Abstract
C23H17F6NOS2, triclinic, P1̄ (no. 2), a = 8.379(3) Å, b = 11.509(2) Å, c = 12.817(3) Å, α = 71.272(3)°, β = 81.342(4)°, γ = 77.553(3)°, V = 1138.7(5) Å3, Z = 2, Rgt(F) = 0.0489, wRref(F2) = 0.1296, T = 296(2) K.
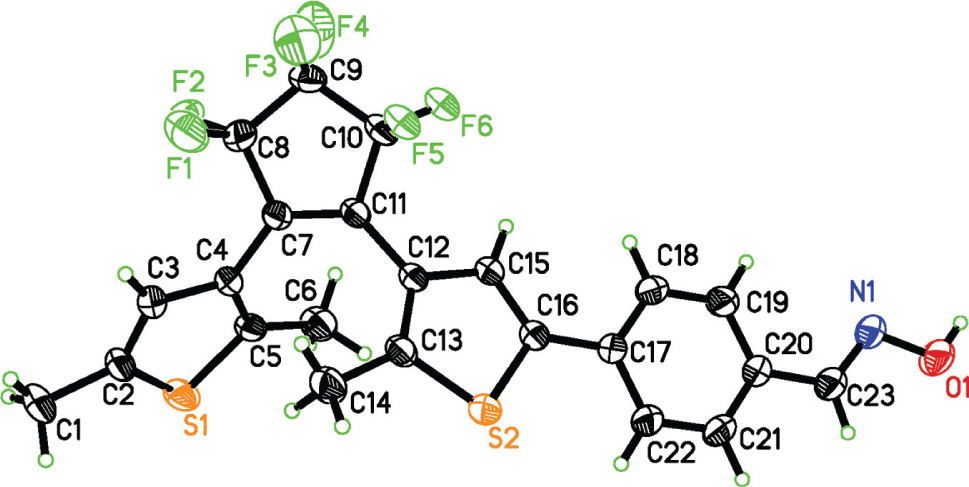
The crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.28 × 0.26 × 0.17 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.30 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω-scans |
| θmax, completeness: | 25°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 5853, 3980, 0.017 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2858 |
| N(param)refined: | 381 |
| Programs: | Bruker programs [1], SHELX [2], [3], [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | 0.9965(4) | 0.1385(3) | 0.4543(2) | 0.0669(8) |
| O1 | 1.0967(3) | 0.0720(2) | 0.3874(2) | 0.0886(8) |
| H1E | 1.0990 | −0.0029 | 0.4170 | 0.133* |
| S1 | 0.59918(11) | 1.04064(8) | 0.82370(8) | 0.0677(3) |
| S2 | 0.53463(12) | 0.76401(7) | 0.53506(7) | 0.0628(3) |
| C8 | 0.1935(4) | 0.7602(3) | 0.9962(3) | 0.0697(9) |
| F1a | 0.0598(8) | 0.8465(5) | 0.9990(9) | 0.155(3) |
| F2a | 0.2725(12) | 0.7392(9) | 1.0888(3) | 0.138(3) |
| F1′b | 0.0493(12) | 0.8362(12) | 0.9577(14) | 0.069(3) |
| F2′b | 0.2053(15) | 0.7993(13) | 1.0812(9) | 0.073(4) |
| C9 | 0.1548(3) | 0.6354(3) | 1.0059(2) | 0.0549(7) |
| F3c | −0.0003(8) | 0.6230(9) | 1.0358(12) | 0.082(3) |
| F4c | 0.2421(19) | 0.5580(8) | 1.0901(6) | 0.083(3) |
| F3′d | −0.0035(9) | 0.6623(14) | 0.9817(16) | 0.101(3) |
| F4′d | 0.159(2) | 0.5446(6) | 1.1010(5) | 0.082(3) |
| C10 | 0.2385(4) | 0.6033(3) | 0.9044(3) | 0.0638(8) |
| F5a | 0.1430(11)a | 0.5787(10) | 0.8424(4) | 0.146(3) |
| F6a | 0.3485(9)a | 0.4952(4) | 0.9419(6) | 0.118(2) |
| F5′b | 0.0987(12) | 0.6305(11) | 0.8500(12) | 0.062(3) |
| F6′b | 0.2983(16) | 0.4852(6) | 0.9105(12) | 0.065(3) |
| C1 | 0.3505(6) | 1.2412(3) | 0.8404(4) | 0.0916(13) |
| H1A | 0.2334 | 1.2604 | 0.8543 | 0.137* |
| H1B | 0.3835 | 1.2914 | 0.7678 | 0.137* |
| H1C | 0.4020 | 1.2584 | 0.8945 | 0.137* |
| C2 | 0.4014(5) | 1.1053(3) | 0.8474(3) | 0.0637(9) |
| C3 | 0.3037(4) | 1.0192(3) | 0.8737(3) | 0.0574(8) |
| H3A | 0.1909 | 1.0368 | 0.8907 | 0.069* |
| C4 | 0.3906(3) | 0.8987(2) | 0.8729(2) | 0.0448(6) |
| C5 | 0.5545(4) | 0.8953(3) | 0.8479(2) | 0.0501(7) |
| C6 | 0.6879(5) | 0.7887(4) | 0.8421(4) | 0.0688(10) |
| C7 | 0.3121(3) | 0.7897(2) | 0.8973(2) | 0.0447(6) |
| C11 | 0.3319(3) | 0.7049(2) | 0.8430(2) | 0.0437(6) |
| C12 | 0.4210(3) | 0.7031(2) | 0.7362(2) | 0.0439(6) |
| C13 | 0.4194(4) | 0.8045(3) | 0.6440(2) | 0.0516(7) |
| C14 | 0.3326(6) | 0.9357(3) | 0.6273(4) | 0.0684(10) |
| C15 | 0.5164(3) | 0.5933(2) | 0.7155(2) | 0.0471(7) |
| H15A | 0.5287 | 0.5161 | 0.7694 | 0.056* |
| C16 | 0.5876(4) | 0.6102(3) | 0.6116(2) | 0.0495(7) |
| C17 | 0.6920(4) | 0.5195(3) | 0.5613(2) | 0.0500(7) |
| C18 | 0.7212(5) | 0.3940(3) | 0.6188(3) | 0.0723(10) |
| H18A | 0.6752 | 0.3672 | 0.6912 | 0.087* |
| C19 | 0.8158(5) | 0.3083(3) | 0.5719(3) | 0.0736(10) |
| H19A | 0.8319 | 0.2245 | 0.6128 | 0.088* |
| C20 | 0.8877(4) | 0.3432(3) | 0.4659(3) | 0.0563(8) |
| C21 | 0.8621(5) | 0.4678(3) | 0.4091(3) | 0.0723(10) |
| H21A | 0.9101 | 0.4943 | 0.3372 | 0.087* |
| C22 | 0.7673(5) | 0.5545(3) | 0.4559(3) | 0.0685(9) |
| H22A | 0.7538 | 0.6386 | 0.4155 | 0.082* |
| C23 | 0.9867(4) | 0.2543(3) | 0.4124(3) | 0.0663(9) |
| H23A | 1.0450 | 0.2844 | 0.3447 | 0.080* |
| H14A | 0.232(5) | 0.937(3) | 0.671(3) | 0.077(12)* |
| H14B | 0.309(4) | 0.971(3) | 0.556(3) | 0.082(12)* |
| H14C | 0.401(5) | 0.982(4) | 0.646(3) | 0.085(12)* |
| H6A | 0.665(4) | 0.712(4) | 0.893(3) | 0.083(12)* |
| H6C | 0.787(6) | 0.802(4) | 0.850(4) | 0.125(17)* |
| H6B | 0.702(6) | 0.776(4) | 0.767(4) | 0.123(17)* |
Occupancies: a = 0.748(15), b = 0.0.252(15), c = 0.54(2), d = 0.46(2)
Source of materials
To a stirred solution of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldehyde-4-yl)-3-thenyl)]perfluorocyclopentene [5] (0.24 g; 0.5 mmol) in ethanol (2.5 mL), hydroxylamine hydrochloride (0.035 g; 0.5 mmol) was added and continuously stirred. After refluxing for 5 h, the mixture was cooled to room temperature and concentrated under vacuum. The product was purified by flash chromatography with petroleum ether/ethyl acetate = 4/1 as the eluant to give the title compound with a yield of 75%. The title compound crystallized from hexane at room temperature. Mp. 306–307 K; 1H NMR (400 MHz), CD3CN, TMS): (ppm): 1.80 (s, 3H, -CH3), 1.88 (s, 3H, -CH3), 2.08 (s, 1H, -OH), 2.3280 (s, 3H, -CH3), 6.71 (s, 1H, thiophene-H), 8.95 (d, 1H, J = 4 Hz, = CH-), 7.51 (m, 2H, phenyl-H), 8.01 (d, 2H, J = 4 Hz, phenyl-H), 8.95 (d, 1H, J = 4 Hz, = CH-); 13C NMR (100 MHz), CD3CN, TMS): (ppm): 13.85, 13.96, 14.62, 102.80, 113.62, 114.27, 123.44, 123.89, 124.37, 125.29, 125.55, 126.95, 128.65, 129.84, 134.99, 135.13, 138.17, 139.66, 140.69, 142.10, 154.12.
Experimental details
All non-hydrogen atoms were refined anisotropically. The fluorine atoms of the central cyclopentene ring show extensive disorder. A two-site split model was used in the refinement yielding ratios of 0.748(15) : 0.252(15) for F1/F2 : F1′/F2′, 0.54(2) : 0.46(2) for F3/F4 : F3′/F4′ and 0.748(15) : 0.252(15) for F5/F6 : F5′/F6′. All H atoms attached to C were fixed geometrically and treated as riding with C—CH = 0.96 Å (methyl) with Uiso(H) = 1.2Ueq (thienyl) or Uiso(H) = 1.5Ueq (methyl, hydroxy).
Comment
Organic photochromic dyes represent a rapidly expanding area of molecular switches due to their wide applications in optical memory media [6], photo- and supramolecular chemistry [7], materials science [8], optogenetics [9], and bioimaging [10]. As representative of photochromic compounds, diarylethene derivatives can undergo reversible changes due to high thermal stability [6], rapid response [6], and fatigue resistance [11], and high reactivity in the solid state [12].
On one hand, molecular modification is one of the most important methods to improve the photochromic performance of diarylethenes. Generally, the photochromic characteristics of diarylethenes strongly depend on the functional substituents [13]. Recently we have reported a series of photochromic diarylethenes bearing different substituents, such as trifluoromethyl, fluorine, chlorine, methoxy and methyl. Aryl aldoximes are important reaction ragents that exhibit biological activity. However, photochromic diarylethenes with aldoxime substituents have not hitherto been reported.
On the other hand, most research on photochromic diarylethenes concentrate on their tautomerization between the bistable states in solution or in polymer blends and limited examples show tunable tautomerization in constraint crystal states. The application of fluorescent photo switching molecules would be facilitated in the solid state, in particular in the crystalline state for ease of use. Several photochromic diarylethenes have been reported but reports of their crystal structures remain limited [14], [15], [16], [17]. On the basis of these considerations, we have explored a aldoxime-containing diarylethene 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldehyde)-3-thienyl]perfluorocyclopentene and investigated its photochromism both in crystalline solids and in solution. The title compound, C23H17F6NOS2, is a new asymmetrical hybrid photochromic diarylethene derivative.
The title molecule adopts a photo-active antiparallel conformation. In the cyclopentene ring, the two thiophene rings are linked by the C7 = C11 double bond [1.340(4) Å]. The two methyl groups are located on opposite sides of the C7 = C11 double bond and this configuration is crucial to allow the compound to exhibit photochromic and photoinduced properties [18]. Upon UV-light irradiation the open-ring isomer of the diarylethene undergoes a 6π-electrocyclization to furnish the closed-ring isomer which can be back-converted upon exposure to visible light. The distance between the two photo-reactive C atoms (C5⋯C13) is 3.515 Å. This distance indicates that the crystal can be expected to undergo photochromic reaction to form the closed ring isomer because photochromic reactivity usually appears when the distance between the reactive C atoms is less than 4.2 Å [19], [20]. Upon irradiation with 297 nm light, the color change of the single-crystals from colorless to purple as the ring-closed isomer is observed. When the purple crystals were dissolved in acetonitrile, the acetonitrile solution also showed a purple color, with a band in the visible region centred at 599 nm, accompanied with the formation of the closed-ring isomer. It should be noted that the purple crystals can revert to their initial colorless state upon irradiation with visible light (λ > 500 nm), and the absorption spectrum of an acetonitrile solution prepared from the colourless crystals is the same as that of a solution of the open ring form, with an absorption maximum at 319 nm.
Funding source: Natural Science Foundation of Jiangxi Province
Award Identifier / Grant number: 20171BAB213018
Funding statement: We gratefully acknowledge support by the Natural Science Foundation of China (41867053), the Science Funds of Natural Science Foundation of Jiangxi Province (20171BAB213018). We thank the editor for providing the figure.
References
1. Bruker. APEX3, SAINT-Plus, XPREP. Bruker AXS Inc., Madison, WI, USA (2016).Suche in Google Scholar
2. Sheldrick, G. M.: SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M.: SADABS. University of Götting, Germany (2014).Suche in Google Scholar
5. Ren, P. P.; Wang, R. J.; Pu, S. Z.; Fan, C. B.: A multi-addressable molecular switch based on a novel diarylethene with an imidazo [4,5-f][1,10] phenanthroline unit. J. Phys. Org. Chem. 27 (2014) 183–190.10.1002/poc.3257Suche in Google Scholar
6. Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S.: Photochromism of diarylethene molecules and crystals: memories, switches, and actuators. Chem. Rev. 114 (2014) 12147–12277.10.1021/cr500249pSuche in Google Scholar PubMed
7. Zhang, C. C.; Fan, C. B.; Pu, S. Z.; Liu, G.: A highly selective chemosensor for Cu2+ based on a diarylethene linking an aminoquinoline unit. Chin. J. Chem. 33 (2015) 1310–1316.10.1002/cjoc.201500578Suche in Google Scholar
8. Peters, G. M.; Tovar, J. D.: Pendant photochromic conjugated polymers incorporating a highly functionalizable thieno[3,4-b]thiophene switching motif. J. Am. Chem. Soc. 141 (2019) 3146–3152.10.1021/jacs.8b12617Suche in Google Scholar PubMed
9. Nakashima, T.; Tsuchie, K.; Kanazawa, R.; Li, R.; Iijima, S.; Galangau, O.; Nakagawa, H.; Mutoh, K.; Kobayashi, Y.; Abe, J.; Kawai, T.: Self-contained photoacid generator triggered by photocyclization of triangle terarylene backbone. J. Am. Chem. Soc. 137 (2015) 7023–7026.10.1021/jacs.5b02826Suche in Google Scholar PubMed
10. Osakada, Y.; Fukaminato, T.; Ichinose, Y.; Fujitsuka, M.; Harada, Y.; Majima, T.: Live cell imaging using photoswitchable diarylethene-doped fluorescent polymer dots. Chem. -Asian J. 12 (2017) 2660–2665.10.1002/asia.201701038Suche in Google Scholar PubMed
11. Yun, C.; You, J.; Kim, J.; Huh, J.; Kim, E.: Photochromic fluorescence switching from diarylethenes and its applications. J. Photoch. Photobio. C: Photoch. Rev. 10 (2009) 111–129.10.1016/j.jphotochemrev.2009.05.002Suche in Google Scholar
12. Kobaka, S.; Uchida, K.; Tsuchida, E.; Irie, M.: Singlecrystalline photochromism of diarylethenes: reactivity structure relationship. Chem. Commun. (2002) 2804–2805.10.1039/B208419HSuche in Google Scholar
13. Fan, C. B.; Fu, Y. L.; Pu, S. Z.: Crystal structure of photochromic 1-(2-methyl-5-phenyl-3-thienyl-2-[2-methyl-5-(4-ethoxylphenyl)-3-thienyl]3,3,4,4,5,5-hexafluorocyclopent-1-ene, C29H22F6OS2. Z. Kristallogr. NCS 231 (2016) 215–217.10.1515/ncrs-2015-0095Suche in Google Scholar
14. Lv, J. F.; Li, H.; Pu, S. Z.: Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2, Z. Kristallogr. NCS 233 (2018) 999–1002.10.1515/ncrs-2018-0116Suche in Google Scholar
15. Liu, F. F.; Li, H.; Pu, S. Z.: Structure and photochromism of 1-[2-methyl-5-phenyl-3-thienyl]-2-[2-methyl-5-(4-chlorophenyl)-3-thienyl]3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16ClF6S2. Z. Kristallogr. NCS 234 (2019) 49–51.10.1515/ncrs-2018-0129Suche in Google Scholar
16. Fan, C. B.; Liu, Y.; Zhang, D. B.; Pu, S. Z.: Crystal structure and crystalline state multiphotochromism properties of a fused diarylethene dimer, Chinese. J. Struct. Chem. 38 (2019) 251–256.Suche in Google Scholar
17. Xu, D. M.; Zheng, C. H.; Pu, S. Z.: Crystal structure and photochromic properties of 1-(2-methyl-5-phenyl-3-thienyl)-2-{2-methyl-5-[4-(9-fluorenone hydrazone)-phenyl]-3-thienyl}perfluorocyclopentene, C41H26F6N2S2. Z. Kristallogr. NCS 234 (2019) 979–981.10.1515/ncrs-2019-0197Suche in Google Scholar
18. Woodward, R. B.; Hoffmann, R.: The conservation of orbital symmetry. Verlag Chemie GmbH, Weinheim (1970).10.1016/B978-1-4832-3290-4.50006-4Suche in Google Scholar
19. Ramamurthy, V.; Venkatesan, K.: Photochemical reactions of organic crystals. Chem. Rev. 87 (1987) 433–481.10.1021/cr00078a009Suche in Google Scholar
20. Kobatake, S.; Irie, M.: Singe-crystalline photochromism of diarylethenes. Bull. Chem. Soc. Jpn. 77 (2004) 195–210.10.1246/bcsj.77.195Suche in Google Scholar
©2019 Xiaoxiao Wu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2
Artikel in diesem Heft
- Frontmatter
- Synthesis and crystal structure of bis{5-fluorine-2-(((4-(1-(methoxy-imino)ethyl)phenyl) imino)methyl)phenolato-κ2N,O}copper(II), C32H28CuF2N4O4
- Redetermination of the crystal structure of N′-(3-ethoxy-2-hydroxybenzylidene)-4-fluorobenzohydrazide monohydrate, C16H17FN2O4
- The crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl) ethylidene)-2-hydroxybenzohydrazide, C15H12ClFN2O2
- Crystal structure of (E)-N-[4-(1H)-imidazolyl phenyl]-(2-methylphenyl)methanimine, C17H15N3
- The crystal structure of 1-benzyl-4-(2-(phenylethynyl)phenyl)-1H-1,2,3-triazole, C23H17N3
- Crystal structure of catena-poly[{μ2-1,5-bis(diphenylphosphanyl)pentane-κ2P:P′}dichloridocadmium(II)], C29H30CdCl2P2
- Crystal structure of methyl (E)-N2-((3-methylquinolin-8-yl)sulfonyl)-Nω′-nitro-L-argininate - ethanol (1/1), C19H28N6O7S
- The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine-κP)iodidomethyl-(2-oxopyridin-1(2H)-olato-κ2O,O′)rhodium(III), C25H28INO3PRh
- Crystal structure of N-(amino(pyrazin-2-yl)methylene)-6-methylpyridin-1-ium-3-carbohydrazonate-κ3O,N,N′)-(dinitrato-κ1O)zinc(II), C12H12N8O7Zn
- The crystal structure of dichlorido-(tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′)chromium(III) chloride — methanol (1/3), CrC27H33Cl3N7O3
- Crystal structure of catena-poly[aqua(μ4-piperazine-1,4-bis(2-hydroxypropanesulfonato-κ8O,O′:O′,N:N′,O′′:O′′,O′′′))silver(I)], C10H24Ag2N2O10S2
- Crystal structure of bis(μ3-oxido)-bis(μ2–2,3,4,5-tetrafluorobenzoato-κ2O:O′)-bis(2,3,4,5-tetrafluorobenzoato-κO)-oktakis(3-chlorobenzyl-κC)tetratin(IV), C84H52Cl8F16O10Sn4
- Crystal structure of (E)-1-{4-[(4-fluoro-2-hydroxybenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15FN2O2
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-N,N′)zinc(II)], {C20H30N4O4P2S4Zn}n
- Crystal structure of methyl 2-(4-(3-iodopyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C15H12IN3O2
- Crystal structure of hexacarbonyl-(μ2-methanoato-k2O:O′)-(μ2–bis(di-p-tolylphosphino)cyclohexylamine-κ2P:P′)dirhenium(I), C42H45NO8P2Re2
- The cocrystal structure of 1′-hydroxy-1H,1′H-[5,5′-bitetrazol]-1-olate and 1,10-phenanthrolin-1-ium, C14H10N10O2
- The crystal structure of 1-benzyl-2-((4-(tert-butyl)phenyl)ethynyl)pyridin-1-ium bromide,C24H24BrN
- Crystal structure of (5,5′-bitetrazole-1,1′-diolate)-bis(1,10-phenanthroline)-copper(II), C26H16CuN12O2
- Crystal structure of bis(ammonium) diaqua-tetrakis(4-hydroxybenzoato)-manganese(II) tetrahydrate, [NH4]2[C28H24MnO14] ⋅ 4(H2O)
- The crystal structure of 3-chloro-1-hydrazino-2,4,6-trinitrobenzene, C6H4ClN5O6
- Crystal structure of catena-[(μ2-pyrazine-κ2N:N′)-bis(O,O′-di-ethyldithiophosphato-κ2S,S′)cadmium(II)], {C12H24CdN2O4P2S4}n
- Crystal structure of catena-poly[(μ2-pyrazine-N,N′)-bis(O,O′-di-isopropyldithiophosphato-S,S′)cadmium(II) acetonitrile di-solvate], [C16H32CdN2O4P2S4⋅2(C2H3N)]n
- Crystal structure of catena-poly{(μ2-N1,N2-bis[(pyridin-4-yl)methyl]ethanediamide-κ2N:N′)-bis(O,O′-di-isopropyldithiophosphato-κ1S)zinc(II)} — acetonitrile (1/1), C26H42N4O6P2S4Zn⋅C2H3N
- Crystal structure of tetraqua-bis(4-(hydroxymethyl)benzoato-κO)cobalt(II), C16H22O10Co
- Crystal structure of catena-[(bis(O,O′-diethyl dithiophosphato-S,S′)-μ2-1,2-bis(4-pyridylmethylene)hydrazine-N,N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- Crystal structure of catena-poly[(μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)-bis(O,O′-dimethyl dithiophosphato-κ2-S,S′)cadmium(II)], {C16H22CdN4O4P2S4}n
- Crystal structure of catena-poly[(bis(O,O′-diethyl dithiophosphato-κ2S,S′)-μ2-1,2-bis(3-pyridylmethylene)hydrazine-κ2N:N′)cadmium(II)], {C20H30CdN4O4P2S4}n
- The crystal structure of catena-poly[(E)-2-(((5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol], C12H15N3OS2Sn
- Crystal structure of dichlorido(N-o-tolyl-1,1-di-p-tolylphosphanamine–κ1P)-(methoxydi-p-tolylphosphane-κ1P)palladium(II), C36H39Cl2NOP2Pd
- The crystal structure of the triclinic polymorph of hexameric (trimethylsilyl)methyllithium, C24H66Li6Si6
- Crystal structure of bis(hydroxydi(pyridin-2-yl)methanolato-κ3N,N′O)cobalt(III) 7,7,8,8-tetracyanoquinodimethane, C34H22CoN8O4
- Synthesis and crystal structure of benzyl 5-oxo-5-phenyl-2-(quinolin-2-yl)pentanoate, C27H23NO3
- Crystal structure of 5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-(2,2-dichloroacetyl)-3,4-dihydro-2 H-benzo[b][1,4]oxazine-7-carboxylate, C19H19Cl2NO5
- Crystal structure of dipentyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate, C18H28O6
- The crystal structure of catena-poly[diaqua-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)-(μ4-5-(benzo[d]thiazol-2-yl)benzene-1,3-dicarboxylate-κ4O,O′:O′′,O′′′)dicadmium(II)], C30H18Cd2N2O10S2
- Crystal structure of 2,7-diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4
- A dinuclear Eu(III) complex in the crystal structure of dodecaaqua-bis(μ2-4-(1H-tetrazol-5-yl)benzoato-κ2O:O′) bis(5-(4-carboxylatophenyl)tetrazol-1-ide) tetrahydrate, C32H50Eu2N16O24
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2
- Crystal structure of 4-dimethylamino-pyridin-1-ium uracil-1-acetate, C13H16N4O4
- Crystal structure of dimethylammonium 5-fluorouracil-1-acetate, C8H12N3O4F
- Crystal structure of bis(N′-((5-(ethoxycarbonyl)-1H-pyrrol-2-yl)methylene)-N-ethylcarbamohydrazonothioato-κ2N,O)nickel(II), C22H30N8O4S2Ni
- Crystal structure of chlorido-(η5-pentamethylcyclopentadienyl)-((bis-pyrazol-1-yl)methane-κ2N,N′) rhodium(III) hexafluorophosphate. (C17H23ClN4RhF6P)
- The crystal structure of 5-(benzofuran-2-carbonyl)-N-cyclohexyl-5,6-dihydrophenanthridine-6-carboxamide, C29H26N2O3
- The crystal structure of 2-oxo-2H-chromen-4-yl acetate, C11H8O4
- The crystal structure of 2-nitroisophthalic acid, C8H5NO6
- Crystal structure of 3-fluoro-9-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino [2′,1′:1,6]pyrazino[2,3-b]quinoxaline, C19H17FN4O
- Crystal structure of (4-fluorobenzyl-κC)(bis(2-hydroxyethyl) carbamodithioato-κ2S,S′)(2,2′-imino-diethanolato-κ3N,O,O′)tin(IV), C16H25FN2O4S2Sn
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-bromophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18BrF3N2O3S
- Crystal structure and anti-inflammatory activity of (3E,5E)-1-((4-chlorophenyl)sulfonyl)-3-(pyridin-4-ylmethylene)-5-(2-(trifluoromethyl)benzylidene)piperidin-4-one, C25H18ClF3N2O3S
- The crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium tetrachloridomanganate(II), C10H16Cl4MnN2
- The crystal structure of 3-carboxy-5-methylpyridin-1-ium-2-carboxylate, C8H7NO4
- Crystal structure of bis(3-methoxy-N-(1-(pyridin-2-yl)ethylidene)benzohydrazonato κ3O,N,N′)zinc(II), C30H28N6O4Zn
- Crystal structure of dichlorido-(4,4′-dichloro-2,2′-bipyridine-κ2N,N′)platinum(II) — acetone (1/1), C13H12Cl4N2PtO
- Crystal structure of diethyl 6,12-bis(4-fluorophenyl)-2,10-dimethoxy-3,9-diphenyl-3,9-diazatetracyclo[6.4.0.02,7.04,11]dodecane-1,5-dicarboxylate, C42H42F2N2O6
- Synthesis and crystal structure of (1E,3E)-2-hydroxy-5-methylisophthalaldehyde O,O-di(2-((((E)-(2-hydroxynaphthalen-1-yl)methylene)amino)oxy)ethyl) dioxime, C35H32N4O7
- The crystal structure of 2-phenyl-4,6-bis(prop-2-yn-1-yloxy)-1,3,5-triazine, C15H11N3O2
- Crystal structure of 7-(2-{4-[(4-bromophenyl)methyl]piperazin-1-yl}ethoxy)-2H-chromen-2-one, C22H23BrN2O3
- Crystal structure of bis-[N-(3-ethyl-1-pyrazin-2-yl-ethylidene)-3-bromo-benzoic acid-hydrazonato-κ3O,N,N′)]-cadmium(II), C30H28N8O2Br2Cd
- Crystal structure of 6-(4-fluorophenyl)-4-methoxy-2H-pyran-2-one, C12H9FO3
- Crystal structure of 3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)butanoic acid, C14H18O4
- The crystal structure of 3-bromo-6-methoxy-2-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine, C13H19BBrNO3
- The crystal structure of 6-methyl-3,20-dioxo-19-norpregna-4,6-dien-17-yl acetate–2,4-dihydroxybenzoic acid (1/1), C30H36O8
- The crystal structure of (5-chloro-2-hydroxy-N-(4-methoxy-2-oxidobenzylidene)benzohydrazonato-κ3N,O,O′)-(pyridine-κ1N)copper(II), C20H16ClCuN3O4
- Crystal structure of (E)-2-cyano-N′-(1-(3-ethylpyrazin-2-yl)ethylidene)acetohydrazide, C11H3N5O
- Crystal structure of (2,7-dihexyl-9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane), C51H56OP2
- Crystal structure of 5-((bis(pyridin-2-ylmethyl)amino)methyl)quinolin-8-ol, C22H20N4O
- Crystal structure of 3-(2-(5-(4-fluorophenyl)-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-2H-chromen-2-one, C28H20FN3O2S
- The crystal structure of [(tetra-μ2-2,6-difluorobenzoato-κ2O:O′)-bis-(2,6-difluorobenzoato-κ2O:O′)-bis-(1,10-phenanthroline-κ2N:N′)]dierbium(III) C66H34N4O12F12Er2
- Crystal structure of bis(3-chloro-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-k3N,N′,O)nickel(II), C26H20N8O2Cl2Ni
- Crystal structure of (E)-3-(3-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)-1-phenylprop-2-en-1-one, C27H21N5O
- Crystal structure of (E)-N′-((4-aminophenyl)sulfonyl)-N,N-dimethylformimidamide, C9H13N3O2S
- Crystal structure of η6-p-cymene-iodido-(N-isopropyl-1-(pyridin-2-yl)methanimine-κ2N,N′)ruthenium(II) hexafluorophosphate(V), C19H26IN2F6Ru
- Crystal structure of 6-iodo-3-phenyl-2-propylquinazolin-4(3H)-one, C17H15IN2O
- Low temperature redetermination of the crystal structure of catena-poly[[tri-4-fluorobenzyltin(IV)]μ2-pyridine-4-carboxylato-κ2N:O], {C27H22F3NO2Sn}n
- Crystal structure of bis(2-propyl-1H-benzo[d]imidazol-3-ium) tetrachloridozincate(II), C10H13Cl4N2Zn
- The crystal structure of (Z)-3-hydrazono-5-nitroindolin-2-one – dimethyl sulfoxide (1/1), C8H6N4O3
- Crystal structure of bis-[N-(1-pyrazin-2-yl-ethylidene)-cyanoacetic acid-hydrazonato-κ3O,N,N′)]-zinc(II), C18H16N10O2Zn
- Crystal structure and photochromism of 1-(2,5-dimethyl-3-thienyl)-2-[2-methyl-5-(benzaldoxime)-3-thienyl] perfluorocyclopentene, C23H17F6NOS2

