Abstract
C15H12ClFN2O2, monoclinic, P21/c (no. 14), a = 13.9210(13) Å, b = 13.2329(15) Å, c = 18.7191(19) Å, β = 126.250(2)°, V = 2780.9(5) Å3, Z = 8, Rgt(F) = 0.0499, wRref(F2) = 0.1420, T = 293(2) K.
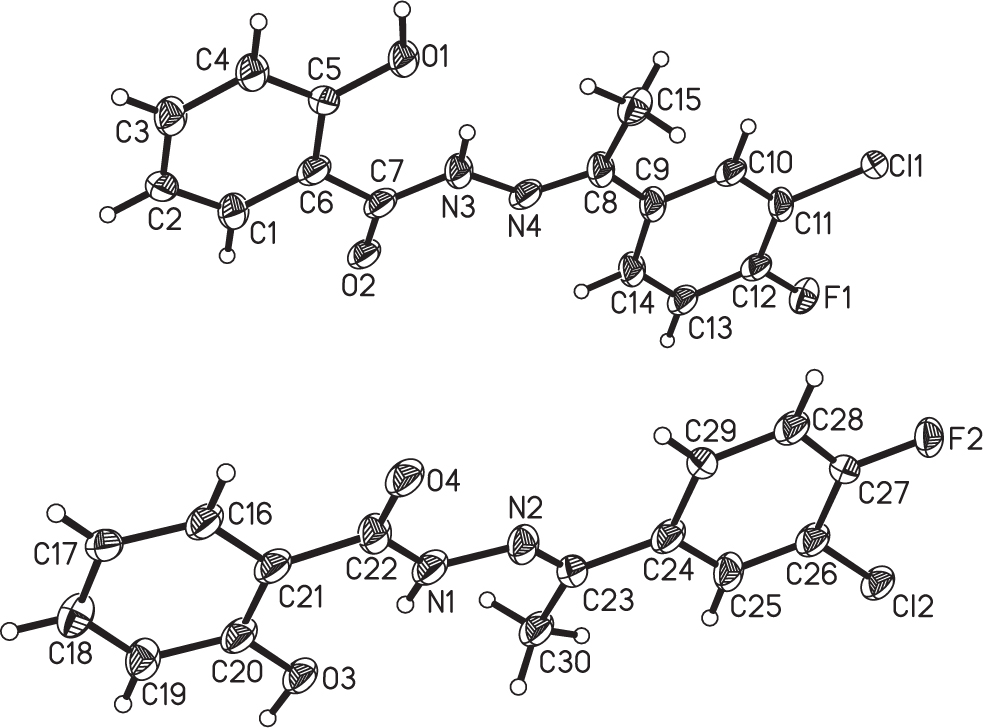
The asymmetric unit of the molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.15 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.29 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 27.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 19612, 6361, 0.084 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4081 |
| N(param)refined: | 381 |
| Programs: | Bruker [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 1.0604(2) | 0.1301(2) | 0.87188(19) | 0.0507(7) |
| H1 | 1.0593 | 0.1998 | 0.8651 | 0.061* |
| C2 | 1.0925(2) | 0.0916(2) | 0.95159(19) | 0.0513(7) |
| H2 | 1.1138 | 0.1346 | 0.9982 | 0.062* |
| C3 | 1.0924(2) | −0.0121(2) | 0.96110(19) | 0.0514(7) |
| H3 | 1.1128 | −0.0391 | 1.0143 | 0.062* |
| C4 | 1.0626(2) | −0.0756(2) | 0.89280(18) | 0.0513(7) |
| H4 | 1.0640 | −0.1451 | 0.9004 | 0.062* |
| C5 | 1.0304(2) | −0.0370(2) | 0.81273(18) | 0.0497(7) |
| C6 | 1.0294(2) | 0.06784(19) | 0.80076(18) | 0.0504(7) |
| C7 | 0.9952(2) | 0.1184(2) | 0.71837(18) | 0.0502(7) |
| C8 | 0.8638(2) | 0.0364(2) | 0.49458(19) | 0.0525(8) |
| C9 | 0.8362(2) | 0.0955(2) | 0.41578(18) | 0.0505(7) |
| C10 | 0.8011(2) | 0.0419(2) | 0.33914(18) | 0.0510(7) |
| H10 | 0.8037 | −0.0283 | 0.3409 | 0.061* |
| C11 | 0.7634(2) | 0.08997(19) | 0.26193(18) | 0.0494(7) |
| C12 | 0.7603(2) | 0.1938(2) | 0.25817(19) | 0.0514(7) |
| C13 | 0.7950(2) | 0.2521(2) | 0.33139(18) | 0.0533(7) |
| H13 | 0.7933 | 0.3223 | 0.3287 | 0.064* |
| C14 | 0.8322(2) | 0.20163(19) | 0.40882(19) | 0.0538(8) |
| H14 | 0.8557 | 0.2397 | 0.4585 | 0.065* |
| C15 | 0.8372(2) | −0.07834(19) | 0.49145(18) | 0.0527(7) |
| H15A | 0.8685 | −0.1016 | 0.5500 | 0.079* |
| H15B | 0.8741 | −0.1150 | 0.4693 | 0.079* |
| H15C | 0.7527 | −0.0892 | 0.4531 | 0.079* |
| C16 | 0.5796(2) | 0.14467(19) | 0.87763(18) | 0.0494(7) |
| H16 | 0.5765 | 0.0748 | 0.8714 | 0.059* |
| C17 | 0.6282(2) | 0.18537(19) | 0.96035(19) | 0.0512(7) |
| H17 | 0.6581 | 0.1437 | 1.0093 | 0.061* |
| C18 | 0.6317(2) | 0.28978(19) | 0.96899(18) | 0.0487(7) |
| H18 | 0.6653 | 0.3184 | 1.0244 | 0.058* |
| C19 | 0.5861(2) | 0.3513(2) | 0.89660(18) | 0.0511(7) |
| H19 | 0.5871 | 0.4210 | 0.9032 | 0.061* |
| C20 | 0.5385(2) | 0.31049(19) | 0.81365(18) | 0.0483(7) |
| C21 | 0.5347(2) | 0.20525(19) | 0.80263(18) | 0.0489(7) |
| C22 | 0.4876(2) | 0.1529(2) | 0.71805(18) | 0.0496(7) |
| C23 | 0.3919(2) | 0.2306(2) | 0.50090(19) | 0.0532(8) |
| C24 | 0.3444(2) | 0.16985(19) | 0.41862(18) | 0.0500(7) |
| C25 | 0.2960(2) | 0.2210(2) | 0.33834(17) | 0.0510(7) |
| H25 | 0.2888 | 0.2909 | 0.3370 | 0.061* |
| C26 | 0.2592(2) | 0.17142(19) | 0.26227(18) | 0.0504(7) |
| C27 | 0.2680(2) | 0.06791(19) | 0.26216(18) | 0.0497(7) |
| C28 | 0.3124(2) | 0.0105(2) | 0.33861(18) | 0.0509(7) |
| H28 | 0.3163 | −0.0597 | 0.3382 | 0.061* |
| C29 | 0.3502(2) | 0.06377(19) | 0.41518(18) | 0.0510(7) |
| H29 | 0.3810 | 0.0274 | 0.4670 | 0.061* |
| C30 | 0.4129(2) | 0.34686(19) | 0.50572(18) | 0.0559(8) |
| H30A | 0.3633 | 0.3804 | 0.5183 | 0.084* |
| H30B | 0.3932 | 0.3703 | 0.4500 | 0.084* |
| H30C | 0.4950 | 0.3615 | 0.5518 | 0.084* |
| Cl1 | 0.71888(6) | 0.02090(5) | 0.16886(5) | 0.0551(2) |
| Cl2 | 0.20314(5) | 0.23827(5) | 0.16556(4) | 0.0528(2) |
| F1 | 0.72159(12) | 0.24023(11) | 0.18107(10) | 0.0539(4) |
| F2 | 0.23185(12) | 0.01976(11) | 0.18642(10) | 0.0520(4) |
| N1 | 0.45966(17) | 0.21313(16) | 0.64992(15) | 0.0488(6) |
| H1A | 0.4694 | 0.2772 | 0.6590 | 0.059* |
| N2 | 0.41626(17) | 0.17763(16) | 0.56630(15) | 0.0497(6) |
| N3 | 0.95095(17) | 0.05715(16) | 0.64765(15) | 0.0493(6) |
| H3A | 0.9464 | −0.0064 | 0.6547 | 0.059* |
| N4 | 0.91240(18) | 0.09048(16) | 0.56430(15) | 0.0519(6) |
| O1 | 1.00053(14) | −0.09903(13) | 0.74459(12) | 0.0511(5) |
| H1E | 1.0059 | −0.1680 | 0.7625 | 0.077* |
| O2 | 1.00823(14) | 0.21007(13) | 0.71545(12) | 0.0520(5) |
| O3 | 0.49501(14) | 0.37106(12) | 0.74203(12) | 0.0500(5) |
| H3E | 0.5034 | 0.4405 | 0.7596 | 0.075* |
| O4 | 0.47437(15) | 0.06042(13) | 0.71121(12) | 0.0533(5) |
Source of material
A mixture of 2-hydroxy-benzoic acid hydrazide (1 mmol, 152.2 mg) and 1-(3-chloro-4-fluoro-phenyl)-ethanone (1 mmol, 172.6 mg) in anhydrous ethanol (25 mL) was kept under reflux conditions (351 K) for 3 h. The solvent was removed under reduced pressure and the solid product was recrystallized from 15 mL of anhydrous ethanol. After 4 days, colourless block-shaped crystals were obtained.
Experimental details
Coordinates of hydrogen atoms were refined without any constraints or restraints. The Uiso values were set to be 1.5Ueq of the carrier atom for methyl H atoms and 1.2Ueq for the remaining H atoms.
Comment
In recent years, Schiff bases as one of the important synthetic intermediates have attracted more and more attention due to the wide applications in the fields of coordination chemistry, biochemistry, pharmacy, nanotechnology, and photochemistry [3], [4], [5], [6], [7], [8], [9], [10]. From the structural point of view, o-hydroxy Schiff bases are attractive because of their intramolecular H-bond, and thus have been extensively investigated and reported [11], [12], [13], [14], [15], [16], [17]. In addition, the heteroatoms (such as N, F, Cl, Br, S) can act as hydrogen bonding acceptors for bioactive molecules in order to exhibit higher biological activity [18], [19]. But, the unsymmetrical o-hydroxy Schiff bases containing heteroatoms especial of F and Cl in one molecule are less investigated. Herein, in order to search for new o-hydroxy Schiff bases containing F and Cl heteroatoms, we have undertaken the synthesis and single crystal structure determination of the title compound (cf. the figure).
In the title compound, there are two crystallographically independent molecules in the asymmetric unit, which have very similar conformations. Compared with those of closely related structure, such a structural feature is uncommon [15]. The bond lengths and angles are in the expected ranges which fit with those of related compounds in literature [11], [12], [13], [14], [15], [16], [17]. The C=N double bond lengths in the independent molecule are 1.277(3) Å (C8=N4) and 1.271(4) Å (C23=N2), respectively, exhibiting the double bond character. The aromatic rings form dihedral angles of 3.9° and 4.2° in the two independent molecules. In the crystal structure, the molecules are linked into double infinite chains by O—H⋯O hydrogen bonds. There also exist intramolecular N—H⋯O hydrogen bonds, which further consolidate the crystal packing.
Acknowledgements
This work was financially supported by East China University of Technology.
References
1. Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA (2008).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Zhang, L. S.; Chen, Q.; Hou, G. G.; Zhao, W.; Hou, Y.: Hydroxyl-substituted double Schiff-base condensed 4-piperidone/cyclohexanones as potential anticancer agents with biological evaluation. J. Enzyme Inhib. Med. Chem. 34 (2019) 264–271.10.1080/14756366.2018.1501042Suche in Google Scholar PubMed PubMed Central
4. Pu, X.: The crystal structure of (E)-4-chloro-2-(((5-methylpyridin-2-yl)imino)methyl)phenol, C13H11ClN2O. Z. Kristallogr. NCS 233 (2018) 253–254.10.1515/ncrs-2017-0254Suche in Google Scholar
5. Chang, F. F.; Zhang, L.; Zhao, P. C.; Huang, W.: Transmetalation for flexible pendant-armed Schiff-base macrocyclic complexes influenced by halide effects. Inorg. Chem. 57 (2018) 1438–1448.10.1021/acs.inorgchem.7b02835Suche in Google Scholar PubMed
6. Aboura, W.; Benabdallah, T.; Zhang, F.; Therrien, B.: Alkoxylation of the imine carbon atom of a Schiff-base ligand upon coordination to arene ruthenium. Inorg. Chim. Acta 483 (2018) 93–97.10.1016/j.ica.2018.07.025Suche in Google Scholar
7. Kadwa, E.; Bala, M. D.; Friedrich, H. B.: Base metal Schiff base complexes applied as catalysts for the oxidation of n-octane. Inorg. Chim. Acta 463 (2017) 112–117.10.1016/j.ica.2017.04.032Suche in Google Scholar
8. Shanty, A.; Philip, J.; Sneha, E.; Kurup, M.; Balachandran, S.; Mohanan, P.: Synthesis, characterization and biological studies of Schiff bases derived from heterocyclic moiety. Bioorg. Chem. 70 (2017) 67–73.10.1016/j.bioorg.2016.11.009Suche in Google Scholar PubMed
9. Abu-Dief, A. M.; Mohamed, I. M. A.: A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-suef Univ. J. Basic Appl. Sci. 4 (2015) 119–133.10.1016/j.bjbas.2015.05.004Suche in Google Scholar PubMed PubMed Central
10. Li, P.; Niu, M.-J.; Hong, M.; Cheng, S.; Dou, J.-M.: Effect of structure and composition of nickel(II) complexes with salicylidene Schiff base ligands on their DNA/protein interaction and cytotoxicity. J. Inorg. Biochem. 137 (2014) 101–108.10.1016/j.jinorgbio.2014.04.005Suche in Google Scholar PubMed
11. Fan, C. G.; Song, M. Z.: (E)-N′-[1-(4-Bromophenyl)ethylidene]-2-hydroxybenzohydrazide. Acta Crystallogr. E65 (2009) o2792.10.1107/S1600536809042081Suche in Google Scholar PubMed PubMed Central
12. Li, M. L.; Huang, X.; Feng, R. K.: (E)-N′-[1-(4-Chlorophenyl)ethylidene]-2-hydroxybenzohydrazide. Acta Crystallogr. E65 (2009) o369.10.1107/S1600536809002311Suche in Google Scholar PubMed PubMed Central
13. Yue, S. Y.; Lu, J. F.: N′-[1-(2-Hydroxyphenyl)ethylidene]-2-methoxybenzohydrazide. Acta Crystallogr. E66 (2010) o1550.10.1107/S1600536810020477Suche in Google Scholar PubMed PubMed Central
14. Qiu, X. Y.; Luo, Q. Y.; Yang, S. L.; Liu, W. S.: (E)-2-Hydroxy-N′-[1-(4-methoxyphenyl)-ethylidene]benzohydrazide. Acta Crystallogr. E62 (2006) o4291–o4292.10.1107/S1600536806029345Suche in Google Scholar
15. Patra, D.; Biswas, N.; Kumari, B.; Das, P.; Sepay, N.; Chatterjee, S.; Drew, M. G. B.; Ghosh, T.: A family of mixed-ligand oxidovanadium(V) complexes with aroylhydrazone ligands: a combined experimental and computational study on the electronic effects of para substituents of hydrazone ligands on the electronic properties, DNA binding and nuclease activities. RSC Adv. 5 (2015) 92456–92472.10.1039/C5RA17844DSuche in Google Scholar
16. Shan, S.; Tian, Y. L.; Wang, S. H.; Wang, W. L.; Xu, Y. L.: (E)-N′-[1-(4-Aminophenyl)ethylidene]benzohydrazide. Acta Crystallogr. E64 (2008) o1363.10.1107/S1600536808019004Suche in Google Scholar PubMed PubMed Central
17. Shi, X. F.; Xing, Z. F.: (E)-N′-[1-(4-Aminophenyl)ethylidene]-2-hydroxybenzohydrazide methanol solvate. Acta Crystallogr. E63 (2007) o4771.10.1107/S1600536807058552Suche in Google Scholar
18. Yao, B. R.; Li, N.; Wang, C. H.; Hou, G. G.; Meng, Q. G.; Yan, K.: Novel asymmetric 3,5-bis(arylidene)piperidin-4-one derivatives: synthesis, crystal structures and cytotoxicity. Acta Crystallogr. C74 (2018) 659–665.10.1107/S2053229618006605Suche in Google Scholar PubMed
19. Li, N.; Bai, X. Y.; Zhang, L. S.; Hou, Y.: Synthesis, crystal structures and anti-inflammatory activity of four 3,5-bis(arylidene)-N-benzenesulfonyl-4-piperidone derivatives. Acta Crystallogr. C74 (2018) 1171–1179.10.1107/S2053229618013232Suche in Google Scholar PubMed
© 2019 Xifeng Yan et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2
Artikel in diesem Heft
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2


