Abstract
C19H17FN4O, orthorhombic, Pbca (no. 61), a = 7.0607(2) Å, b = 18.4459(5) Å, c = 23.8955(7) Å, V = 3112.17(15) Å3, Z = 8, Rgt(F) = 0.0543, wRref(F2) = 0.1540, T = 100(2) K.
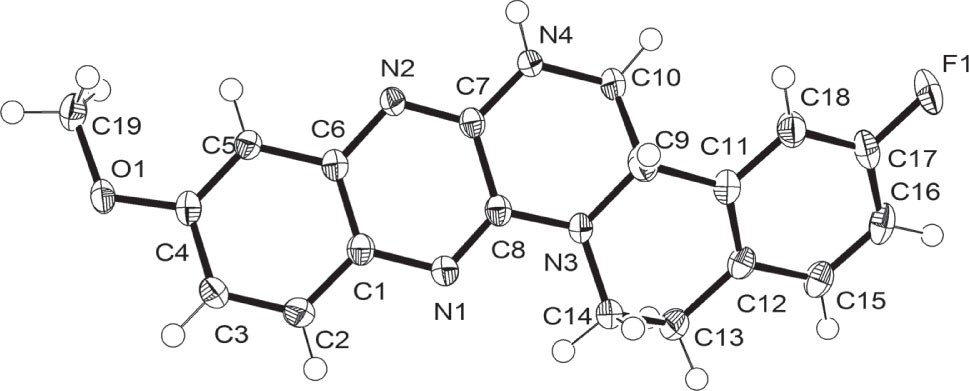
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.13 × 0.12 × 0.11 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.83 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 73.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 7304, 3064, 0.041 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2487 |
| N(param)refined: | 227 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.7725(3) | 0.47235(10) | 0.34176(8) | 0.0161(4) |
| C2 | 0.9126(3) | 0.49070(11) | 0.30257(8) | 0.0187(4) |
| H2 | 0.901149 | 0.474712 | 0.265810 | 0.022* |
| C3 | 1.0667(3) | 0.53198(11) | 0.31769(8) | 0.0180(4) |
| H3 | 1.157475 | 0.544076 | 0.291079 | 0.022* |
| C4 | 1.0875(3) | 0.55595(10) | 0.37319(8) | 0.0159(4) |
| C5 | 0.9500(2) | 0.54038(10) | 0.41260(8) | 0.0147(4) |
| H5 | 0.962842 | 0.556866 | 0.449189 | 0.018* |
| C6 | 0.7895(3) | 0.49913(10) | 0.39680(8) | 0.0144(4) |
| C7 | 0.5124(3) | 0.44158(10) | 0.42174(8) | 0.0145(4) |
| C8 | 0.5011(3) | 0.41067(10) | 0.36528(8) | 0.0151(4) |
| C9 | 0.1866(3) | 0.36515(11) | 0.38792(9) | 0.0221(5) |
| H9 | 0.119976 | 0.410116 | 0.378469 | 0.027* |
| C10 | 0.2413(3) | 0.36799(12) | 0.44919(8) | 0.0224(5) |
| H10A | 0.128983 | 0.375583 | 0.471801 | 0.027* |
| H10B | 0.297652 | 0.322172 | 0.460129 | 0.027* |
| C11 | 0.0545(3) | 0.30193(11) | 0.37700(9) | 0.0202(4) |
| C12 | 0.0912(3) | 0.24947(11) | 0.33620(8) | 0.0178(4) |
| C13 | 0.2700(3) | 0.25356(11) | 0.30203(9) | 0.0230(5) |
| H13A | 0.245921 | 0.234952 | 0.264734 | 0.028* |
| H13B | 0.366849 | 0.223596 | 0.319174 | 0.028* |
| C14 | 0.3404(3) | 0.33130(12) | 0.29804(8) | 0.0213(4) |
| H14A | 0.461899 | 0.332479 | 0.279115 | 0.026* |
| H14B | 0.251493 | 0.360171 | 0.276566 | 0.026* |
| C15 | −0.0403(3) | 0.19428(11) | 0.32749(8) | 0.0205(4) |
| H15 | −0.016332 | 0.159589 | 0.300143 | 0.025* |
| C16 | −0.2058(3) | 0.18966(11) | 0.35845(9) | 0.0222(5) |
| H16 | −0.292595 | 0.152509 | 0.352564 | 0.027* |
| C17 | −0.2367(3) | 0.24238(12) | 0.39834(9) | 0.0222(5) |
| C18 | −0.1133(3) | 0.29833(12) | 0.40798(9) | 0.0226(5) |
| H18 | −0.140778 | 0.333404 | 0.434733 | 0.027* |
| C19 | 1.2755(3) | 0.62196(11) | 0.43916(8) | 0.0208(4) |
| H19A | 1.394435 | 0.647061 | 0.441208 | 0.031* |
| H19B | 1.275121 | 0.582677 | 0.465519 | 0.031* |
| H19C | 1.174512 | 0.654911 | 0.447881 | 0.031* |
| F1 | −0.39778(17) | 0.23897(7) | 0.42986(6) | 0.0314(3) |
| N1 | 0.6239(2) | 0.42797(9) | 0.32657(7) | 0.0171(4) |
| N2 | 0.6523(2) | 0.48466(9) | 0.43648(7) | 0.0150(3) |
| N3 | 0.3595(2) | 0.36129(9) | 0.35439(7) | 0.0169(4) |
| N4 | 0.3750(2) | 0.42620(9) | 0.45906(7) | 0.0182(4) |
| H4 | 0.366451 | 0.451380 | 0.489245 | 0.022* |
| O1 | 1.24949(18) | 0.59398(8) | 0.38385(6) | 0.0202(3) |
Source of material
(7-Fluoro-1,2,3,4-tetrahydroisoquinolin-1-yl)methanamine (1.8 g, 10 mmol), 2,3-dichloro-6-methoxyquinoxaline (1.8 g, 8 mmol) and K2CO3 (4.15 g, 0.03 mol) were dissolved in DMF (20 mL). The mixture was stirred at 120 °C under an atmosphere of dry N2 for 10 hours. After cooling to room temperature, the mixture was poured into 500 mL ice-water and filtered. The filter cake was washed with water (50 mL*3) and dried at 80 °C for 5 hours. Recrystallization from methylene dichloride (250 mL) gave a yellow solid (yield 37.6%); m.p. 234–238 °C; 1H NMR (600 MHz, DMSO) δ [ppm] 7.83 (d, J = 3.8 Hz, 1H), 7.41–7.33 (m, 2H), 7.28 (dd, J = 8.3, 6.0 Hz, 1H), 7.10 (d, J = 2.5 Hz, 1H), 6.90–6.78 (m, 2H), 4.94 (ddd, J = 12.5, 4.8, 2.4 Hz, 1H), 4.73 (dd, J = 9.6, 2.9 Hz, 1H), 4.03 (dt, J = 11.6, 3.9 Hz, 1H), 3.79 (s, 3H), 3.35 (s, 2H), 3.06–2.97 (m, 1H), 2.91 (dd, J = 12.8, 6.3 Hz, 1H); 13C NMR (151 MHz, DMSO) δ [ppm] 162.04, 160.45, 157.07, 144.42, 142.08, 138.74, 136.50, 131.60, 131.23, 126.29, 114.50, 114.36, 113.62, 113.12–112.99, 112.90, 105.79), 55.62, 53.19, 45.52, 27.93. Crystals were obtained by evaporation within 3 days at room temperature.
Experimental details
The structure was solved with the SHELXL program using Olex2 [2], [3]. All hydrogen atoms were placed in the calculated positions.
Comment
Quinoxaline and its derivatives exhibit diverse biological activities, such as anticancer, antibacterial, antiviral, anti-inflammatory [4], [5], [6]. Tetrazanbigen (TNBG) was a creatively designed and synthesized leading compound bearing the quinoxaline core and showing anticancer activity [7]. Recently, a series of TNBG monosubstituted derivatives were developed with better physical properties and biological effect compared to TNBG [8]. The title compound is a disubstituted TNBG derivative, which was synthesized from (7-fluoro-1,2,3,4-tetrahydroisoquinolin-1-yl)methanamine [9] and 2,3-dichloro-6-methoxyquinoxaline [10]. It showed excellent anticancer activities against hepatoma cells, colon cancer cells and lung cancer cells in vitro, which can be deduced that the introduction of an electron-withdraw fluorine group and an electron-donating methoxy group at position C-3 and position C-9 of TNBG (see the figure) could improve their anticancer activities. All bond lengths and angles are in the normal ranges [11]. In conclusion the title compound is a potential antitumor agent, which can be used for further development.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 30772595
Award Identifier / Grant number: 30371632
Award Identifier / Grant number: 30171070
Funding source: Program of Chongqing Medical and Pharmaceutical College
Award Identifier / Grant number: YGZ2018102
Funding statement: This work was supported by the National Natural Science Foundation of China (30772595, 30371632, 30171070) and Program of Chongqing Medical and Pharmaceutical College (YGZ2018102).
References
1. Agilent Technologies: CrysAlisPro, Version 1.171.37.35. Agilent Technologies UK Ltd., Yarnton, England (2014).Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Search in Google Scholar
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. El Newahie, A. M. S.; Ismail, N. S. M.; Abou, E. E. D. A.; Abouzid, K. A. M.: Quinoxaline-based scaffolds targeting tyrosine kinases and their potential anticancer activity. Arch. Pharm. 349 (2016) 309–326.10.1002/ardp.201500468Search in Google Scholar PubMed
5. Montana, M.; Mathias, F.; Terme, T.; Vanelle, P.: Antitumoral activity of quinoxaline derivatives: a systematic review. Eur. J. Med. Chem. 163 (2019) 136–147.10.1016/j.ejmech.2018.11.059Search in Google Scholar PubMed
6. Kaushal, T.; Srivastava, G.; Sharma, A.; Negi, A. S.: An insight into medicinal chemistry of anticancer quinoxalines. Bioorg. Med. Chem. 27 (2019) 16–35.10.1016/j.bmc.2018.11.021Search in Google Scholar PubMed
7. Zheng, X. H.; Li, W.; Lan, Z. P.; Yang, X.; Li, L. J.; Yuan, Y. H.; Zhu, X.; Cen, X. G.; Zhang, X. Y.; Yu, Y.: Antitumour effects of tetrazanbigen against human hepatocellular carcinoma qgy-7701 through inducing lipid accumulation in vitro and in vivo. J. Pharm. Pharmacol. 67 (2015) 1593–1602.10.1111/jphp.12467Search in Google Scholar PubMed
8. Xiang, C.; Gan, Y. J.; Yu, Y.; Zhang, Y.: Synthesis and evaluation of new sterol derivatives as potential antitumor agents. RSC Adv. 8 (2018) 26528–26537.10.1039/C8RA04152KSearch in Google Scholar
9. Xi, M. Y.; Jia, J. M.; Sun, H. P.; Sun, Z. Y.; Jiang, J. W.; Wang, Y. J.; Zhang, M. Y.; Zhu, J. F.; Xu, L. L.; Jiang, Z. Y.; Xue, X.; Ye, M.; Yang, X.; Gao, Y.; Tao, L.; Guo, X. K.; Xu, X. L.; Zhang, X. J.; Guo, Q. L.; Hu, R.; You, Q. D.: 3-Aroylmethylene-2,3,6,7-tetrahydro-1H-pyrazino[2,1-a] isoquinolin-4(11bH) ones as potent Nrf2/ARE inducers in human cancer cells and AOM-DSS treated mice. J. Med. Chem. 56 (2013) 7925–7938.10.1021/jm400944kSearch in Google Scholar PubMed
10. Zhang, P. M.; Li, Y. W.; Zhou, J.; Chen, Y. J.; Gan, Z. J.; Yu, Y.: A one-pot facile synthesis of 2,3-dihydroxyquinoxaline and 2,3-dichloroquinoxaline derivatives using silica gel as an efficient catalyst. J. Heterocycl. Chem. 55 (2018) 1809–1814.10.1002/jhet.3224Search in Google Scholar
11. Lindner, B. D.; Paulus, F.; Appleton, A. L.; Schaffroth, M.; Engelhart, J. U.; Schelkle, K. M.; Tverskoy, O.; Rominger, F.; Hamburger, M.; Bunz, U. H. F.: Electron-transporting phenazinothiadiazoles with engineered microstructure. J. Mater. Chem. C 2 (2014) 9609–9612.10.1039/C4TC01992JSearch in Google Scholar
©2019 Yu Hang Chen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2
Articles in the same Issue
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2

