Abstract
C26H20Cl2N6O4, orthorhombic, Pbca (no. 61), a = 7.7316(6) Å, b = 24.8021(19) Å, c = 26.074(2) Å, V = 4999.9(7) Å3, Z = 8, R gt (F) = 0.0674, wR ref (F 2) = 0.1625, T = 296(2) K.
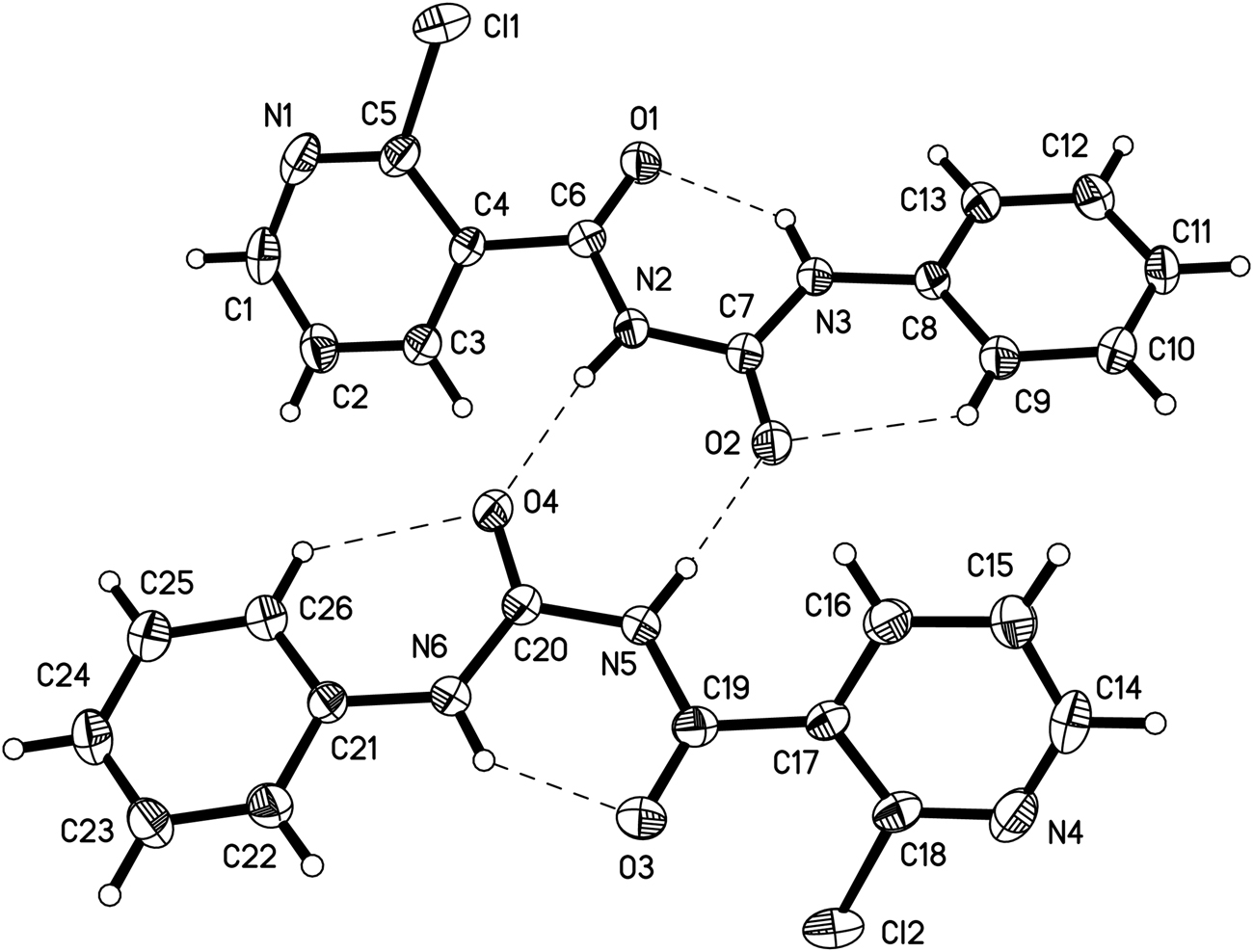
The asymmetric unit of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.48 × 0.34 × 0.22 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.31 mm−1 |
| Diffractometer, scan mode: | φ and ω |
| θ max, completeness: | 27.5°, >99% |
| N(hkl)measured , N(hkl)unique, R int: | 95,911, 5739, 0.121 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 3667 |
| N(param)refined: | 343 |
| Programs: | Olex2 [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Cl1 | 0.19308 (13) | 0.49008 (3) | −0.25012 (3) | 0.0740 (3) |
| O1 | 0.3155 (3) | 0.48839 (7) | −0.14194 (7) | 0.0606 (6) |
| O2 | 0.1657 (3) | 0.40173 (7) | −0.01569 (7) | 0.0608 (6) |
| N1 | 0.3167 (4) | 0.39981 (12) | −0.28105 (9) | 0.0617 (7) |
| N2 | 0.2225 (3) | 0.41379 (8) | −0.09979 (7) | 0.0428 (5) |
| H2 | 0.1889 | 0.3811 | −0.1047 | 0.051* |
| N3 | 0.2599 (3) | 0.48372 (8) | −0.04193 (8) | 0.0425 (5) |
| H3 | 0.2898 | 0.5013 | −0.0689 | 0.051* |
| C1 | 0.3787 (4) | 0.34948 (14) | −0.27533 (11) | 0.0610 (8) |
| H1 | 0.4088 | 0.3302 | −0.3046 | 0.073* |
| C2 | 0.3994 (4) | 0.32534 (12) | −0.22898 (11) | 0.0548 (8) |
| H2A | 0.4394 | 0.2901 | −0.2267 | 0.066* |
| C3 | 0.3601 (3) | 0.35435 (10) | −0.18552 (10) | 0.0460 (6) |
| H3A | 0.3730 | 0.3385 | −0.1534 | 0.055* |
| C4 | 0.3014 (3) | 0.40683 (10) | −0.18934 (9) | 0.0373 (6) |
| C5 | 0.2798 (4) | 0.42680 (11) | −0.23897 (10) | 0.0470 (7) |
| C6 | 0.2784 (3) | 0.44087 (10) | −0.14247 (9) | 0.0406 (6) |
| C7 | 0.2131 (4) | 0.43245 (10) | −0.04920 (9) | 0.0419 (6) |
| C8 | 0.2661 (3) | 0.51260 (9) | 0.00480 (9) | 0.0388 (6) |
| C9 | 0.1936 (4) | 0.49477 (11) | 0.05044 (10) | 0.0519 (7) |
| H9 | 0.1386 | 0.4615 | 0.0522 | 0.062* |
| C10 | 0.2046 (4) | 0.52757 (12) | 0.09334 (11) | 0.0583 (8) |
| H10 | 0.1573 | 0.5157 | 0.1241 | 0.070* |
| C11 | 0.2826 (4) | 0.57676 (11) | 0.09175 (11) | 0.0554 (8) |
| H11 | 0.2868 | 0.5983 | 0.1209 | 0.066* |
| C12 | 0.3551 (4) | 0.59421 (11) | 0.04670 (11) | 0.0559 (8) |
| H12 | 0.4092 | 0.6277 | 0.0453 | 0.067* |
| C13 | 0.3478 (4) | 0.56224 (10) | 0.00341 (10) | 0.0460 (7) |
| H13 | 0.3982 | 0.5741 | −0.0269 | 0.055* |
| Cl2 | 0.16199 (10) | 0.22435 (3) | 0.12346 (3) | 0.0614 (2) |
| O3 | −0.0509 (3) | 0.22324 (7) | 0.02733 (7) | 0.0605 (6) |
| O4 | 0.0987 (3) | 0.30349 (7) | −0.10350 (6) | 0.0523 (5) |
| N4 | 0.0579 (4) | 0.31406 (11) | 0.16181 (9) | 0.0664 (7) |
| N5 | 0.0432 (3) | 0.29560 (8) | −0.01890 (7) | 0.0426 (5) |
| H5 | 0.0693 | 0.3292 | −0.0160 | 0.051* |
| N6 | 0.0168 (3) | 0.22144 (8) | −0.07201 (8) | 0.0453 (5) |
| H6 | −0.0115 | 0.2058 | −0.0438 | 0.054* |
| C14 | −0.0120 (5) | 0.36290 (15) | 0.15962 (12) | 0.0767 (10) |
| H14 | −0.0191 | 0.3826 | 0.1899 | 0.092* |
| C15 | −0.0742 (5) | 0.38623 (12) | 0.11591 (12) | 0.0642 (9) |
| H15 | −0.1190 | 0.4210 | 0.1163 | 0.077* |
| C16 | −0.0687 (4) | 0.35660 (11) | 0.07120 (11) | 0.0513 (7) |
| H16 | −0.1101 | 0.3712 | 0.0407 | 0.062* |
| C17 | −0.0011 (3) | 0.30489 (10) | 0.07205 (9) | 0.0393 (6) |
| C18 | 0.0609 (4) | 0.28672 (11) | 0.11880 (10) | 0.0453 (6) |
| C19 | −0.0044 (3) | 0.27025 (10) | 0.02540 (10) | 0.0418 (6) |
| C20 | 0.0552 (4) | 0.27397 (10) | −0.06836 (9) | 0.0409 (6) |
| C21 | 0.0169 (4) | 0.18842 (10) | −0.11617 (10) | 0.0448 (7) |
| C22 | −0.0522 (4) | 0.13721 (10) | −0.11018 (11) | 0.0531 (7) |
| H22 | −0.1008 | 0.1273 | −0.0789 | 0.064* |
| C23 | −0.0493 (5) | 0.10108 (12) | −0.14993 (13) | 0.0716 (10) |
| H23 | −0.0942 | 0.0666 | −0.1455 | 0.086* |
| C24 | 0.0196 (6) | 0.11595 (13) | −0.19595 (13) | 0.0799 (11) |
| H24 | 0.0230 | 0.0913 | −0.2228 | 0.096* |
| C25 | 0.0841 (5) | 0.16708 (13) | −0.20291 (12) | 0.0747 (10) |
| H25 | 0.1282 | 0.1770 | −0.2347 | 0.090* |
| C26 | 0.0839 (5) | 0.20396 (11) | −0.16286 (11) | 0.0589 (8) |
| H26 | 0.1282 | 0.2385 | −0.1675 | 0.071* |
Source of material
According to our previous work [4, 5], to a solution of 2-chloronicotinoyl isocyanate (0.18 g, 1 mmol) in toluene (10 mL), aniline (0.10 g, 1.1 mmol) was added dropwise. The mixture was stirred at room temperature for 12 h. The solvent was evaporated. The crude product was recrystallized from ethanol as colorless block crystals.
Experimental details
Using Olex2 [1], the structure was solved with the ShelXS [2] program using Direct Methods and refined with the ShelXL [3] refinement package.
Comment
Nicotinamide derivatives are natural products, which often possessed different activities, such as herbicidal activity [6], fungicidal activity [7]. On the other hand, the acyl urea group is an active group in many bioactive molecules [8].
The bond lengths and bond angles in both crystallographic independent molecules (see the figure) are all in the normal ranges [9], [10], [11]. The torsion angles of O2–C7–N2–C6 and O1–C6–N2–C7 were −177.3(3)°, and −6.4(4)° respectively in the molecule 1 (see upper part of the figure), which indicated the two carbonyl groups are opposite. The other torsion angle of C6–N2–C7–N3 was 2.2(4)°, which showed the acyl urea group is nearly in the same plane, which is the same as in the molecule 2 (lower part of the figure) and in a reported structure [12]. The compound also shows intramolecular and intermolecular hydrogen bonding (see the figure).
Funding source: Zhejiang Shuren University
Award Identifier / Grant number: 2020XZ011
Funding source: Zhejiang Provincial Natural Science Foundation of China
Award Identifier / Grant number: LY19C140002
Funding source: Chemical Company for Research
Award Identifier / Grant number: KYY-HX-20210140
Award Identifier / Grant number: KYY-HX-20190720
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Zhejiang Shuren University Basic Scientific Research Special Funds (No. 2020XZ011), Zhejiang Provincial Natural Science Foundation of China (No. LY19C140002), the Chemical Company for Research (KYY-HX-20210140, KYY-HX-20190720).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Zhang, P. P., Wang, Q., Min, L. J., Wu, H. K., Weng, J. Q., Tan, C. X., Zhang, Y. G., Liu, X. H. Synthesis, crystal structure, fungicidal activity and molecular docking of nicotinic acyl urea derivatives. J. Mol. Struct. 2020, 1205, 127485; https://doi.org/10.1016/j.molstruc.2019.127485.Search in Google Scholar
5. Zhu, J. L., Liu, X. H. The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9CI2N3O2. Z. Kristallogr. N. Cryst. Struct. 2022, 237; https://doi.org/10.1515/ncrs-2022–0255.10.1515/ncrs-2022-0255Search in Google Scholar
6. Yu, C. S., Wang, Q., Bajsa-Hirschel, J., Cantrell, C., Duke, S. O., Liu, X. H. Synthesis, crystal structure, herbicidal activity and SAR study of novel N-(arylmethoxy)-2-chloronicotinamides derived from nicotinic acid. J. Agric. Food Chem. 2021, 69, 6423–6430; https://doi.org/10.1021/acs.jafc.0c07538.Search in Google Scholar PubMed
7. Sun, G. X., Wang, Q., Min, L. J., Han, L., Liu, X. H. Synthesis, crystal structure, fungicidal activities and molecular docking of acyl urea derivatives containing 2-chloronicotine motif. Chin. J. Struct. Chem. 2022, 41, 114–122.Search in Google Scholar
8. Pinheiro, A. C., de Souza, M. V. N., Wardell, J. L., Wardell, S. M. S. V. Structures of N-(arenecarbonyl)-N,N′-dicyclohexylureas, cyclohexyl-NH-C(O)-N(cyclohexyl)-C(O)Ar, (Ar = Ph, pyridin-3-yl and pyrazinyl). Z. für Kristallogr. Cryst. Mater. 2012, 227, 166–177; https://doi.org/10.1524/zkri.2012.1447.Search in Google Scholar
9. Min, L. J., Wang, Q., Tan, C. X., Weng, J. Q., Liu, X. H. Synthesis, crystal structure and fungicidal activity of 2-chloro-N-(o-tolylcarbamoyl)nicotinamide. Chin. J. Struct. Chem. 2020, 39, 452–458.Search in Google Scholar
10. Sun, G. X., Min, L. J., Han, L., Liu, X. H. The crystal structure of (E)-3-chloro-2-(2-(4-methylbenzylidene)hydrazinyl)pyridine, C13H12ClN3. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 129–131; https://doi.org/10.1515/ncrs-2021-0432.Search in Google Scholar
11. Zheng, B. Y., Sun, N. B., Chen, X. S., Liu, X. H. The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 355–356; https://doi.org/10.1515/ncrs-2021-0489.Search in Google Scholar
12. Chen, W. T., Wang, Q., Min, L. J., Wu, H. K., Weng, J. Q., Tan, C. X., Zhang, Y. G., Hu, B. Z., Liu, X. H. Synthesis, crystal structure, fungicidal activity, molecular docking, and density functional theory study of 2-chloro-N-(p-tolylcarbamoyl)nicotinamide. Indian J. Heterocycl. Chem. 2019, 29, 429–435.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

