8294 (6) | 0.1997 (5) | 0.2785 (2) | 0.0169 (9) |
| C13 | 0.7973 (6) | 0.0888 (5) | 0.2378 (2) | 0.0145 (9) |
| C14 | 0.8128 (6) | −0.0458 (5) | 0.2655 (2) | 0.0165 (9) |
| H14 | 0.827070 | −0.062825 | 0.310684 | 0.020* |
| C15 | 0.8069 (6) | −0.1557 (5) | 0.2258 (2) | 0.0133 (8) |
| C16 | 0.7715 (6) | −0.1266 (5) | 0.1596 (2) | 0.0166 (9) |
| H16 | 0.768023 | −0.199819 | 0.133118 | 0.020* |
| C17 | 0.7415 (6) | 0.0111 (5) | 0.1326 (2) | 0.0152 (9) |
| C18 | 0.7663 (6) | 0.1133 (5) | 0.1712 (2) | 0.0144 (8) |
| C19 | 0.8421 (6) | −0.3046 (5) | 0.2573 (2) | 0.0172 (9) |
| C20 | 0.6722 (7) | 0.0564 (5) | 0.0646 (2) | 0.0191 (10) |
| C22 | 0.8884 (7) | 0.2623 (5) | 0.0903 (2) | 0.0233 (10) |
| H22 | 0.992806 | 0.185713 | 0.069206 | 0.028* |
| C23 | 0.6854 (8) | 0.4694 (6) | 0.1189 (3) | 0.0332 (12) |
| H23 | 0.621033 | 0.570262 | 0.120204 | 0.040* |
-
aOccupancy: 0.399 (14). bOccupancy: 0.601 (14). cOccupancy: 0.606 (16). dOccupancy: 0.394 (16).
Source of material
All chemicals were available from commercial sources. MnCl2·6H2O (0.171 mmol), 2-(4H-1,2,4-triazol-4-yl)benzene-1,3,5-tricarboxylic acid (0.097 mmol) and H2O/CH3CN (4 mL, 1:3 v/v) were placed in a 15 mL PTFE vessel sealed with stainless steel reactor, heated to 120 °C for three days. Colorless crystals of the title compound with a yield of 49% were obtained through the cooling process (2 °C h−1). Elemental analysis found/calcd. (%) for C44H42Mn5N12O36: C 33.22/33.34, H 2.64/2.56, N 10.57/10.43. IR data (KBr, cm−1): 3426 (s), 1037 (s), 1614 (s), 1380 (m), 668 (m), 651 (m), 469 (w), 3727 (w), 2967 (w), 1285 (w), 784 (w), 589 (w), 530 (w), 415 (w).
Experimental details
Hydrogen atoms were generated geometrically. CCDC number 2034133 contains the crystallographic data, which has been deposited in Cambridge Crystallographic Database.
Comment
As a class of porous metal-organic framework materials, MOFs have aroused great attention and focused research due to their structural diversities and the prospects of application in the area of specific gas separation and subsequent storage, heterogeneous catalysis, fluorescence, magnetism, and electrochemistry, etc. [5], [6], [7 and references cited there].
With the development of coordination chemistry, the functionally bi-topic ligands including amino polycarboxylic acid [8] and N-containing aromatic carboxylic acid [9] are of considerable interest. Triazole-modified aromatic carboxylic compounds represent the most important class of heteropolydentate ligands capable of forming mono-, bi-, and polynuclear complexes with inorganic metals [10–13]. We herein report the synthesis and crystal structure of a novel complex [Mn5(μ4–L3−)2(μ5–HL2−)2(H2O)10]·2H2O (1) (H3L = 2-(4H-1,2,4-triazol-4-yl)benzene-1,3,5-tricarboxylic acid). The formula of 1 is established by elemental analysis. Single crystal X-ray diffraction analysis reveals that compound 1 crystallized in the triclinic space group P
Funding source: Natural Science Foundation of Shanxi Province
Award Identifier / Grant number: 202103021223002
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 21671124
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: We gratefully acknowledge support by the Natural Science Foundation of Shanxi Province (202103021223002) and National Natural Science Foundation of China (21671124).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlisPRO; Agilent Technologies: Santa Clara, CA, USA, 2013.Suche in Google Scholar
2. Fejfarová, K., Aem, B., Dehno, A. Absorption Correction: Analytical; CrysAlis PRO: Oxford Refinement, 2010.Suche in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
5. Liu, X. P., Fan, W. D., Zhang, M. H., Li, G. X., Liu, H. J., Sun, D. F., Zhao, L. M., Zhu, H. Y., Guo, W. Y. Enhancing light hydrocarbon storage and separation through introducing lewis basic nitrogen sites within a carboxylate-decorated copper-organic framework. Mater. Chem. Front. 2018, 2, 1146–1154; https://doi.org/10.1039/c8qm00105g.Suche in Google Scholar
6. Li, X. Y., Shi, W. J., Wang, X. Q., Ma, L. N., Hou, L., Wang, Y. Y. Luminescence modulation, white light emission, and energy transfer in a family of lanthanide metal-organic frameworks based on a planar π-conjugated ligand. Cryst. Growth Des. 2017, 17, 4217–4224; https://doi.org/10.1021/acs.cgd.7b00530.Suche in Google Scholar
7. Oar–Arteta, L., Wezendonk, T., Sun, X., Kapteijn, F., Gascon, J. Metal organic frameworks as precursors for the manufacture of advanced catalytic materials. Mater. Chem. Front. 2017, 1, 1709–1745; https://doi.org/10.1039/c7qm00007c.Suche in Google Scholar
8. Truong, K. N., Müller, P., Dronskowski, R., Englert, U. Dynamic uptake and release of water in the mixed-metal EDTA complex M3[Yb(EDTA)(CO3)] (M = K, Rb, Cs). Cryst. Growth Des. 2017, 17, 80–88; https://doi.org/10.1021/acs.cgd.6b01227.Suche in Google Scholar
9. Li, Y. F., Wang, D., Liao, Z., Kang, Y., Ding, W. H., Zheng, X. J., Jin, L. P. Luminescence tuning of the Dy–Zn metal-organic framework and its application in the detection of Fe(III) ions. J. Mater. Chem. C 2016, 4, 4211–4217; https://doi.org/10.1039/c6tc00832a.Suche in Google Scholar
10. Liu, K., Shi, W., Cheng, P. The coordination chemistry of Zn(II), Cd(II) and Hg(II) complexes with 1,2,4-triazole derivatives. Dalton Trans. 2011, 40, 8475–8490; https://doi.org/10.1039/c0dt01578d.Suche in Google Scholar PubMed
11. Yan, J. Z., Lu, L. P. Syntheses, structures, and luminescence properties of two new open-framework zinc phosphites based on 1,2,4-triazole derivatives. Chin. J. Struct. Chem. 2018, 37, 1331–1338; https://doi.org/10.14102/j.cnki.0254-5861.2011-1929.Suche in Google Scholar
12. Yan, J. Z., Lu, L. P., Feng, S. S., Zhu, M. L. Synthesis and structure of a ladder-like co-crystal CuICl with 3,5-dipropyl-4-amino-1,2,4-triazole. Chin. J. Struct. Chem. 2015, 34, 401–407; https://doi.org/10.14102/j.cnki.0254-5861.2011-0495.Suche in Google Scholar
13. Chen, X., Shang, L., Cui, H., Yang, H., Liu, L., Ren, Y., Wang, J. Four novel Zn(II)/Cu(II) coordination polymers containing hydroxyl groups: synthesis, crystal structure, luminescence sensing and photocatalysis properties. CrystEngComm 2020, 22, 5900–5913; https://doi.org/10.1039/d0ce00950d.Suche in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

