Abstract
C22H26Cl2N2O2Zn, monoclinic, P21/n (no. 14), a = 10.488(7) Å, b = 10.139(7) Å, c = 22.760(15) Å, β = 99.705°, V = 2386(3) Å3, Z = 4, R gt (F) = 0.0395, wR ref (F2) = 0.1053, T = 296(2) K.
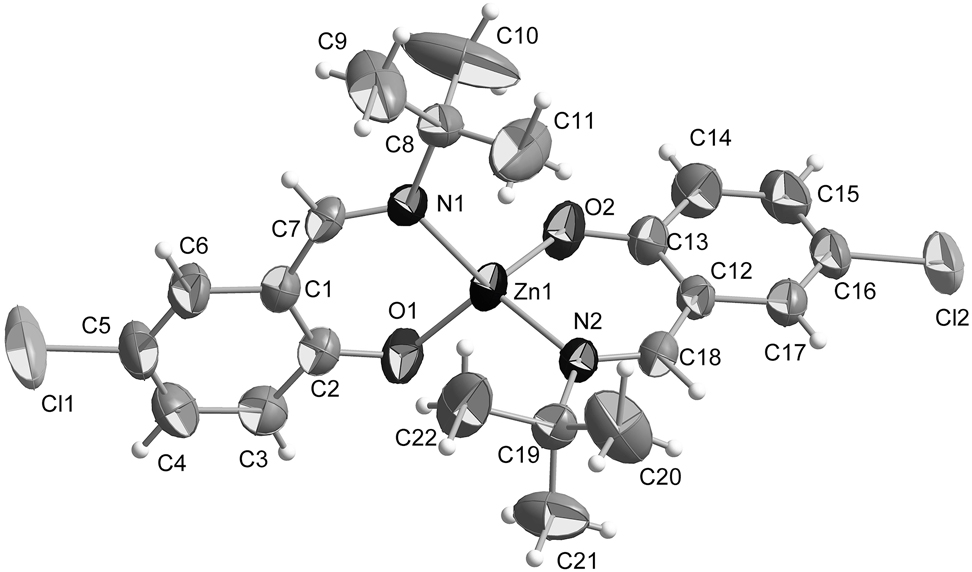
The molecular structure is shown in Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block |
| Size: | 039×0.37×0.32 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.27 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 21,975, 4208, 0.022 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3749 |
| N(param)refined: | 269 |
| Programs: | Bruker [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.5995 (3) | 0.2509 (3) | 0.83077 (13) | 0.0468 (7) |
| C2 | 0.5307 (3) | 0.3638 (3) | 0.84439 (13) | 0.0479 (7) |
| C3 | 0.5220 (3) | 0.3852 (4) | 0.90477 (15) | 0.0602 (8) |
| H3 | 0.477820 | 0.459169 | 0.914832 | 0.072* |
| C4 | 0.5759 (4) | 0.3019 (4) | 0.94885 (16) | 0.0676 (10) |
| H4 | 0.569398 | 0.319941 | 0.988300 | 0.081* |
| C5 | 0.6403 (4) | 0.1904 (4) | 0.93484 (16) | 0.0724 (10) |
| C6 | 0.6530 (4) | 0.1659 (4) | 0.87754 (16) | 0.0656 (9) |
| H6 | 0.697992 | 0.091333 | 0.868879 | 0.079* |
| C7 | 0.6265 (3) | 0.2160 (3) | 0.77272 (14) | 0.0487 (7) |
| H7 | 0.681693 | 0.144516 | 0.771691 | 0.058* |
| C8 | 0.6306 (3) | 0.2224 (3) | 0.66711 (14) | 0.0540 (8) |
| C9 | 0.6852 (7) | 0.0843 (5) | 0.6723 (2) | 0.120 (2) |
| H9A | 0.757108 | 0.080710 | 0.704495 | 0.180* |
| H9B | 0.713665 | 0.060668 | 0.635735 | 0.180* |
| H9C | 0.619537 | 0.023707 | 0.679854 | 0.180* |
| C10 | 0.7323 (7) | 0.3157 (7) | 0.6552 (3) | 0.173 (4) |
| H10A | 0.699773 | 0.404319 | 0.654814 | 0.259* |
| H10B | 0.756006 | 0.295834 | 0.617272 | 0.259* |
| H10C | 0.806776 | 0.307431 | 0.685913 | 0.259* |
| C11 | 0.5185 (6) | 0.2248 (8) | 0.6173 (2) | 0.145 (3) |
| H11A | 0.451245 | 0.168738 | 0.626949 | 0.217* |
| H11B | 0.545499 | 0.193922 | 0.581494 | 0.217* |
| H11C | 0.486592 | 0.313433 | 0.611474 | 0.217* |
| C12 | 0.3291 (3) | 0.6265 (3) | 0.61896 (12) | 0.0434 (6) |
| C13 | 0.4603 (3) | 0.6507 (3) | 0.64277 (14) | 0.0491 (7) |
| C14 | 0.5204 (4) | 0.7571 (3) | 0.61840 (18) | 0.0690 (10) |
| H14 | 0.606939 | 0.774886 | 0.633021 | 0.083* |
| C15 | 0.4561 (4) | 0.8352 (4) | 0.57401 (18) | 0.0712 (10) |
| H15 | 0.498993 | 0.904379 | 0.558884 | 0.085* |
| C16 | 0.3279 (4) | 0.8111 (3) | 0.55180 (15) | 0.0609 (9) |
| C17 | 0.2658 (3) | 0.7094 (3) | 0.57353 (14) | 0.0545 (8) |
| H17 | 0.179186 | 0.694087 | 0.558065 | 0.065* |
| C18 | 0.2506 (3) | 0.5234 (3) | 0.63836 (13) | 0.0441 (6) |
| H18 | 0.163975 | 0.523567 | 0.620653 | 0.053* |
| C19 | 0.1828 (3) | 0.3364 (3) | 0.69065 (15) | 0.0517 (7) |
| C20 | 0.0961 (5) | 0.2880 (5) | 0.6346 (2) | 0.1034 (17) |
| H20A | 0.043649 | 0.359561 | 0.616584 | 0.155* |
| H20B | 0.041286 | 0.218665 | 0.644755 | 0.155* |
| H20C | 0.148417 | 0.255011 | 0.607097 | 0.155* |
| C21 | 0.1029 (5) | 0.4042 (5) | 0.7313 (2) | 0.0962 (15) |
| H21A | 0.158349 | 0.432471 | 0.766995 | 0.144* |
| H21B | 0.039513 | 0.343726 | 0.741398 | 0.144* |
| H21C | 0.060000 | 0.479384 | 0.711273 | 0.144* |
| C22 | 0.2519 (4) | 0.2214 (5) | 0.7239 (3) | 0.1036 (18) |
| H22A | 0.304695 | 0.178342 | 0.699035 | 0.155* |
| H22B | 0.189494 | 0.160066 | 0.734129 | 0.155* |
| H22C | 0.305548 | 0.252541 | 0.759530 | 0.155* |
| Cl1 | 0.7118 (2) | 0.08261 (17) | 0.99087 (6) | 0.1442 (7) |
| Cl2 | 0.24555 (13) | 0.91344 (12) | 0.49620 (5) | 0.0953 (4) |
| N1 | 0.5846 (2) | 0.2705 (2) | 0.72221 (10) | 0.0431 (5) |
| N2 | 0.2841 (2) | 0.4317 (2) | 0.67665 (11) | 0.0420 (5) |
| O1 | 0.4771 (2) | 0.4484 (2) | 0.80495 (10) | 0.0632 (6) |
| O2 | 0.5291 (2) | 0.5823 (2) | 0.68545 (11) | 0.0637 (6) |
| Zn1 | 0.46723 (3) | 0.42824 (3) | 0.72054 (2) | 0.04662 (14) |
Source of material
(E)-2-((tert-Butylimino)methyl)-4-chlorophenol (1.16 g, 5.5 mmol), zinc acetate monohydrate (0.50 g, 2.5 mmol) and ethanol (80 mL) were heated and stirred at 373 K for 6 h, then cooled to room temperature, resulting in a yellow solution. Crystals of the title compound were obtained by slow evaporation within three days.
Experimental details
All hydrogen atoms were identified in difference Fourier syntheses. The Uiso values of the hydrogen atoms of methyl groups were set to 1.5Ueq(C) and the Uiso values of all other hydrogen atoms were set to 1.2Ueq(C).
Comment
Salicylaldehyde Schiff base is a kind of ligand, which can form complexes with many metal ions [4], [5], [6], [7]. Salicylaldehyde Schiff base metal complexes are widely used in catalysis and so on [8], [9], [10].
The crystal structure shows that the zinc complex has a distorted tetrahedral structure and the Zn is four-coordinated by two phenol oxygen atoms and imine nitrogen atoms of two Schiff bases. The Zn–O distance (O1–Zn1 = 1.917(3) Å; O2–Zn1 = 1.917(2) Å) and the Zn–N length (N1–Zn1 = 2.014(3) Å; N2–Zn1 = 2.010(3) Å) are similar to those seen in related complexes [11, 12]. The C=N bond distance is 1.285(4) Å (C7=N1) and 1.282(4) Å (C18=N2), respectively. The O–Zn–N bond angles are 97.81(9)° (O1–Zn1–N1) and 98.11(10)° (O2–Zn1–N2). Moreover, the coordination of the two NO bidentate chelate ligands to the Zn ion results in the formation of two six-membered rings.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Natural Science Foundation of Hunan Province of China (2021JJ30291), the Scientific Research Fund of Hunan Provincial Education Department (21A0518, 21C0691), Undergraduate Training Program for Innovation and Entrepreneurship of Hunan Province of China ([2021]197-3585), Undergraduate Training Program for Innovation and Entrepreneurship of Hunan University of Science and Enginerring (2021), Yongzhou Guiding Science and Technology Plan (2021), the construct program of applied characteristic discipline in Hunan University of Science and Engineering.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX3, SAINT-Plus, XPREP; Bruker AXS Inc.: Madison, Wisconsin, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Khorshidifard, M., Rudbari, H. A., Askari, B., Sahihi, M., Farsani, M. R., Jalilian, F., Bruno, G. Cobalt(II), copper(II), zinc(II) and palladium(II) Schiff base complexes: synthesis, characterization and catalytic performance in selective oxidation of sulfides using hydrogen peroxide under solvent-free conditions. Polyhedron 2015, 95, 1–13; https://doi.org/10.1016/j.poly.2015.03.041.Search in Google Scholar
5. Behzad, M., Ghomi, L. S., Damercheli, M., Mehravi, B., Ardestani, M. S., Jahromi, H. S., Abbasi, Z. Crystal structures and in vitro anticancer studies on new unsymmetrical copper(II) Schiff base complexes derived from meso-1,2-diphenyl-1,2-ethylenediamine: a comparison with related symmetrical ones. J. Coord. Chem. 2016, 69, 2469–2481; https://doi.org/10.1080/00958972.2016.1198786.Search in Google Scholar
6. Peng, G., Qian, Y.-F., Wang, Z.-W., Chen, Y., Yadav, T., Fink, K., Ren, X.-M. Tuning the coordination geometry and magnetic relaxation of Co(II) single-ion magnets by varying the ligand substitutions. Cryst. Growth Des. 2021, 21, 1035–1044; https://doi.org/10.1021/acs.cgd.0c01372.Search in Google Scholar
7. Dayan, S., Tercan, M., Özdemir, F. A., Aykutog̃lu, G., Özdemir, N., Şerbetçi, Z., Dinçer, M., Dayan, O. Catalytic and biological activities of homoleptic palladium(II) complexes bearing the 2-aminobenzothiazole moiety. Polyhedron 2021, 1996, 115106; https://doi.org/10.1016/j.poly.2021.115106.Search in Google Scholar
8. Cozzi, P. G. Metal-Salen Schiff base complexes in catalysis: practical aspects. Chem. Soc. Rev 2004, 33, 410–421; https://doi.org/10.1039/b307853c.Search in Google Scholar
9. Raman, N., Raja, S. J. DNA cleavage, structural elucidation and anti-microbial studies of three novel mixed ligand Schiff base complexes of copper(II). J. Serb. Chem. Soc. 2007, 72, 983–992; https://doi.org/10.2298/jsc0710983r.Search in Google Scholar
10. Yilmaz, I., Cukurovali, A. Synthesis, characterization and antimicrobial activity of the Schiff bases derived from 2,4-disubstituted thiazoles and 3-methoxysalicylaldehyde, and their cobalt(II), copper(II), nickel(II) and zinc(II) complexes. Trans. Met. Chem. 2003, 28, 399–404.10.1023/A:1023630209043Search in Google Scholar
11. Tsai, M.-J., Su, Y.-T., Wu, J.-Y. Anion effect on the formation of zinc-salicyaldimine compounds in neutral and anionic complex forms: synthesis, characterization, 1H NMR studies, and photophysical properties. Eur. J. Inorg. Chem. 2021, 21, 1035–1044.10.1002/ejic.202100377Search in Google Scholar
12. Khorshidifard, M., Rudbari, H. A., Askari, B., Sahihi, M., Farsani, M. R., Jalilian, F., Bruno, G. Cobalt(II), copper(II), zinc(II) and palladium(II) Schiff base complexes: synthesis, characterization and catalytic performance in selective oxidation of sulfides using hydrogen peroxide under solvent-free conditions. Polyhedron 2015, 95, 1–13; https://doi.org/10.1016/j.poly.2015.03.041.Search in Google Scholar
© 2022 Qian-Zhi Yang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of (E)-(4-imidazol-1-yl-phenyl)-(2-methoxy-benzylidene)-amine monohydrate, C17H17N3O2
- Crystal structure of 6-methyl-3-(pyrrolidine-1-carbonyl)-2H-chromen-2-one, C15H15N1O3
- Crystal structure of 4-methyl-4-nitropentanoic acid, C6H11NO4
- The crystal structure of (E)-3-(furan-2-yl)acrylonitrile, C7H5NO
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)-1H-pyrazole-4-carboxamide monohydrate, C23H26F2N4O3
- Crystal structure of 2-(4-bromobenzyloxy)-6-chloropyridine, C12H9BrClNO
- Crystal structure of N-(4-bromo-2,6-dichloro-phenyl)pyrazin-2-amine, C10H6BrCl2N3
- Crystal structure of (E)-1-(2–nitrophenyl)-3-phenylprop-2-en-1-one, C15H11NO3
- The crystal structure of (E)-3-chloro-2-(2-(4-fluorobenzylidene)hydrazinyl)pyridine, C12H9ClFN3
- Crystal structure of (E)-amino(2-(thiazol-2-ylmethylene)hydrazineyl)methaniminium nitrate, C10H16N12O6S2
- Crystal structure of 9-methoxy-2,3,4,4a,5,6-hexahydro-1H-pyrido [1′,2′:1,6]pyrazino[2,3-b]quinoxaline, C15H18N4O
- The crystal structure bis(dimethylsulfoxide-κ1O)-dipyridine-κ1 N-bis(m2-(Z)-3-methyl-2-oxido-N-((Z)-oxido(phenyl)methylene)benzohydrazonato-κ5)trinickel(II) - dimethylsulfoxide (1/2), C48H56N6Ni3O10S4
- Crystal structure of bis(bis(triphenylphosphine)iminium) tetradecacarbonyltetratelluridopentaferrate(2-), (PPN)2[Fe5Te4(CO)14]
- Crystal structure of 4-Hydroxy-3-(naphthalen-2-ylthio)pent-3-en-2-one, C15H14O2S
- The crystal structure of [(1,10-phenanthroline-κ2 N,N)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)nickel(II)] monohydrate, C36H26N4O5Ni
- Crystal structure of 3,3′-(pyridine-2,6-diylbis(methylene))bis(1-propyl-1H-imidazol-3-ium) ditetrafluoroborate, C19H27B2F8N5
- The crystal structure of (E)-1-(4-aminophenyl)-3-(p-tolyl)prop-2-en-1-one, C16H15NO
- The crystal structure of poly[(μ2-terephthalato-κ4O,O′: O″,O‴)-(μ4-terephthalato-κ4O:O′:O″:O‴)-{μ4-(1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ4O:O′:O″,O‴)}dicadmium(II)] – water – acetronitrile (1/2/2), C38H36N14O10Cd2
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)cobalt(II)–water–N,N-dimethylformamide(1/2/1), C27H31N3O9Co
- The co-crystal structure of 4-hydroxy-3-methoxybenzoic acid – 4,4′-bipyridine, C8H8O4·C10H8N2
- Crystal structure of catena-poly[(μ2-1,1′-(biphenyl-4,4′-diyl)bis(1H-imidazol)-κ2N:N′)-bis(4-bromobenzoate-κ1O)cobalt(II)], C32H22Br2CoN4O4
- Crystal structure of (E)-5-propyl-4-((pyridin-2-ylmethylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione – methanol (1/1), C11H13N5S
- The crystal structure of (Z)-4-bromo-6-(((1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)amino)methylene)cyclohexa-2,4-dien-1-one monohydrate, C11H16BrNO5
- Crystal structure of bis(tetrapropylammonium) nonaselenidotetrastannate(IV), (Pr4N)2[Sn4Se9]
- Crystal structure of 2,6-di-tert-butyl-4-(4-chlorobenzylidene)cyclohexa-2,5-dien-1-one, C21H25ClO
- Crystal structure of (2,2′-((naphthalen-1-ylmethyl)azanediyl)diacetato-κ3 N,O,O′)-(1,10-phenanthroline-κ2 N,N′)-copper(II) trihydrate, CuC27H27N3O7
- The crystal structure of tetrakis(6-phenylpyridine-2-carboxylato-κ2N,O)-bis(1H-pyrazol-3-ylamine-κ2 N:N)dicobalt(II) dihydrate, C27H23N5O5Co
- The crystal structure of bis((E)-2-((tert-butylimino)methyl)-4-chlorophenolato-κ2N,O)zinc(II), C22H26Cl2N2O2Zn
- The crystal structure of poly[diaqua-(μ3-5-nitrobenzene-1,2,3-tricarboxylato-κ3O:O′:O′)-(μ2-4,4′-dipyridylamine-κ2N:N′)copper(II)], C38H30Cu3N8O20
- The crystal structure of (E)-1-ferrocenyl-3-(naphthalen-1-yl)prop-2-en-1-one, C23H18FeO
- The crystal structure of (E)-1-ferrocenyl-3-(4-isopropylphenyl)prop-2-en-1-one, C22H22FeO
- Crystal structure of 6-hydroxy-2,2-dimethyl-4Hbenzo[d][1,3]dioxin-4-one, C10H10O4
- The crystal structure of (2E,4E)-1-ferrocenyl-5-phenylpenta-2,4-dien-1-one, C21H18FeO
- Crystal structure of alaninato-κ2N,O-bis(hydroxylamido-κ2N,O)-oxido-vanadium(V), C3H10N3O5V
- Crystal structure of catena-poly[aqua-bis[μ2-6-(1H-imidazol-1-yl)nicotinato-κ2 N,O]copper(II)], C18H14N6O5Cu
- Crystal structure of diethyl 4,6-diphenyl-1,9-di-p-tolylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,5(2H)-dicarboxylate, C42H42N2O4
- The crystal structure of cobalt cadmium bis(hydrogenphosphate) bis(phosphate(V)) tetrahydrate, H10O20P4Co3.14Cd1.86
- Crystal structure of dimethyl 1,4,6,9-tetraphenylhexahydro-3H-2,7,3,5-(epimethanetriyliminomethanetriyl)cyclopenta[b]pyridine-3,7(2H)-dicarboxylate, C38H34N2O4
- Crystal structure of (Z)-4-(furan-2-yl((4-iodophenyl)amino)methylene)-5-methyl-2(p-tolyl)-2,4-dihydro-3H-pyrazol-3-one, C21H16I N3O2
- Crystal structure of (E)-1-(4-(3,5-dimethoxystyryl)phenyl)-7-ethylheptanedioate, C25H30O6
- Crystal structure of 6-bromo-2-(4-chlorophenyl)chroman-4-one (6-bromo-4′-chloroflavanone), C15H10BrClO2
- The crystal structure of 2-(benzhydryloxy)-3-nitropyridine, C18H14N2O3
- The crystal structure of 1,3(4,1)-dipyridin-1-iuma-2(1,8)-diethynylanthracena-5(1,3)-benzenacyclohexaphane-11,31-diium bis(hexafluoridophosphate), C36H24F12N2P2
- Crystal structure of 3,6-di-tert-butyl-1-iodo-9-methyl-8-(pyren-1-ylethynyl)-9H-carbazole, C39H34IN
- The cocrystal 2-(dimethylammonio)-5-nitrobenzoate – 2-(dimethylamino)-5-nitrobenzoic acid, C9H10N2O4
- Crystal structure of 5-nitroquinazolin-4(3H)-one, C8H5N3O3

