Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
Abstract
C44H43N11O12Co2, triclinic, P
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Purple block |
| Size: | 0.18 × 0.15 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.86 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 25.0°, 99% |
| N(hkl) measured , N(hkl) unique, R int: | 10,985, 7503, 0.029 |
| Criterion for I obs, N(hkl) gt: | I obs > 2 σ(I obs), 5201 |
| N(param)refined: | 648 |
| Programs: | Bruker [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.3879 (4) | 0.1110 (3) | 0.3604 (3) | 0.0274 (9) |
| C2 | 0.4536 (4) | 0.2238 (3) | 0.4345 (2) | 0.0252 (9) |
| C3 | 0.5784 (4) | 0.2481 (3) | 0.4761 (3) | 0.0308 (10) |
| H3 | 0.623960 | 0.195684 | 0.455387 | 0.037* |
| C4 | 0.6354 (4) | 0.3499 (3) | 0.5479 (3) | 0.0303 (10) |
| H4 | 0.719467 | 0.365467 | 0.574119 | 0.036* |
| C5 | 0.5692 (4) | 0.4285 (3) | 0.5812 (3) | 0.0282 (10) |
| C6 | 0.4446 (4) | 0.4046 (3) | 0.5394 (3) | 0.0387 (12) |
| H6 | 0.398785 | 0.456715 | 0.560308 | 0.046* |
| C7 | 0.3888 (4) | 0.3034 (3) | 0.4668 (3) | 0.0379 (11) |
| H7 | 0.305956 | 0.288977 | 0.439237 | 0.046* |
| C8 | 0.6243 (4) | 0.5326 (3) | 0.6615 (3) | 0.0267 (9) |
| C9 | 0.7500 (4) | 0.6526 (3) | 0.7785 (3) | 0.0249 (9) |
| C10 | 0.8560 (4) | 0.7113 (3) | 0.8530 (3) | 0.0257 (9) |
| C11 | 0.9415 (4) | 0.6544 (3) | 0.8603 (3) | 0.0361 (11) |
| H11 | 0.931307 | 0.581923 | 0.818010 | 0.043* |
| C12 | 1.0409 (4) | 0.7041 (3) | 0.9294 (3) | 0.0356 (11) |
| H12 | 1.098930 | 0.665642 | 0.932324 | 0.043* |
| C13 | 1.0560 (4) | 0.8096 (3) | 0.9944 (3) | 0.0262 (9) |
| C14 | 0.9697 (4) | 0.8660 (3) | 0.9887 (3) | 0.0352 (11) |
| H14 | 0.977279 | 0.936575 | 1.033182 | 0.042* |
| C15 | 0.8711 (4) | 0.8183 (3) | 0.9170 (3) | 0.0365 (11) |
| H15 | 0.816005 | 0.858311 | 0.912341 | 0.044* |
| C16 | 1.1592 (4) | 0.8599 (3) | 1.0734 (3) | 0.0268 (9) |
| C17 | −0.0552 (4) | −0.2869 (3) | 0.1583 (2) | 0.0238 (9) |
| C18 | −0.1650 (3) | −0.3556 (3) | 0.0828 (2) | 0.0212 (9) |
| C19 | −0.1792 (4) | −0.4673 (3) | 0.0276 (3) | 0.0276 (10) |
| H19 | −0.121121 | −0.501797 | 0.037775 | 0.033* |
| C20 | −0.2794 (4) | −0.5275 (3) | −0.0426 (2) | 0.0264 (9) |
| H20 | −0.287924 | −0.602121 | −0.079108 | 0.032* |
| C21 | −0.3674 (3) | −0.4768 (3) | −0.0587 (2) | 0.0220 (9) |
| C22 | −0.3541 (4) | −0.3658 (3) | −0.0033 (3) | 0.0284 (10) |
| H22 | −0.413149 | −0.331431 | −0.012582 | 0.034* |
| C23 | −0.2521 (4) | −0.3057 (3) | 0.0665 (3) | 0.0276 (10) |
| H23 | −0.242519 | −0.230722 | 0.102591 | 0.033* |
| C24 | −0.4723 (3) | −0.5406 (3) | −0.1342 (2) | 0.0212 (9) |
| C25 | −0.5955 (3) | −0.6628 (3) | −0.2525 (2) | 0.0217 (9) |
| C26 | −0.6481 (3) | −0.7666 (3) | −0.3331 (2) | 0.0214 (9) |
| C27 | −0.5836 (4) | −0.8472 (3) | −0.3611 (2) | 0.0255 (9) |
| H27 | −0.505495 | −0.834942 | −0.328760 | 0.031* |
| C28 | −0.6339 (3) | −0.9460 (3) | −0.4369 (2) | 0.0243 (9) |
| H28 | −0.589998 | −0.999828 | −0.454519 | 0.029* |
| C29 | −0.7503 (3) | −0.9650 (3) | −0.4867 (2) | 0.0220 (9) |
| C30 | −0.8144 (4) | −0.8840 (3) | −0.4591 (3) | 0.0276 (10) |
| H30 | −0.891362 | −0.895400 | −0.492235 | 0.033* |
| C31 | −0.7653 (4) | −0.7863 (3) | −0.3828 (3) | 0.0288 (10) |
| H31 | −0.810492 | −0.733410 | −0.364307 | 0.035* |
| C32 | −0.8017 (4) | −1.0718 (3) | −0.5680 (2) | 0.0245 (9) |
| C33 | 0.0147 (4) | 0.1560 (3) | 0.3035 (3) | 0.0358 (11) |
| H33 | 0.093369 | 0.202487 | 0.299681 | 0.043* |
| C34 | −0.1301 (4) | 0.0159 (4) | 0.3037 (3) | 0.0382 (11) |
| H34 | −0.171273 | −0.055442 | 0.299786 | 0.046* |
| C35 | −0.1872 (4) | 0.0979 (4) | 0.3230 (3) | 0.0423 (12) |
| H35 | −0.272753 | 0.093985 | 0.334042 | 0.051* |
| C36 | −0.1073 (5) | 0.2999 (4) | 0.3462 (4) | 0.0531 (14) |
| H36A | −0.024813 | 0.349647 | 0.344180 | 0.064* |
| H36B | −0.127010 | 0.329331 | 0.406262 | 0.064* |
| C37 | −0.2116 (4) | 0.3000 (4) | 0.2870 (3) | 0.0478 (13) |
| H37A | −0.286519 | 0.235094 | 0.275246 | 0.057* |
| H37B | −0.179958 | 0.291345 | 0.231172 | 0.057* |
| C38 | −0.2558 (4) | 0.4021 (3) | 0.3214 (3) | 0.0431 (12) |
| H38A | −0.314243 | 0.398790 | 0.275192 | 0.052* |
| H38B | −0.304809 | 0.402445 | 0.369836 | 0.052* |
| C39 | −0.0844 (4) | 0.5516 (3) | 0.3011 (3) | 0.0284 (10) |
| H39 | −0.104639 | 0.520312 | 0.240085 | 0.034* |
| C40 | −0.0913 (4) | 0.5771 (4) | 0.4375 (3) | 0.0439 (12) |
| H40 | −0.114927 | 0.568785 | 0.488157 | 0.053* |
| C41 | 0.0054 (4) | 0.6610 (4) | 0.4329 (3) | 0.0388 (11) |
| H41 | 0.060557 | 0.720810 | 0.481298 | 0.047* |
| C42 | 0.5167 (4) | 0.9317 (4) | 0.8532 (3) | 0.0417 (12) |
| H42 | 0.583760 | 0.957964 | 0.825472 | 0.050* |
| C43 | 0.3216 (5) | 0.9538 (5) | 0.9212 (4) | 0.0633 (16) |
| H43A | 0.325772 | 0.888710 | 0.928576 | 0.095* |
| H43B | 0.240335 | 0.936662 | 0.886481 | 0.095* |
| H43C | 0.327428 | 1.014132 | 0.977922 | 0.095* |
| C44 | 0.4384 (5) | 1.0850 (4) | 0.8610 (4) | 0.0562 (15) |
| H44A | 0.507387 | 1.094781 | 0.826336 | 0.084* |
| H44B | 0.456681 | 1.150016 | 0.916436 | 0.084* |
| H44C | 0.357138 | 1.075325 | 0.829959 | 0.084* |
| Co1 | 0.12432 (5) | −0.04372 (4) | 0.26195 (3) | 0.02256 (15) |
| Co2 | 0.13780 (5) | −0.25823 (4) | 0.29449 (3) | 0.02151 (15) |
| N1 | 0.5670 (3) | 0.6119 (3) | 0.6928 (2) | 0.0344 (9) |
| N2 | 0.6491 (3) | 0.6885 (3) | 0.7679 (2) | 0.0331 (9) |
| H2 | 0.637801 | 0.750821 | 0.803415 | 0.040* |
| N3 | 0.7377 (3) | 0.5538 (3) | 0.7122 (2) | 0.0281 (8) |
| N4 | −0.5693 (3) | −0.5042 (3) | −0.1497 (2) | 0.0265 (8) |
| H4A | −0.579942 | −0.440714 | −0.116252 | 0.032* |
| N5 | −0.6492 (3) | −0.5808 (2) | −0.2254 (2) | 0.0259 (8) |
| N6 | −0.4845 (3) | −0.6411 (2) | −0.1984 (2) | 0.0227 (7) |
| N7 | −0.0034 (3) | 0.0520 (3) | 0.2907 (2) | 0.0280 (8) |
| N8 | −0.0929 (3) | 0.1883 (3) | 0.3228 (2) | 0.0369 (9) |
| N9 | −0.1475 (3) | 0.5069 (3) | 0.3523 (2) | 0.0321 (9) |
| N10 | 0.0108 (3) | 0.6458 (3) | 0.3474 (2) | 0.0262 (8) |
| N11 | 0.4295 (3) | 0.9870 (3) | 0.8766 (2) | 0.0402 (10) |
| O1 | 0.2751 (2) | 0.0990 (2) | 0.32642 (18) | 0.0321 (7) |
| O2 | 0.4434 (3) | 0.0372 (2) | 0.33751 (19) | 0.0382 (8) |
| O3 | 1.1577 (3) | 0.9508 (2) | 1.13595 (17) | 0.0326 (7) |
| O4 | 1.2389 (3) | 0.8072 (2) | 1.07241 (18) | 0.0362 (7) |
| O5 | −0.0371 (2) | −0.1834 (2) | 0.19469 (18) | 0.0306 (7) |
| O6 | 0.0105 (2) | −0.3381 (2) | 0.18025 (17) | 0.0300 (7) |
| O7 | −0.7561 (3) | −1.1515 (2) | −0.58065 (18) | 0.0331 (7) |
| O8 | −0.8919 (2) | −1.0835 (2) | −0.62484 (17) | 0.0294 (7) |
| O9 | 0.2604 (3) | −0.1510 (2) | 0.24192 (18) | 0.0263 (6) |
| O10 | 0.2457 (3) | −0.3655 (2) | 0.25684 (19) | 0.0286 (7) |
| O11 | 0.3705 (3) | 0.6984 (3) | 0.9298 (2) | 0.0488 (9) |
| O12 | 0.5167 (3) | 0.8481 (3) | 0.8651 (2) | 0.0487 (9) |
| H11A | 0.411 (3) | 0.7516 (13) | 0.9170 (15) | 0.026 (12)* |
| H11B | 0.326 (2) | 0.7269 (19) | 0.9698 (14) | 0.063 (18)* |
| H10A | 0.3189 (14) | −0.355 (2) | 0.2376 (18) | 0.050 (15)* |
| H10B | 0.236 (3) | −0.4229 (13) | 0.2671 (16) | 0.042 (14)* |
| H9B | 0.326 (2) | −0.0922 (19) | 0.2647 (18) | 0.068 (17)* |
| H9A | 0.258 (4) | −0.172 (3) | 0.1865 (3) | 0.073 (18)* |
Source of material
The mixture of 3,5-bis(4′-carboxy-phenyl)-1,2,4-triazole 15.45 mg (0.05 mmol), cobalt nitrate hexahydrate 43.65 mg (0.075 mmol), 1,3-di(1H-imidazol-1-yl)propane 17.6 mg (0.05 mmol), NaOH 12 mg (0.15 mmol) and N,N-dimethylformamide (6 mL) were placed in the autoclave lined with PTFE and heated at 120 ∘C for 24 h, then cooled down to room temperature over 24 h. The light pink crystals after cooling to room temperature were collected.
Experimental details
The structure was solved by direct methods and refined with the SHELX crystallographic software package [1]. The hydrogen atoms were placed at calculated positions and refined as riding atoms.
Comment
Coordination polymers (CPs) have raised special interest as a class of organic-inorganic hybrid materials due to their unique infinite structures, rich structural diversities formed by the combination of metal ions with organic ligands and important potential applications. Mixed ligands-CPs are often constructed from metal ions, carboxylates and nitrogen-containing ligands. In most situations, N containing ligands are electrically neutral, while carboxylate ligands are negatively charged in such coordination polymers. Multicarboxylates like the 3,5-bis(4′-carboxy-phenyl)-1,2,4-triazole have been widely utilized in such networks [4], [5], [6], [7]. On the other hand, 1,3-di(1H-imidazol-1-yl)propane (diim) is known to serve as bridging ligand [8], [9], [10]. Meanwhile, similar coordination polymer has been reported [11] which is constructed by same metal ion and ligands, the title compound exhibts a totally different structure by changing the synthesis conditions.
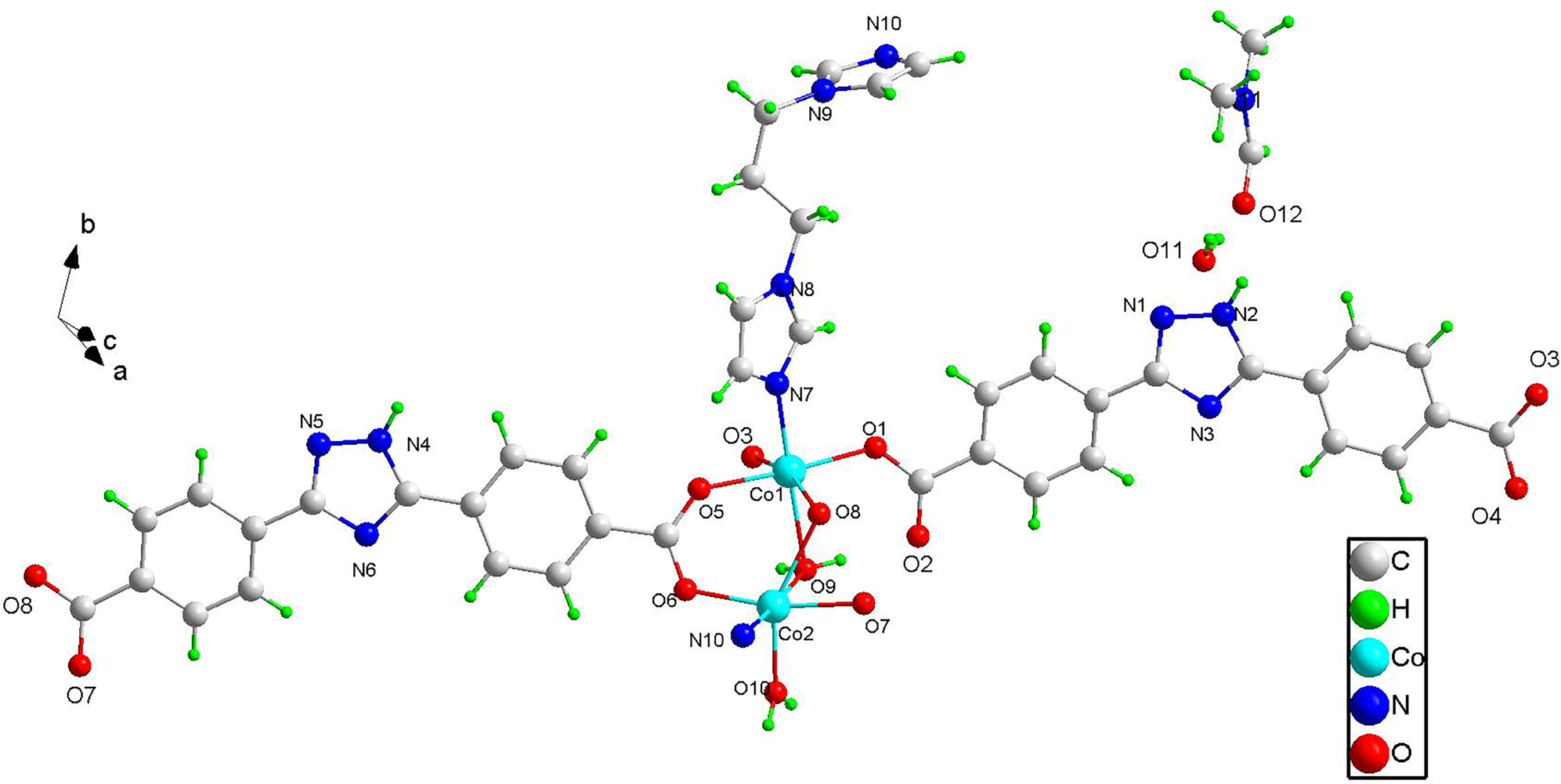
The asymmetric unit of title structure contains two independent Co(II) cations (Co1, Co2), two deprotonated 3,5-bis(4′-carboxy-phenyl)-1,2,4-triazole ligands, one 1,3-di(1H-imidazol-1-yl)propane ligand, one bridging water molecule, one unidentate coordinated water molecule, one free DMF and one free water. As shown in the figure, the two Co(II) exhibit similar coordination modes which exhibit distorted octahedral geometry. The six atoms that coordinate with Co1 come from one nitrogen atom (N7) of 1,3-di(1H-imidazol-1-yl)propane, one oxygen atom (O9) of bridged water, three unidentate coordinated oxygen atoms (O1,O3,O5) of three 4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoate ligands, one bridged carboxyl oxygen atom (O8) of one 4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoate ligand. Meanwhile, Co2 exhibits another distorted octahedral geometry with one nitrogen atom (N10) of 1,3-di(1H-imidazol-1-yl)propane, one oxygen atom (O9) of bridging coordinated water molecule, one oxygen atom (O10) of one unidentate water, three atoms (O6,O7,O8) of three 4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoate ligands. An interesting two dimensional double layer framework is formed.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 22177066
Funding source: Natural Science Foundation of Shaanxi Province
Award Identifier / Grant number: 2021KJXX-51
Funding source: Hanzhong City and Shaanxi University of Technology
Award Identifier / Grant number: SLGRCQD2007
Funding source: Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education
Award Identifier / Grant number: KLSNFM2020007
Funding source: Shaanxi Provincial Science and Technology Department
Award Identifier / Grant number: 2021JQ-752
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The National Natural Science Foundation of China (No. 22177066), the Natural Science Foundation of Shaanxi Province (No. 2021KJXX-51), the Co-construction Project of Hanzhong City and Shaanxi University of Technology (SLGRCQD2007), and the Open Foundation of Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education (KLSNFM2020007). Shaanxi Provincial Science and Technology Department project (Program No. 2021JQ-752).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT, APEX2 and SADABS. Bruker AXS Inc., Madison, WI, 2009.Suche in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXTL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Hou, X. Y., Wang, X., Fu, F., Wang, J. J., Tang, L. Synthesis and characterization of two- and three-dimensional coordination polymers built with 3,5-bis(4′-carboxy-phenyl)-1,2,4-triazole and/or 4-bis(imidazol-1-ylmethyl) benzene. J. Coord. Chem. 2013, 66, 3126–3136. https://doi.org/10.1080/00958972.2013.827178.Suche in Google Scholar
5. Li, Y. W., He, K. H., Bu, X. H. Bottom-up assembly of a porous MOF based on nanosized nonanuclear zinc precursors for highly selective gas adsorption. J. Mater. Chem. 2013, 1, 4186–4189. https://doi.org/10.1039/c3ta01322g.Suche in Google Scholar
6. Hou, X. Y., Wang, X., Ren, Y. X., Wang, J. J., Jin, W., Kang, W. W., Ma, X., Han, X. X. Syntheses, crystal structures, and photoluminescent properties for one new Cd(II) CP based on 3,5-bis(4′-carboxy-phenyl)-1,2,4-triazole and 1,4-bis(imidazol-1-ylmethyl)benzene. Chin. J. Struct. Chem. 2017, 36, 2067–2072.Suche in Google Scholar
7. Hou, X. Y., Wang, X., Wang, X., Lou, J. G., Fu, F., Wang, J. J., Tang, L., Cao, J. Six lanthanide(III) coordination polymers with 3,5-bis(4′-carboxy-phenyl)-1,2,4-triazole: syntheses, structures, and photoluminescence. J. Coord. Chem. 2015, 68, 1814–1828. https://doi.org/10.1080/00958972.2015.1025768.Suche in Google Scholar
8. Li, P.-X. Lu, J.-F. A novel 3D twofold Zn(II) coordination polymer constructed by mixed flexible ligands: synthesis, crystal structure and fluorescence property. Chin. J. Struct. Chem. 2017, 36, 303–309.Suche in Google Scholar
9. Lu, J. F., Liu, Z. H. Three metal induced 3D coordination polymers based on H3BTC and 1,3–BIP as co-ligands: synthesis, structures and flfluorescent properties. Polyhedron 2016, 107, 19–26. https://doi.org/10.1016/j.poly.2016.01.018.Suche in Google Scholar
10. Lu, J. F., Wang, M. Z., Liu, Z. H. Two novel coordination polymers constructed by the same mixed ligands of 1,3-bip and H2bpdc: syntheses, structures and catalytic properties. J. Mol. Struct. 2015, 1098, 41–46. https://doi.org/10.1016/j.molstruc.2015.05.030.Suche in Google Scholar
11. Qiu, X., Zhao, J., Liu, M. L., Ge, H. G., Ji, X. H., Wang, Q., Jin, L. X., Lu, J. F. Crystal structure of C28H34N8O8Co. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 697–699; https://doi.org/10.1515/ncrs-2021-0034.Suche in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

