Abstract
C20H22F2N2O4, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange block |
| Size | 0.14 × 0.13 × 0.11 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.94 mm−1 |
| Diffractometer, scan mode: | Bruker D8 Venture, φ and ω |
| θ max, completeness: | 72.1°, 99% |
| N(hkl)measured, N(hkl)unique, R int: | 5378, 1812, 0.027 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 1625 |
| N(param)refined: | 128 |
| Programs: | Bruker [1], SHELX [2], Olex2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| F1 | 0.9087 (2) | 0.95953 (9) | 0.37137 (9) | 0.0681 (3) |
| O1 | 0.4406 (2) | 0.68236 (10) | 0.79894 (9) | 0.0500 (3) |
| H1 | 0.327936 | 0.615097 | 0.836080 | 0.075* |
| O2 | 0.26550 (18) | 0.16591 (9) | 0.94055 (8) | 0.0415 (2) |
| N1 | 0.1157 (2) | 0.48530 (11) | 0.81784 (10) | 0.0401 (3) |
| C1 | 0.4911 (3) | 0.71494 (13) | 0.66568 (12) | 0.0394 (3) |
| C2 | 0.6761 (3) | 0.82331 (13) | 0.58499 (14) | 0.0476 (3) |
| H2 | 0.763873 | 0.874412 | 0.622674 | 0.057* |
| C3 | 0.7281 (3) | 0.85425 (14) | 0.44989 (14) | 0.0480 (3) |
| C4 | 0.6085 (3) | 0.78502 (15) | 0.38838 (13) | 0.0509 (3) |
| H4 | 0.652106 | 0.808851 | 0.294496 | 0.061* |
| C5 | 0.4224 (3) | 0.67951 (16) | 0.46897 (13) | 0.0490 (3) |
| H5 | 0.333138 | 0.631312 | 0.428880 | 0.059* |
| C6 | 0.3613 (3) | 0.64133 (13) | 0.60785 (12) | 0.0397 (3) |
| C7 | 0.1663 (3) | 0.52811 (14) | 0.68993 (12) | 0.0421 (3) |
| H7 | 0.073585 | 0.484701 | 0.646429 | 0.051* |
| C8 | −0.0805 (3) | 0.37050 (14) | 0.89368 (13) | 0.0447 (3) |
| H8A | −0.156143 | 0.341219 | 0.829930 | 0.054* |
| H8B | −0.245025 | 0.409331 | 0.935891 | 0.054* |
| C9 | 0.0682 (3) | 0.23775 (13) | 1.00365 (12) | 0.0414 (3) |
| H9A | 0.171781 | 0.268451 | 1.058767 | 0.050* |
| H9B | −0.075274 | 0.169673 | 1.065146 | 0.050* |
| C10 | 0.3923 (3) | 0.03154 (13) | 1.03798 (11) | 0.0403 (3) |
| H10A | 0.243123 | −0.037841 | 1.088844 | 0.048* |
| H10B | 0.488978 | 0.048919 | 1.104164 | 0.048* |
Source of material
All chemicals were purchased from commercial sources and used without further purification. The title compound was prepared following the literature procedures [4, 5]. A flask was charged with 4-fluoro-2-hydroxy-benzaldehyde (280.22 mg, 2 mmol) and 1,8-diamino-3,6-dioxaoctane (148.21 mg, 1 mmol) and 20 mL methanol. The mixture was stirred at 238 K for 6 h. After cooling to room temperature, the mixture was filtered, washed successively with methanol and methanol/hexane (1:4), respectively. The isolated product was dried under reduced pressure and purified by recrystallization with methanol solution. Several clear orange crystals were obtained.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
In the past decades, various Schiff base compounds and their derivatives have been designed and synthesized through different strategies. For example, Salen-type compound [6], Salamo-type compound [7] and so on. These compounds and their derivatives have excellent applications in materials science [8], biochemistry [9] and coordination chemistry [10]. The Schiff base compounds form stable metal complexes with one, two or more transition metal centers [11], [12], and rare earth metals [13].
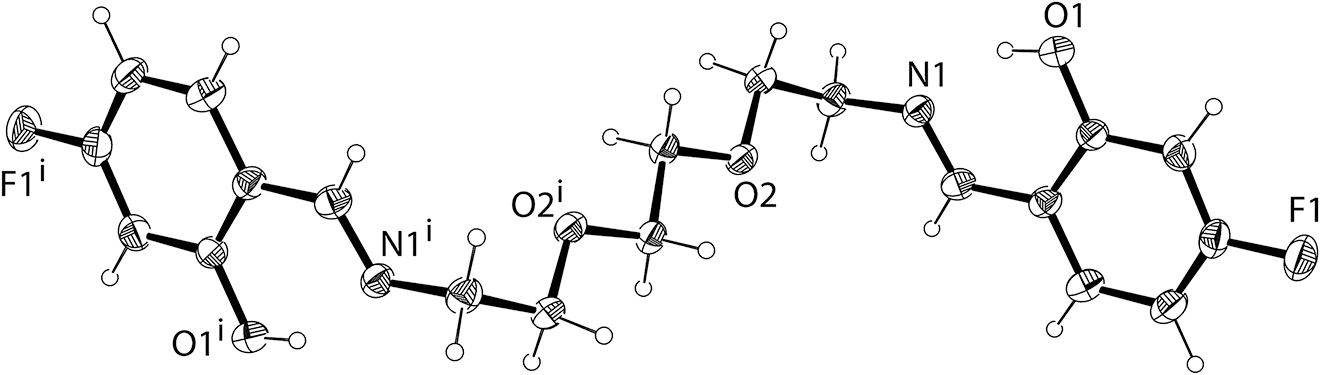
The crystal structure of the title compound was analyzed by X-ray diffraction (cf. the figure), in which all bond lengths and bond angles are within the normal ranges [7]. There is one strong intramolecular O1—H1⃛N1 hydrogen bond interaction (d(N1⃛H1) = 1.85 Å, d(O1—H1) = 0.84 Å and d(O1⃛N1) = 2.5953(13) Å) in the title structure at both ends of the centrosymmetric molecule. In addition, there are two intermolecular C2—H2⃛F1#2 (#2 −x + 2, −y + 2, −z + 1) and C4—H4⃛O2#3 (#3 −x + 1, −y + 1, −z + 1) hydrogen bonds, which are (d(F1#2⃛H1) = 2.45 Å, d(C2—H2) = 0.95 Å, d(C2⃛F1#2) = 3.3474(17) Å) and (d(O2#3⃛H4) = 2.47 Å, d(C4—H4) = 0.95 Å, d(C4⃛O2#3) = 3.4078(15) Å), respectively.
Funding source: Open Project Program of Shihezi University
Award Identifier / Grant number: ZZZC2021117
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This project was supported by the Open Project Program of Shihezi University (ZZZC2021117).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX3, SAINT; Bruker AXS Inc.: Madison, WI, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
4. Beyramabadi, S. A., Morsali, A., Bozorgmehr, M. R., Khoshkholgh, M. J., Esmaeili, A. A. N, N′–dipyridoxyl(1,8-diamino-3,6-dioxaoctane) Schiff-base: synthesis, experimental and theoretical identification. Bull. Chem. Soc. Ethiop. 2013, 27, 273–280; https://doi.org/10.4314/bcse.v27i2.12.Search in Google Scholar
5. Xu, X., Wang, J. F., Bian, R. N., Zhao, L. Synthesis, structure, Hirshfeld analysis and fluorescence properties of a new asymmetric salamo-based ligand and its Cu(II) complex involving oxime oxygen coordination. J. Coord. Chem. 2020, 73, 2209–2223; https://doi.org/10.1080/00958972.2020.1822524.Search in Google Scholar
6. Schmid, J., Frey, W., Peters, R. Polynuclear enantiopure Salen-mesoionic carbene hybrid complexes. Organometallics 2017, 36, 4313–4324; https://doi.org/10.1021/acs.organomet.7b00729.Search in Google Scholar
7. Li, J., Zhang, H. J., Chang, J., Jia, H. R., Sun, Y. X., Huang, Y. Q. Solvent-induced unsymmetric Salamo-like trinuclear NiII complexes: syntheses, crystal structures, fluorescent and magnetic properties. Crystals 2018, 8, 176; https://doi.org/10.3390/cryst8040176.Search in Google Scholar
8. Liu, P. P., Wang, C. Y., Zhang, M., Song, X. Q. Pentanuclear sandwich-type ZnII–LnIII clusters based on a new Salen-like salicylamide ligand: structure, near-infrared emission and magnetic properties. Polyhedron 2017, 129, 133–140; https://doi.org/10.1016/j.poly.2017.03.019.Search in Google Scholar
9. Li, P., Niu, M. J., Hong, M., Cheng, S., Dou, J. M. Effect of structure and composition of nickel(II) complexes with salicylidene Schiff base ligands on their DNA/protein interaction and cytotoxicity. J. Inorg. Biochem. 2014, 137, 101–108; https://doi.org/10.1016/j.jinorgbio.2014.04.005.Search in Google Scholar PubMed
10. Yu, T. Z., Su, W. M., Li, W. L., Hong, Z. R., Hua, R. N., Li, M. T., Chu, B., Li, B., Zhang, Z. Q., Hu, Z. Z. Synthesis, crystal structure and electroluminescent properties of a Schiff base zinc complex. Inorg. Chim. Acta 2006, 359, 2246–2251; https://doi.org/10.1016/j.ica.2006.01.019.Search in Google Scholar
11. Ebrahimpour, M., Behzad, M. Spectroscopic studies and determination of the formation constants and thermodynamic parameters for a new water-soluble Co(II) Schiff-base complex and some imidazole derivatives. J. Coord. Chem. 2011, 64, 1426–1435; https://doi.org/10.1080/00958972.2011.570756.Search in Google Scholar
12. Zhou, X. X., Cai, Y. P., Zhu, S. Z., Zhan, Q. G., Liu, M. S., Zhou, Z. Y., Chen, L. Self-assembly of two novel cadmium(II) complexes: one from tripodal imine-phenol ligand and the other from in situ partial degradation of dipolar imine-phenol ligand. Cryst. Growth Des. 2008, 8, 2076–2079; https://doi.org/10.1021/cg8002228.Search in Google Scholar
13. Lee, J. C., Jeong, Y. K., Kim, J. M., Kang, J. G. Sensitized luminescence of Eu(III) complexes with Schiff-base and 1,10-phenanthroline: role of Schiff-base as a sensitizer. Spectrochim. Acta 2014, A124, 256–264; https://doi.org/10.1016/j.saa.2013.12.117.Search in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

