Abstract
C28H32Fe2N4O13, monoclinic, P2/n (no. 13), a = 8.0964(3) Å, b = 13.4889(5) Å, c = 14.0543(5) Å, β = 95.190(3)°, V = 1528.60(10) Å3 , Z = 2, Rgt (F) = 0.0405, wR ref(F 2) = 0.0972, T = 173(2) K.
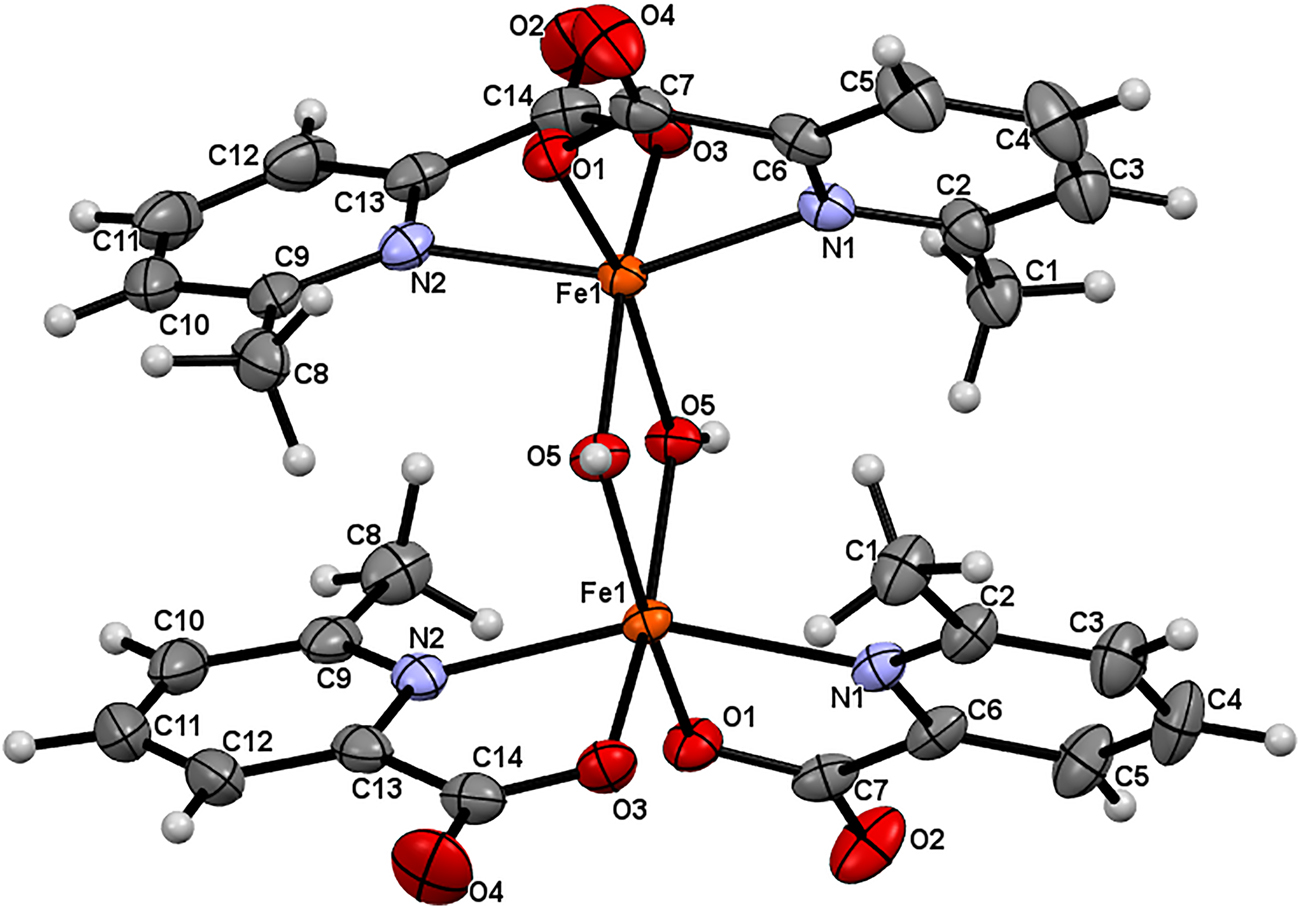
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.17 × 0.10 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.02 mm−1 |
| Diffractometer, scan mode: | Xcalibur, φ and ω |
| θ max, completeness: | 28.9°, 98% |
| N(hkl)measured, N(hkl)unique, R int: | 10,287, 3566, 0.034 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2670 |
| N(param)refined: | 226 |
| Programs: | CrysAlisPRO [1], Olex2 [2, 3], SHELX [3], Mercury [4, 5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Fe1 | 0.86752 (4) | 0.72914 (3) | 0.84619 (2) | 0.01856 (11) |
| O1 | 1.11457 (19) | 0.71986 (12) | 0.86174 (12) | 0.0262 (4) |
| O2 | 1.3528 (2) | 0.80230 (16) | 0.86847 (15) | 0.0405 (5) |
| O3 | 0.8007 (2) | 0.72871 (13) | 0.97914 (12) | 0.0278 (4) |
| O4 | 0.6850 (3) | 0.63967 (18) | 1.08788 (14) | 0.0510 (6) |
| O5 | 0.6330 (2) | 0.73046 (13) | 0.79354 (12) | 0.0212 (3) |
| H5 | 0.563 (4) | 0.743 (2) | 0.818 (3) | 0.048 (11)* |
| N1 | 0.9388 (2) | 0.88338 (15) | 0.86761 (14) | 0.0225 (4) |
| N2 | 0.8682 (2) | 0.57198 (16) | 0.87877 (14) | 0.0241 (4) |
| C1 | 0.6627 (3) | 0.9509 (2) | 0.8846 (2) | 0.0343 (6) |
| H1A | 0.609291 | 0.928190 | 0.823111 | 0.051* |
| H1B | 0.613702 | 1.014059 | 0.901874 | 0.051* |
| H1C | 0.646227 | 0.901373 | 0.933914 | 0.051* |
| C2 | 0.8442 (3) | 0.96457 (19) | 0.87692 (18) | 0.0284 (6) |
| C3 | 0.9155 (3) | 1.0585 (2) | 0.8802 (2) | 0.0380 (7) |
| H3 | 0.847687 | 1.115410 | 0.885441 | 0.046* |
| C4 | 1.0840 (4) | 1.0696 (2) | 0.8760 (2) | 0.0427 (7) |
| H4 | 1.133033 | 1.133645 | 0.877513 | 0.051* |
| C5 | 1.1808 (3) | 0.9851 (2) | 0.8694 (2) | 0.0372 (7) |
| H5A | 1.297379 | 0.990375 | 0.867306 | 0.045* |
| C6 | 1.1053 (3) | 0.89439 (19) | 0.86614 (17) | 0.0260 (5) |
| C7 | 1.2014 (3) | 0.7985 (2) | 0.86437 (17) | 0.0261 (5) |
| C8 | 1.0186 (3) | 0.5124 (2) | 0.7468 (2) | 0.0357 (6) |
| H8A | 0.945174 | 0.533604 | 0.691300 | 0.054* |
| H8B | 1.075693 | 0.451183 | 0.731283 | 0.054* |
| H8C | 1.100660 | 0.564315 | 0.763743 | 0.054* |
| C9 | 0.9185 (3) | 0.49403 (19) | 0.82930 (19) | 0.0298 (6) |
| C10 | 0.8784 (4) | 0.3976 (2) | 0.8568 (2) | 0.0414 (7) |
| H10 | 0.911758 | 0.342379 | 0.821183 | 0.050* |
| C11 | 0.7918 (4) | 0.3827 (2) | 0.9344 (2) | 0.0467 (8) |
| H11 | 0.761238 | 0.317477 | 0.951444 | 0.056* |
| C12 | 0.7490 (4) | 0.4622 (2) | 0.9877 (2) | 0.0401 (7) |
| H12 | 0.692877 | 0.452825 | 1.043556 | 0.048* |
| C13 | 0.7890 (3) | 0.5556 (2) | 0.95853 (17) | 0.0286 (6) |
| C14 | 0.7527 (3) | 0.6475 (2) | 1.01402 (18) | 0.0304 (6) |
| O1W | 0.750000 | 0.7497 (5) | 1.250000 | 0.204 (5) |
| H1W | 0.759169 | 0.716755 | 1.191366 | 0.244* |
| O2W | 0.4626 (5) | 0.8189 (3) | 1.0859 (5) | 0.1221 (15) |
| H2WA | 0.500380 | 0.760399 | 1.081962 | 0.183* |
| H2WB | 0.489898 | 0.798188 | 1.153989 | 0.10 (2)* |
Source of material
The starting material 6-methyl-2-pyridinecarboxylic acid was bought from the commercial source and used without further treatment. The title compound was prepared by the solvothermal method. Firstly, 6-methyl-2-pyridinecarboxylic acid (27.4 mg, 0.2 mmol) was dissolved in 5 mL methanol with triethylamine (28 μL, 0.2 mmol). Then, the 5 mL methanolic solution of Fe(NO3)3·H2O (40.4 mg, 0.1 mmol) was slowly dropped into the above solution. The mixture was stirred at room temperature for 30 min, then transferred to the Teflon autoclave and heated at 120 °C for three days. After cooling down to room temperature, red block crystals of the title compound were obtained.
Experimental details
Hydrogen atoms were added using riding models.
Comment
Picolinic acid derivatives have been widely studied as complex ligands [6], or functional materials [7]. However, only a limited number of compounds of its 6-methyl substituted derivative (6-methyl-2-pyridinecarboxylic acid) have been reported, and most of them are mononuclear compounds [8, 9].
Fe(III) complex (see the Figure) was achieved by considering the charge balance of the whole motif. All the bond lengths are within normal ranges, similar to its dinuclear analogue with two hydroxyl bridges [10]. The Fe(III) is bridged by two OH− and chelated by two 6-methyl-2-pyridinecarboxylato groups to form a diunclear complex. The diunclear complexes are interlinked to from a 3D supramolecular network by two intermolecular H-bonds C8–H8A···O4 (symmetry code: x + 1/2, −y + 1, z − 1/2) [length 2.58(3) Å, angle 140.8(2)°], O5–H1···O2 (symmetry code: x − 1, y, z) [length 2.06(2) Å, angle 168.4(4)°] between the dinuclear complexes, one H-bonding C1–H1B···O2W (symmetry code: −x + 1, −y + 2, −z + 2) [length 2.34 (7) Å, angle 165.5(8)°] between the dinuclear complex and the water (lattice solvent) molecules.
Acknowledgments
We are very grateful to Luan Hui-Ni Famous Teacher Studio (2019). We thank Doctor Feng-Lei Yang for the help with crystal data deposition.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction Ltd. CrysAlisPRO, Version 1.171.32.24; Oxford Diffraction Ltd: Abingdon, Oxfordshire, England, 2008.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K., Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment-Olex2 dissected. Acta Crystallogr. 2015, A71, 59–75; https://doi.org/10.1107/s2053273314022207.Search in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Mercury 3.10 (Build 157083) Copyright: Cambridge Crystallographic Data Centre. http://www.ccdc.cam.ac.uk/mercury/.Search in Google Scholar
6. Soldin, Z., Kukovec, B. M., Matkovic-Calogovic, D., Popovic, Z. The solvent effect on composition and dimensionality of mercury(II) complexes with picolinic acid. Molecules 2021, 26, 16; https://doi.org/10.3390/molecules26165002.Search in Google Scholar PubMed PubMed Central
7. Suanzes Pita, J., Urbani, M., Bottari, G., Ince, M., Kumar, S. A., Chandiran, A. K., Yum, J. H., Gratzel, M., Nazeeruddin, M. K., Torres, T. Pyridyl- and picolinic acid substituted zinc(II) phthalocyanines for dye-sensitized solar cells. ChemPlusChem 2017, 82, 1057–1061; https://doi.org/10.1002/cplu.201700048.Search in Google Scholar PubMed
8. García, F., Perles, J., Zamora, F. Amo-Ochoa, P. Rhodium and copper 6-methylpicolinate complexes. Structural diversity and supramolecular interaction study. Inorg. Chim. Acta 2016, 453, 574–582; https://doi.org/10.1016/j.ica.2016.08.040.Search in Google Scholar
9. Chishiro, T., Kon, Y., Nakashima, T., Goto, M., Sato, K. Practical iron-catalyzed hydrogen peroxide epoxidation of aromatic olefins using a combination of two kinds of simple picolinate ligands under halide-free reaction conditions. Adv. Synth. Catal. 2014, 356, 623–627; https://doi.org/10.1002/adsc.201300774.Search in Google Scholar
10. Eshtiagh-Hosseini, H., Alfi, N., Mirzaei, M., Fanwick, P., Fanwick, P. E. Di-μ-hydroxido-bis-[aqua-(pyridine-2,6-dicarboxyl-ato)iron(III)] monohydrate. Acta Crystallogr. 2010, E66, m1450; https://doi.org/10.1107/s1600536810041966.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

