Abstract
C19H17BrO3, triclinic, P
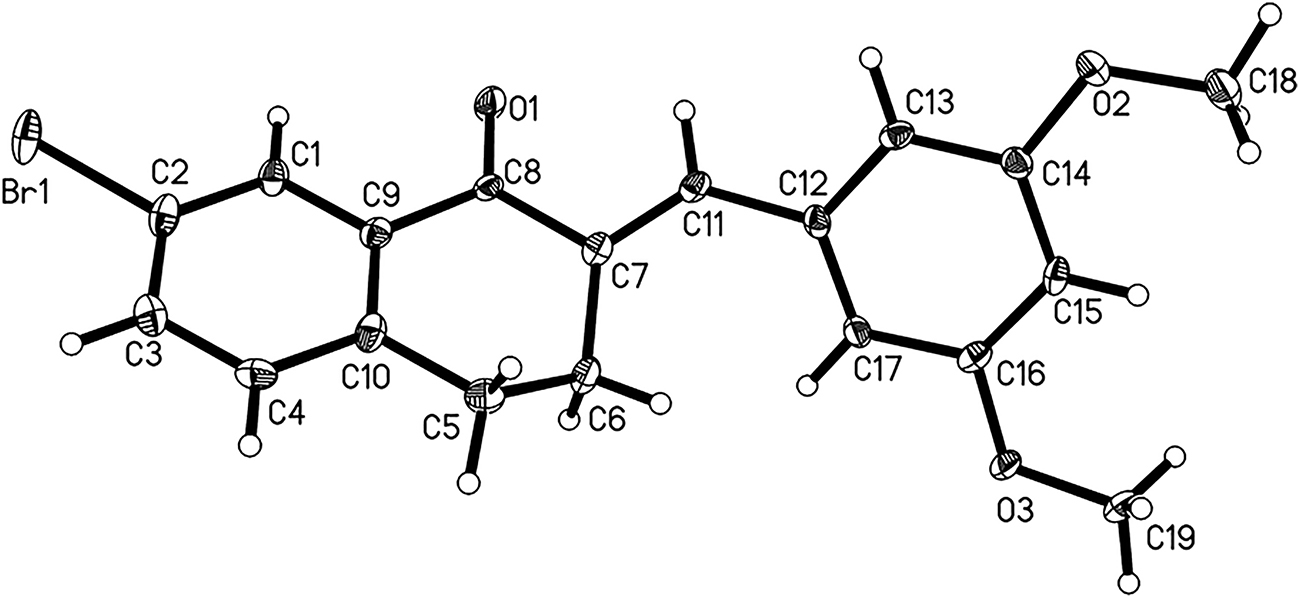
The molecular structure is shown in the figure. Displacement ellipsoids are drawn at the 40% probability level.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.15 × 0.12 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.57 mm−1 |
| Diffractometer, scan mode: | SuperNova |
| θ max, completeness: | 25.5°, >99% |
| N(hkl)measured , N(hkl)unique, R int: | 5429, 2985, 0.069 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2546 |
| N(param)refined: | 211 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Br1 | 1.06665 (8) | 0.74238 (10) | 0.21958 (5) | 0.0303 (3) |
| C1 | 0.8426 (8) | 0.5624 (8) | 0.3815 (4) | 0.0195 (13) |
| H1 | 0.771558 | 0.474543 | 0.330170 | 0.023* |
| C2 | 0.9975 (8) | 0.7158 (8) | 0.3582 (4) | 0.0206 (13) |
| C3 | 1.1045 (8) | 0.8522 (8) | 0.4327 (5) | 0.0231 (13) |
| H3 | 1.207276 | 0.955778 | 0.415461 | 0.028* |
| C4 | 1.0539 (8) | 0.8295 (8) | 0.5334 (5) | 0.0217 (13) |
| H4 | 1.124545 | 0.919343 | 0.584029 | 0.026* |
| C5 | 0.8549 (8) | 0.6535 (9) | 0.6715 (5) | 0.0244 (14) |
| H5A | 0.887366 | 0.771045 | 0.705500 | 0.029* |
| H5B | 0.928711 | 0.607489 | 0.703575 | 0.029* |
| C6 | 0.6522 (8) | 0.5254 (8) | 0.6865 (4) | 0.0204 (13) |
| H6A | 0.636370 | 0.496944 | 0.757402 | 0.024* |
| H6B | 0.580705 | 0.585603 | 0.671510 | 0.024* |
| C7 | 0.5816 (7) | 0.3522 (8) | 0.6186 (4) | 0.0172 (12) |
| C8 | 0.6324 (8) | 0.3708 (8) | 0.5091 (4) | 0.0153 (12) |
| C9 | 0.7936 (8) | 0.5406 (8) | 0.4838 (4) | 0.0169 (12) |
| C10 | 0.9000 (8) | 0.6756 (8) | 0.5605 (4) | 0.0198 (13) |
| C11 | 0.4872 (7) | 0.1826 (8) | 0.6471 (4) | 0.0164 (12) |
| H11 | 0.462205 | 0.091826 | 0.595573 | 0.020* |
| C12 | 0.4174 (7) | 0.1188 (7) | 0.7476 (4) | 0.0158 (12) |
| C13 | 0.4095 (7) | −0.0439 (8) | 0.7722 (4) | 0.0168 (12) |
| H13 | 0.445886 | −0.107472 | 0.725625 | 0.020* |
| C14 | 0.3474 (7) | −0.1101 (8) | 0.8657 (4) | 0.0171 (12) |
| C15 | 0.2896 (8) | −0.0191 (8) | 0.9355 (4) | 0.0179 (12) |
| H15 | 0.249605 | −0.063302 | 0.998698 | 0.021* |
| C16 | 0.2926 (8) | 0.1385 (8) | 0.9093 (4) | 0.0187 (12) |
| C17 | 0.3555 (8) | 0.2097 (8) | 0.8166 (4) | 0.0171 (12) |
| H17 | 0.356464 | 0.316094 | 0.800407 | 0.021* |
| C18 | 0.2627 (9) | −0.3543 (9) | 0.9729 (5) | 0.0281 (15) |
| H18A | 0.331427 | −0.277078 | 1.031810 | 0.042* |
| H18B | 0.262500 | −0.468327 | 0.974168 | 0.042* |
| H18C | 0.139025 | −0.373505 | 0.973577 | 0.042* |
| C19 | 0.2004 (8) | 0.1908 (8) | 1.0759 (4) | 0.0214 (13) |
| H19A | 0.102114 | 0.069609 | 1.077040 | 0.032* |
| H19B | 0.167062 | 0.273034 | 1.112066 | 0.032* |
| H19C | 0.308928 | 0.199309 | 1.108233 | 0.032* |
| O1 | 0.5450 (6) | 0.2496 (6) | 0.4420 (3) | 0.0240 (10) |
| O2 | 0.3450 (6) | −0.2711 (6) | 0.8822 (3) | 0.0263 (10) |
| O3 | 0.2345 (6) | 0.2361 (6) | 0.9728 (3) | 0.0252 (10) |
Source of material
The preparation of the intermediate 7-bromo-3,4-dihydronaphthalen-1(2H)-one was similar to the previous reported method [4, 5], which was used to synthesize the aromatic tetralones in the next step. 7-Bromo-3,4-dihydronaphthalen-1(2H)-one (0.60 g, 2.7 mmol) and 3,5-dimethoxybenzaldehyde (0.67 g, 4.0 mmol) were dissolved in 5 mL of methanol. A NaOH solution (25%, 2 mL) was dropped to the above solution through a constant pressure dropping funnel. The mixture was stirred for 4 h at 298 K, and the progress of the reaction was traced by thin layer chromatography (TLC). After the completion of the reaction, the upper solution was poured off and the remaining solid was washed by the cold methanol and dried. The pure product of the title compound was separated by 200–300 mesh silica gel column (petroleum ether/ethyl acetate/methanol = 10:10:1, v/v/v).
Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d (C–H) = 0.96 Å (methyl), U iso(H) = 1.5U eq(C), and d(C–H) = 0.97 Å (methylene), U iso(H) = 1.2U eq(C), and d(C–H) = 0.93 Å (aromatic), U iso(H) = 1.2U eq(C). The analysis of the F o/F c data and the two difference density peaks point to a twinning with an expected ratio smaller than 4:1. As the constituation of the target molecules is obtained without serious distorsions, a twin refinement was not undertaken.
Comment
Curcumin is a yellow phenolic pigment found mainly in the rhizome of turmeric, a plant of the ginger family. It was found to possess multiple anti-inflammatory, anti-tumour and anti-rheumatic effects without toxicity to humans at higher doses [6]. However, the poor bioavailability and unstable structure limited its clinical application [7]. In order to overcome the shortcoming of curcumin derivatives, a set of curcumin analogues were designed and synthesized in recent years. For example, halogenated bis(methoxybenzyl)-4-piperidone displays significant anti-cancer activity effects [8]. Boc-piperidone chalcones were novel cytotoxic drugs against highly metastatic cancer cells [9]. These results shows that curcumin derivatives have good bioactivity, which can be regarded as anti-inflammatory and anti-tumour agents [10, 11]. Based on the above studies, the target product of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one was synthesized by the Claisen–Schmidt condensation reaction.
Single-crystal structure analysis reveals that there is one molecule in the asymmetric unit. In the molecule, the bond length of C7=C11 is 1.344(8) Å, other bond lengths and bond angles are similar to the values reported by related articles [12], [13], [14], [15]. As shown in the figure, the methoxybenzene ring and carbonyl group are arranged around the double bond with the torsion of C8–C7–C11–C12 being 180.0(5)°. Thus the title compound adopts the E stereochemistry. The C4 atom is deviated from the least-squares plane of the cyclohexanone ring with the value of 0.480(17) Å, which makes the cyclohexyl ring display an envelope conformation. The two benzene rings are not coplanar with the dihedral angle of 59.70(15)°. Through the further observation, it was found that adjacent molecules were linked to a chain structure along the b axis by the weak C18–H18B⃛O3 hydrogen bond. The chains were further interacted to form the bc plane by C18–H18A⃛Br1 interaction. The neighbouring chains further interact with each other via weak C3–H3⃛O1 hydrogen bond to form a 3D architecture.
Considering the bioactive property of aromatic-tetralones, –Br and –OMe substitutes were selected to modify the structure of curcumin analogue, which can act as the hydrogen-bonding acceptor and enhance interactions with proteins [16].
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Shandong Provincial Natural Science Foundation (No. ZR2019MB032), College Youth Innovation Science and Technology Support Programme of Shandong Province (No. 2020KJK003), Key R&D Programme of Shandong Province (No. 2019JZZY011104), Shandong New Drug Loading & Release Technology and Preparation Engineering Laboratory.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Ameer, F., Giles, R. G. F., Green, I. R., Pearce, R. Synthesis of methoxy-2-hydroxy-1,4-naphthoquinones and reaction of one isomer with aldehydes under basic conditions. Synth. Commun. 2004, 34, 1247–1258; https://doi.org/10.1081/scc-120030312.Search in Google Scholar
5. Zhang, Z., Sangaiah, R., Gold, A., Ball, L. M. Synthesis of uniformly 13C-labeled polycyclic aromatic hydrocarbons. Org. Biomol. Chem. 2011, 9, 5431–5435; https://doi.org/10.1039/c0ob01107j.Search in Google Scholar PubMed
6. Soleimani, V., Sahebkar, A., Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother. Res. 2018, 32, 985–995; https://doi.org/10.1002/ptr.6054.Search in Google Scholar PubMed
7. Helal, M., Das, U., Bandy, B., Islam, A., Nazarali, A. J., Dimmock, J. R. Mitochondrial dysfunction contributes to the cytotoxicity of some 3,5-bis(benzylidene)-4-piperidone derivatives in colon HCT-116 cells. Bioorg. Med. Chem. Lett. 2013, 23, 1075–1078; https://doi.org/10.1016/j.bmcl.2012.12.016.Search in Google Scholar PubMed
8. Schmitt, F., Subramaniam, D., Anant, S., Padhye, S., Begemann, G., Schobert, R., Biersack, B. Halogenated bis(methoxybenzylidene)-4-piperidone curcuminoids with improved anticancer activity. ChemMedChem 2018, 13, 1115–1123; https://doi.org/10.1002/cmdc.201800135.Search in Google Scholar PubMed
9. Ocasio-Malavé, C., Donate, M. J., Sánchez, M. M., Sosa-Rivera, J. M., Mooney, J. W., Pereles-De León, T. A., Carballeira, N. M., Zayas, B., Vélez-Gerena, C. E., Martínez-Ferrer, M., Sanabria-Ríos, D. J. Synthesis of novel 4-Boc-piperidone chalcones and evaluation of their cytotoxic activity against highly-metastatic cancer cells. Bioorg. Med. Chem. Lett. 2020, 30, 126760; https://doi.org/10.1016/j.bmcl.2019.126760.Search in Google Scholar PubMed PubMed Central
10. Li, N., Xin, W. Y., Yao, B. R., Cong, W., Wang, C. H., Hou, G. G. N-phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-κB inhibitors in hepatic carcinoma cell lines. Eur. J. Med. Chem. 2018, 155, 531–544; https://doi.org/10.1016/j.ejmech.2018.06.027.Search in Google Scholar PubMed
11. Wang, Y., Hedblom, A., Koerner, S. K., Li, M., Jernigan, F. E., Wegiel, B., Sun, L. Novel synthetic chalcones induce apoptosis in the A549 non-small cell lung cancer cells harboring a KRAS mutation. Bioorg. Med. Chem. Lett. 2016, 26, 5703–5706; https://doi.org/10.1016/j.bmcl.2016.10.063.Search in Google Scholar PubMed PubMed Central
12. El-Sayed, N. N. E., Almaneai, N. M., Ghabbour, H. A., Alafeefy, A. M. Crystal structure of (E)-2-(4-hydroxy-3-methoxybenzylidene)-6-methoxy-3,4-dihydronaphthalen-1(2h)-one, C19H18O4. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 203–205; https://doi.org/10.1515/ncrs-2016-0195.Search in Google Scholar
13. Luan, M. Z., Wang, H. Y., Zhang, M., Song, J., Xu, Y. R., Zhao, F. L., Meng, Q. G. Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl) benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2h)-one, C19H14F4O2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 245–247.10.1515/ncrs-2020-0484Search in Google Scholar
14. Wang, L., Meng, Q. G., Hou, G. G. Crystal structure of (E)-7-methoxy-2-((6-methoxypyridin-2-yl)methylene)-tetralone, C18H17NO3. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 1023–1025; https://doi.org/10.1515/ncrs-2021-0207.Search in Google Scholar
15. Zhang, X. F., Wang, H. Y., Zhao, S. N., Zhang, S. N., Zhao, F. L., Meng, Q. G. Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl) benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 47–49; https://doi.org/10.1515/ncrs-2020-0448.Search in Google Scholar
16. Gao, C. L., Hou, G. G., Liu, J., Ru, T., Xu, Y. Z., Zhao, S. Y., Ye, H., Zhang, L. Y., Chen, K. X., Guo, Y. W., Pang, T., Li, X. W. Synthesis and target identification of benzoxepane derivatives as potential anti-neuroinflammatory agents for ischemic stroke. Angew. Chem. Int. Ed. 2020, 59, 2429–2439; https://doi.org/10.1002/anie.201912489.Search in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8


