Abstract
C18H18N4O5, monoclinic, P21/c (no. 14), a = 11.7553(8) Å, b = 6.4876(4) Å, c = 22.3442(15) Å, β = 91.263(7)°, V = 1703.64(19) Å3, Z = 4, Rgt (F) = 0.0531, wRref (F 2) = 0.1376, T = 160 K.
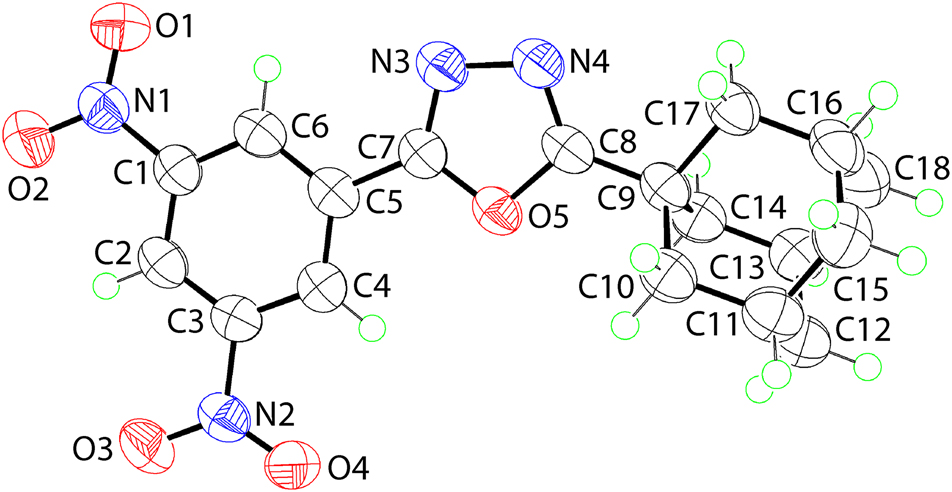
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.12 × 0.07 × 0.01 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.90 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω |
| θ max, completeness: | 74.6°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 6232, 6232 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4033 |
| N(param)refined: | 245 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.8577 (2) | 0.7819 (4) | 0.69232 (10) | 0.0333 (6) |
| C2 | 0.8024 (2) | 0.9481 (4) | 0.71731 (10) | 0.0353 (6) |
| H2 | 0.842743 | 1.053385 | 0.738362 | 0.042* |
| C3 | 0.6859 (2) | 0.9527 (4) | 0.71004 (10) | 0.0323 (5) |
| C4 | 0.6242 (2) | 0.8020 (4) | 0.68018 (10) | 0.0324 (5) |
| H4 | 0.543881 | 0.812750 | 0.675324 | 0.039* |
| C5 | 0.6827 (2) | 0.6341 (4) | 0.65740 (10) | 0.0325 (6) |
| C6 | 0.8009 (2) | 0.6237 (4) | 0.66345 (10) | 0.0340 (6) |
| H6 | 0.841465 | 0.509658 | 0.647932 | 0.041* |
| C7 | 0.6221 (2) | 0.4643 (4) | 0.62800 (10) | 0.0317 (5) |
| C8 | 0.4828 (2) | 0.2888 (4) | 0.59284 (10) | 0.0330 (6) |
| C9 | 0.3623 (2) | 0.2453 (4) | 0.57540 (11) | 0.0328 (6) |
| C10 | 0.2827 (2) | 0.2846 (5) | 0.62800 (11) | 0.0385 (6) |
| H10A | 0.304942 | 0.195270 | 0.662224 | 0.046* |
| H10B | 0.289245 | 0.430014 | 0.641056 | 0.046* |
| C11 | 0.1596 (3) | 0.2382 (5) | 0.60835 (12) | 0.0418 (6) |
| H11 | 0.108048 | 0.262139 | 0.642671 | 0.050* |
| C12 | 0.1254 (3) | 0.3810 (5) | 0.55639 (12) | 0.0422 (6) |
| H12A | 0.045432 | 0.353884 | 0.543939 | 0.051* |
| H12B | 0.131343 | 0.526470 | 0.569435 | 0.051* |
| C13 | 0.2035 (3) | 0.3437 (5) | 0.50361 (11) | 0.0392 (6) |
| H13 | 0.180847 | 0.436678 | 0.469698 | 0.047* |
| C14 | 0.3271 (2) | 0.3877 (4) | 0.52273 (11) | 0.0371 (6) |
| H14A | 0.334896 | 0.533664 | 0.535099 | 0.045* |
| H14B | 0.377670 | 0.363704 | 0.488570 | 0.045* |
| C15 | 0.1504 (3) | 0.0140 (5) | 0.58816 (13) | 0.0456 (7) |
| H15A | 0.172820 | −0.078254 | 0.621692 | 0.055* |
| H15B | 0.070632 | −0.017583 | 0.576332 | 0.055* |
| C16 | 0.2277 (3) | −0.0235 (5) | 0.53526 (12) | 0.0424 (6) |
| H16 | 0.220315 | −0.170242 | 0.521993 | 0.051* |
| C17 | 0.3513 (3) | 0.0192 (4) | 0.55500 (12) | 0.0381 (6) |
| H17A | 0.402608 | −0.006822 | 0.521266 | 0.046* |
| H17B | 0.373608 | −0.073891 | 0.588344 | 0.046* |
| C18 | 0.1941 (3) | 0.1192 (5) | 0.48338 (12) | 0.0434 (7) |
| H18A | 0.244738 | 0.094967 | 0.449281 | 0.052* |
| H18B | 0.114952 | 0.089406 | 0.469918 | 0.052* |
| N1 | 0.9822 (2) | 0.7726 (4) | 0.69664 (9) | 0.0386 (5) |
| N2 | 0.6232 (2) | 1.1268 (4) | 0.73577 (9) | 0.0366 (5) |
| N3 | 0.6651 (2) | 0.2948 (4) | 0.60817 (9) | 0.0364 (5) |
| N4 | 0.5728 (2) | 0.1799 (4) | 0.58514 (9) | 0.0361 (5) |
| O1 | 1.03085 (17) | 0.6403 (3) | 0.66813 (8) | 0.0434 (5) |
| O2 | 1.0322 (2) | 0.8980 (4) | 0.72841 (11) | 0.0627 (7) |
| O3 | 0.6754 (2) | 1.2451 (4) | 0.76899 (10) | 0.0551 (6) |
| O4 | 0.52210 (17) | 1.1428 (3) | 0.72273 (8) | 0.0429 (5) |
| O5 | 0.50776 (16) | 0.4750 (3) | 0.62007 (7) | 0.0334 (4) |
Source of material
A mixture of adamantane-1-carboxylic acid (1.8 g, 0.01 mol), 3,5-dinitrobenzoyl chloride (2.3 g, 0.01 mol) and phosphorus oxychloride (8 mL) was heated under reflux for 1 h. On cooling, crushed ice (50 g) was added cautiously and the mixture stirred for 30 min. The precipitated crude product was filtered, washed with saturated sodium hydrogen carbonate solution and finally with water, dried and crystallised from ethanol to yield 3.41 g (92%) of the title compound (I) as colourless plates. M.pt.: 473–475 K (uncorrected). Anal. Calcd. for C18H18N4O5: C, 58.37; H, 4.90; N, 15.13%. Found: C, 58.33; H, 4.92; N, 15.09%. 1 H NMR (DMSO-d6, 700.17 MHz): δ 1.80–1.82 (m, 6H, Adamantane–H), 2.01–2.13 (m, 9H, Adamantane–H), 8.99 (s, 1H, Ar–H), 9.02 (s, 2H, Ar–H). 13 C{1 H} NMR (DMSO-d6, 176.08 MHz): δ 27.61, 34.53, 36.12, 39.63 (Adamantane–C), 121.34, 126.82, 126.86, 149.21 (Ar–C), 161.55, 173.81 (Oxadiazole–C). Single crystals suitable for X-ray diffraction were obtained by slow evaporation of a solution of (I) in EtOH/CHCl3 (1:1) at room temperature.
Experimental details
The C-bound H atoms were geometrically placed (C–H = 0.95–1.00 Å) and refined as riding with Uiso (H) = 1.2Ueq (C). The crystal was refined as a twin with a 180° rotation about [0 0 1]; the major component of the twin was refined to 0.5597(14).
Comment
Adamantane-containing derivatives were recognized early for their diverse chemotherapeutic properties and several adamantane derivatives are currently used in efficient therapies as anti-viral, anti-cancer and anti-microbial agents [5, 6]. On the other hand, the 1,3,4-oxadiazole heterocycle represents the core pharmacophore of several marketed drugs [7, 8]. In the present study, the crystal structure of an adamantane-1,3,4-oxadiazole hybrid molecule, (I), is described and its features compared to literature precedents [9], [10], [11], [12], [13], [14].
The molecular structure of (I) is shown in Figure (50% probability ellipsoids). The molecule comprises a central 1,3,4-oxadiazole ring connected at the C1-position to an adamantan-1-yl residue and at the C2-position to a 3,5-dinitrophenyl ring. Within the ring, the C7–N3 [1.292(4) Å] and C8–N4 [1.287(4) Å] bond lengths are consistent with substantial double-bond character with the N3–N4 bond length being 1.405(3) Å. The small elongation and shortening of the C–N and N–N bonds from their standard values is indicative of delocalisation of π-electron density in the ring; the r.m.s. deviation of the five atoms comprising the ring is 0.002 Å, consistent with strict planarity. The substituted phenyl ring forms a dihedral angle of 4.73(13)° with the five-membered ring. The N1- and N2-nitro groups are twisted out of the phenyl ring they are connected to, as seen in the dihedral angles between the respective least-squares planes of 9.38(12)° and 9.94(16)°. The dihedral angle between the nitro substituents [10.3(3)°] is indicative of a conrotatory relationship.
There are five literature precedents for (I) which differ only in the nature of the phenyl-bound substituents. These are the 4-fluoro [9], 4-chloro [9], 4-bromo [10], 4-nitro [11] and 3-fluoro [12] derivatives. The molecules adopt approximately the same conformations to that seen in (I) but with a range of nearly 21° in the dihedral angles formed between the five- and six-membered rings. Thus, for the 4-substituted molecules, the dihedral angles are 20.79(15)°, 9.48(7)°, 10.41(5)° and 0° [the molecule is bisected by a mirror plane], respectively. For the two independent molecules comprising the asymmetric-unit of the 3-fluoro species, the independent dihedral angles are 3.0(3)° and 3.3(3)°. Two other molecules worthy of mention are those where the adamantan-1-yl residue of (I) is substituted by a second 3,5-dinitrophenyl substituent [13] and a 2-(4-chlorophenyl)-1H-1,3-benzodiazole [14]. The bond lengths in the 1,3,4-oxadiazole ring of both structures match those noted for (I).
In the molecular packing of (I), helical chains along the 21-screw axis along the b-direction feature phenyl–C–H⃛O(nitro) contacts [C2–H2⃛O1i: H6⃛O2i = 2.60 Å, C6⃛O2i = 3.425(3) Å with angle at H6 = 146° for symmetry operation i: 2 − x, 1/2 + y, 3/2 − z]. Within the individual stacks comprising the chain, there are additional nitro-O⃛π(oxadiazole) interactions whereby the nitro-group is approximately parallel to a translationally related five-membered ring, forming a dihedral angle of 12.2(2)° [O4⃛Cg(oxadiazole)ii = 2.955(2) Å with angle at O4 = 92.42(14)° for ii: x, −1 + y, z]. Centrosymmetrically related helical chains are connected into a double layer by methylene–C—H⃛π(oxadiazole) interactions [C14–H14b⃛Cg(oxadiazole)iii = 2.94° with angle at H10 = 126° for iii: 1 − x, 1 − y, 1 − z]. The layers stack along the c-axis without directional interactions between them.
Given all of the specified interactions identified from a geometric-based analysis of the molecular packing are relatively weak. It was thought worthwhile to conduct a complimentary analysis of the calculated Hirshfeld surfaces, encompassing the full and decomposed two-dimensional fingerprint plots, employing Crystal Explorer 17.5 [15] and following established procedures [16]. Despite their being only one H⃛O contact less than the sum of the van der Waals radii, O⃛H/H⃛O contacts make up 30.3% of all surface contacts. This contribution is only exceeded by H⃛H contacts at 33.4%. The next two most important percentage contributions come from N⃛H/H⃛N [11.5%] and C⃛H/H⃛C [10.1%] contacts. The next two significant surface contacts are O⃛C/C⃛O [5.0%] and O⃛O [4.2%]. The remaining contacts are due to N⃛C/C⃛N [2.5%], O⃛N/N⃛O [2.2%] and N⃛N [0.9%].
Funding source: Princess Nourah bint Abdulrahman University
Award Identifier / Grant number: PNURSP2022R3
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Princess Nourah bint Abdulrahman University Researchers Supporting Project No. PNURSP2022R3, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CRYSALISPRO; Rigaku Corporation: Oxford, UK, 2019.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. Wanka, L., Iqbal, K., Schreiner, P. R. The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604; https://doi.org/10.1021/cr100264t.Search in Google Scholar PubMed PubMed Central
6. Liu, J., Obando, D., Liao, V., Lifa, T., Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963; https://doi.org/10.1016/j.ejmech.2011.01.047.Search in Google Scholar PubMed
7. Ruan, B. F., Guo, Q. L., Li, Q. S., Li, L. Z., Deora, G. S., Zhou, B. G. Review of the biological activities of heterocyclic compounds comprising oxadiazole moieties. Curr. Top. Med. Chem. 2022, 22, 578–599; https://doi.org/10.2174/1568026622666220202123651.Search in Google Scholar PubMed
8. Vaidya, A., Pathak, D., Shah, K. 1,3,4-Oxadiazole and its derivatives: a review on recent progress in anticancer activities. Chem. Biol. Drug Des. 2021, 97, 572–591; https://doi.org/10.1111/cbdd.13795.Search in Google Scholar PubMed
9. Al-Wahaibi, L. H., Alsfouk, A., El-Emam, A. A., Blacque, O. Crystal structures and Hirshfeld surface analysis of 2-(adamantan-1-yl)-5-(4-fluorophenyl)-1,3,4-oxadiazole and 2-(adamantan-1-yl)-5-(4-chlorophenyl)-1,3,4-oxadiazole. Acta Crystallogr. 2019, E75, 611–615; https://doi.org/10.1107/s2056989019004651.Search in Google Scholar
10. Alzoman, N. Z., El-Emam, A. A., Ghabbour, H. A., Chidan Kumar, C. S., Fun, H.-K. Crystal structure of 2-(adamantan-1-yl)-5-(4-bromophenyl)-1,3,4-oxadiazole. Acta Crystallogr. 2014, E70, o1231–o1232; https://doi.org/10.1107/s1600536814023861.Search in Google Scholar
11. El-Emam, A. A., Kadi, A. A., El-Brollosy, N. R., Ng, S. W., Tiekink, E. R. T. 2-(Adamantan-1-yl)-5-(4-nitrophenyl)-1,3,4-oxadiazole. Acta Crystallogr. 2012, E68, o795; https://doi.org/10.1107/s1600536812005302.Search in Google Scholar PubMed PubMed Central
12. Khan, M., Akhtar, T., Al-Masoudi, N. A., Stoeckli-Evans, H., Hameed, S. Synthesis, crystal structure and anti-HIV activity of 2-adamantyl/adamantylmethyl-5-aryl-1,3,4-oxadiazoles. Med. Chem. 2012, 8, 1190–1197; https://doi.org/10.2174/157340612804075232.Search in Google Scholar
13. Wang, Z., Zhang, H., Killian, B. J., Jabeen, F., Pillai, G. G., Berman, H. M., Mathelier, M., Sibble, A. J., Yeung, J., Zhou, W., Steel, P. J., Hall, C. D., Katritzky, A. R. Synthesis, characterization and energetic properties of 1,3,4-oxadiazoles. Eur. J. Org. Chem. 2015, 2015, 5183–5188; https://doi.org/10.1002/ejoc.201500583.Search in Google Scholar
14. Kerimov, I., Ayhan-Kılcıgil, G., Özdamar, E. D., Can-Eke, B., Çoban, T., Özbey, S., Kazak, C. Design and one-pot and microwave-assisted synthesis of 2-amino/5-aryl-1, 3, 4-oxadiazoles bearing a benzimidazole moiety as antioxidants. Arch. Pharm. Chem. Life Sci. 2012, 345, 549–556; https://doi.org/10.1002/ardp.201100440.Search in Google Scholar PubMed
15. Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D., Spackman, M. A. CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011; https://doi.org/10.1107/s1600576721002910.Search in Google Scholar PubMed PubMed Central
16. Tan, S. L., Jotani, M. M., Tiekink, E. R. T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. 2019, E75, 308–318; https://doi.org/10.1107/s2056989019001129.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

