Abstract
C30H33N6O2Cl monoclinic, P21 (no. 4), a = 13.7983(8) Å, b = 7.3771(3) Å, c = 14.3578(7) Å, β = 101.730(5)°, V = 1430.97(12) Å3, Z = 2, R gt (F) = 0.0525, wR ref (F 2) = 0.1285, T = 170 K.
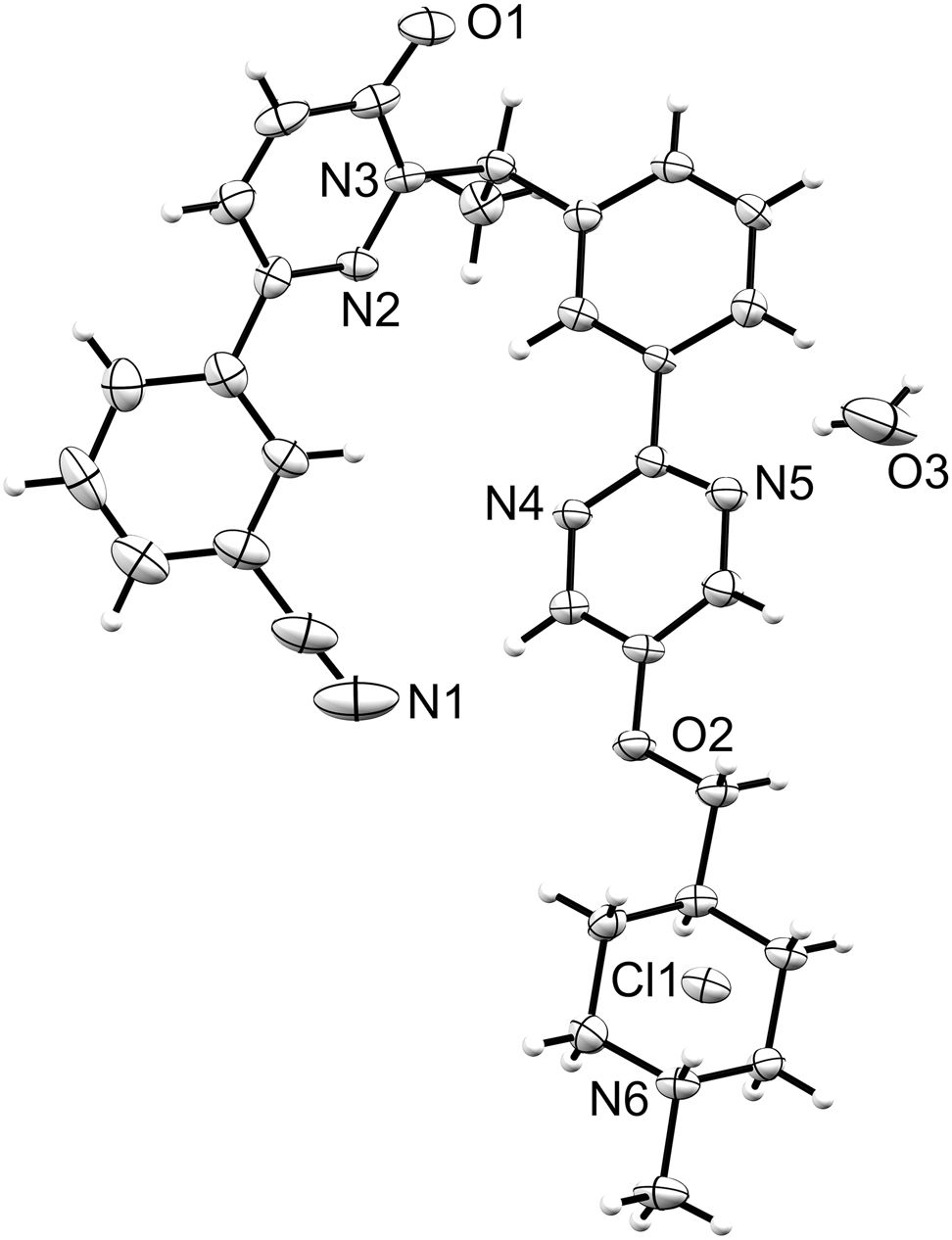
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless needle |
| Size: | 0.15 × 0.11 × 0.09 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.53 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θ max, completeness: | 65.0°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 4900, 3637, 0.050 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3225 |
| N(param)refined: | 367 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Cl01 | 0.91107 (9) | −0.02945 (17) | 0.10094 (8) | 0.0332 (3) |
| O002 | 0.7509 (2) | 0.5505 (5) | 0.3237 (2) | 0.0299 (8) |
| O003 | 0.5322 (3) | 0.5637 (6) | 0.9674 (3) | 0.0442 (10) |
| N004 | 0.6661 (3) | 0.6883 (6) | 0.5332 (3) | 0.0259 (9) |
| N005 | 0.4388 (3) | 0.7122 (5) | 0.7319 (3) | 0.0256 (9) |
| N006 | 0.8370 (3) | 0.6137 (6) | 0.5797 (3) | 0.0291 (10) |
| N007 | 0.8296 (3) | 0.3283 (6) | 0.0080 (3) | 0.0272 (9) |
| H007 | 0.860862 | 0.215316 | 0.034714 | 0.033* |
| N008 | 0.4900 (3) | 0.7014 (5) | 0.8226 (3) | 0.0240 (9) |
| C009 | 0.7486 (4) | 0.8309 (7) | 0.8836 (3) | 0.0277 (11) |
| H009 | 0.748720 | 0.865240 | 0.945896 | 0.033* |
| C00A | 0.5637 (3) | 0.8460 (7) | 0.8516 (3) | 0.0257 (10) |
| H00A | 0.578913 | 0.849418 | 0.921327 | 0.031* |
| C00B | 0.6590 (3) | 0.8074 (7) | 0.8194 (3) | 0.0248 (10) |
| C00C | 0.7501 (3) | 0.7285 (7) | 0.6969 (3) | 0.0226 (10) |
| C00D | 0.7521 (4) | 0.6720 (7) | 0.5983 (3) | 0.0264 (10) |
| C00E | 0.6613 (4) | 0.7589 (7) | 0.7256 (3) | 0.0274 (11) |
| H00E | 0.602093 | 0.746814 | 0.681648 | 0.033* |
| C00F | 0.8368 (4) | 0.8035 (7) | 0.8551 (3) | 0.0308 (12) |
| H00F | 0.895897 | 0.819445 | 0.898833 | 0.037* |
| C00G | 0.6694 (4) | 0.6435 (7) | 0.4444 (3) | 0.0280 (11) |
| H00G | 0.611648 | 0.651261 | 0.398139 | 0.034* |
| C00H | 0.8430 (3) | 0.5124 (7) | 0.2954 (3) | 0.0289 (12) |
| H00B | 0.869101 | 0.396408 | 0.320795 | 0.035* |
| H00C | 0.891376 | 0.605629 | 0.318926 | 0.035* |
| C00I | 0.7551 (4) | 0.5856 (7) | 0.4176 (3) | 0.0249 (10) |
| C00J | 0.3689 (4) | 0.5913 (7) | 0.7021 (4) | 0.0279 (11) |
| C00K | 0.8216 (3) | 0.5083 (7) | 0.1873 (3) | 0.0258 (11) |
| H00K | 0.791678 | 0.624088 | 0.163453 | 0.031* |
| C00L | 0.9183 (3) | 0.4852 (7) | 0.1532 (3) | 0.0259 (10) |
| H00D | 0.950111 | 0.373138 | 0.178150 | 0.031* |
| H00H | 0.962575 | 0.584758 | 0.176369 | 0.031* |
| C00M | 0.3174 (4) | 0.6102 (7) | 0.6014 (4) | 0.0332 (12) |
| C00N | 0.8396 (4) | 0.5694 (7) | 0.4893 (3) | 0.0303 (12) |
| H00N | 0.898253 | 0.527613 | 0.474484 | 0.036* |
| C00O | 0.8979 (3) | 0.4814 (7) | 0.0447 (3) | 0.0265 (10) |
| H00I | 0.868233 | 0.595216 | 0.019895 | 0.032* |
| H00J | 0.959692 | 0.466833 | 0.023215 | 0.032* |
| C00P | 0.4783 (4) | 0.5657 (7) | 0.8874 (3) | 0.0328 (12) |
| C00Q | 0.7512 (4) | 0.3563 (8) | 0.1476 (3) | 0.0311 (12) |
| H00L | 0.688455 | 0.374880 | 0.166858 | 0.037* |
| H00M | 0.778335 | 0.241761 | 0.174151 | 0.037* |
| C00R | 0.3692 (4) | 0.6787 (7) | 0.5348 (4) | 0.0336 (12) |
| H00R | 0.435854 | 0.708270 | 0.553209 | 0.040* |
| C00S | 0.8396 (4) | 0.7527 (7) | 0.7625 (4) | 0.0307 (11) |
| H00S | 0.899832 | 0.734831 | 0.744324 | 0.037* |
| C00T | 0.5211 (4) | 1.0288 (7) | 0.8172 (4) | 0.0325 (12) |
| H00O | 0.510106 | 1.033108 | 0.748993 | 0.049* |
| H00P | 0.566696 | 1.122623 | 0.843541 | 0.049* |
| H00Q | 0.459441 | 1.046593 | 0.837078 | 0.049* |
| O00U | 1.0231 (4) | 0.4242 (9) | 0.6737 (3) | 0.086 (2) |
| H00V | 0.961615 | 0.448626 | 0.660622 | 0.129* |
| H00W | 1.047185 | 0.498214 | 0.717746 | 0.129* |
| C00V | 0.3203 (5) | 0.7022 (8) | 0.4409 (4) | 0.0418 (14) |
| C00W | 0.7335 (4) | 0.3469 (8) | 0.0390 (4) | 0.0347 (12) |
| H00T | 0.691427 | 0.243970 | 0.016494 | 0.042* |
| H00U | 0.699960 | 0.456021 | 0.011880 | 0.042* |
| N00X | 0.4191 (6) | 0.8427 (8) | 0.3222 (4) | 0.0723 (19) |
| C00Y | 0.3472 (4) | 0.4531 (8) | 0.7635 (4) | 0.0368 (12) |
| H00Y | 0.296008 | 0.371762 | 0.742116 | 0.044* |
| C00Z | 0.4006 (4) | 0.4399 (7) | 0.8528 (4) | 0.0392 (13) |
| H00Z | 0.386721 | 0.347873 | 0.892421 | 0.047* |
| C010 | 0.8139 (4) | 0.3160 (8) | −0.0990 (3) | 0.0403 (14) |
| H01A | 0.777212 | 0.419771 | −0.127039 | 0.060* |
| H01B | 0.777690 | 0.207649 | −0.120411 | 0.060* |
| H01C | 0.876946 | 0.312914 | −0.117562 | 0.060* |
| C011 | 0.3745 (5) | 0.7802 (9) | 0.3738 (4) | 0.0511 (17) |
| C012 | 0.2180 (4) | 0.5650 (8) | 0.5724 (4) | 0.0423 (14) |
| H012 | 0.182465 | 0.520608 | 0.615972 | 0.051* |
| C013 | 0.2218 (5) | 0.6568 (9) | 0.4113 (4) | 0.0501 (16) |
| H013 | 0.189744 | 0.673327 | 0.348364 | 0.060* |
| C014 | 0.1722 (5) | 0.5871 (9) | 0.4767 (5) | 0.0530 (18) |
| H014 | 0.106258 | 0.553391 | 0.456972 | 0.064* |
Source of material
The compound was synthesized according to the procedure in our previous work [4]. A mixture of 3-(6-oxo-1,6-dihydropyridazin-3-yl)benzonitrile (197.2 mg, 1 mmol), (S)-1-(3-(5-((1-methylpiperidin-4-yl)methoxy)pyrimidin-2-yl)phenyl) ethan-1-ol (327.4 mg, 1 mmol), and PPh3 (393.4 mg, 1.5 mmol) in anhydrous tetrahydrofuran (THF) (8.0 mL) were added diisopropyl azodicarboxylate (DIAD) (303.3 mg, 1.5 mmol) slowly at 0 °C. The reaction mixture was allowed to warm to room temperature. After 1 h, the reaction was quenched with ice water (20.0 mL) and the mixture was extracted with CH2Cl2 (20.0 × 3). The organic layers were separated, dried and concentrated under reduced pressure. The crude products were purified by flash column chromatography using CH2Cl2/CH3OH (20/1, v/v) as eluents to give (R)-3-(1-(1-(3-(5-((1-methylpiperidin-4-yl)methoxy)pyrimidin-2-yl) phenyl)ethyl)-6-oxo-1,6-dihydropyridazin-3-yl)benzonitrile. The hydrochloride salt of the title compound (506.6 mg, 1 mmol) was obtained by adding hydrogen chloride solution in ethyl acetate (1.0 M, 1 mL) to ethanol. After drying at the rotavapor, the solid product was recrystallized from a mixture of CH2Cl2 and ethyl acetate (v/v, 1/3, 20 mL). Pale yellow block crystals were obtained after three days.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
Chirality is the basic feature of nature, and the main substances that makeup life, such as amino acids, carbohydrates, nucleic acids, proteins, enzymes, and receptors are chiral [5]. Enantiomers do not differ in their physical and chemical properties but could differ in their pharmacological activity, metabolic process, metabolic rate, and toxicity [6, 7]. Pyridazine and its derivatives have attracted wide increasing attention due to its biological activities, such as anticancer and antibacterial [8, 9]. As a part of our current research interest in the exploration of the chiral compounds with anticancer activities [10], we herein report the molecular structure of a new chiral pyridazine derivative.
The asymmetric unit of the title compound contains the hydrochloride of (R)-3-(1-(1-(3-(5-((1-methylpiperidin-4-yl)methoxy)pyrimidin-2-yl) phenyl)ethyl)-6-oxo-1,6-dihydropyridazin-3-yl)benzonitrile and an additional water molecule. The single-crystal structure of this salt monhydrate with (R)-configuration is shown in the figure. A classical hydrogen bond of the N–H⃛Cl type is observed in the structure. All bond lengths and angles of this molecule are in the expected ranges.
Funding source: Foundation of Guangdong Basic and Applied Basic Research
Award Identifier / Grant number: 2019A1515110266
Funding source: Foundation for Young Talents
Award Identifier / Grant number: 2019KQNCX159
Funding source: Jiangmen Program for Young Talents
Award Identifier / Grant number: 2019td04
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financially supported by the Foundation of Guangdong Basic and Applied Basic Research (2019A1515110266), the Foundation for Young Talents (2019KQNCX159), and Jiangmen Program for Young Talents (2019td04). We also thank Guangzhou Synmepilin Pharmaceuticals Co. Ltd. for the support of the fund.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2009.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
4. Li, X., Chen, X., Yang, W., Guo, J. Preparation method of pyrimidine derivative and application thereof. Patent WO 2020/052539, 2020 Al.Suche in Google Scholar
5. MacKenzie, L. E., Stachelek, P. The twists and turns of chiral chemistry. Nat. Chem. 2021, 13, 521–522; https://doi.org/10.1038/s41557-021-00729-8.Suche in Google Scholar PubMed
6. Kasprzyk-Hordern, B. Pharmacologically active compounds in the environment and their chirality. Chem. Soc. Rev. 2010, 39, 4466–4503; https://doi.org/10.1039/c000408c.Suche in Google Scholar PubMed
7. Diao, X., Han, Y., Liu, C. The fungicidal activity of tebuconazole enantiomers against Fusarium graminearum and its selective effect on DON production under different conditions. J. Agric. Food Chem. 2018, 66, 3637–3643; https://doi.org/10.1021/acs.jafc.7b05483.Suche in Google Scholar PubMed
8. He, Z.-X., Gong, Y.-P., Zhang, X., Ma, L.-Y., Zhao, W. Pyridazine as a privileged structure: an updated review on anticancer activity of pyridazine containing bioactive molecules. Eur. J. Med. Chem. 2021, 209, 112946–112976; https://doi.org/10.1016/j.ejmech.2020.112946.Suche in Google Scholar PubMed
9. Garrido, A., Vera, G., Delaye, P.-O., Enguehard-Gueiffier, C. Imidazo[1,2-b]pyridazine as privileged scaffold in medicinal chemistry: an extensive review. Eur. J. Med. Chem. 2021, 226, 113867–113899; https://doi.org/10.1016/j.ejmech.2021.113867.Suche in Google Scholar PubMed
10. Zhang, N., An, B., Zhou, Y., Li, X., Yan, M. Synthesis, evaluation, and mechanism study of new tepotinib derivatives as antiproliferative agents. Molecules 2019, 24, 1173–1189; https://doi.org/10.3390/molecules24061173.Suche in Google Scholar PubMed PubMed Central
© 2022 Han Yao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

