Abstract
This study evaluates the efficacy of pembrolizumab for the treatment of advanced/metastatic melanoma. The literature search was conducted in electronic databases for studies that evaluated the efficacy and safety of pembrolizumab either alone or in combination with other treatments advanced/metastatic melanoma patients. Random-effects meta-analyses were performed to achieve pooled effect sizes of response and survival rates. The overall objective response rate (ORR) was 34.2% [95% confidence interval (CI): 30.4, 38.0]. However, ORR differed with respect to the history of prior systemic therapy. ORR was lower in studies with over 50% patients with prior therapy (25.5% [22.4, 28.5]) than in studies with under 50% patients with prior therapy (40.1% [34.1, 46.1]). ORR was higher in pembrolizumab monotherapy (32.9% [28.1, 37.7]) than in pembrolizumab–ipilimumab combination (27.6% [24.0, 31.2]). Overall ORR was inversely associated with visceral metastasis and prior systemic therapy. With pembrolizumab treatment, either alone or in combination, the progression-free survival (PFS) was 5.73 months; 12-, 24-, and 60-month PFS rate were 44%, 27%, and 25%, respectively; and 12-, 24-, and 60-month overall survival rates were 65%, 50%, and 41%, respectively. The percentage of AEs that led to treatment discontinuation was 13%. Pembrolizumab monotherapy is a valuable option for the treatment of advanced/metastatic melanoma patients.
1 Introduction
Melanoma is a tumor of melanocytes, which most commonly arise in the skin but may also appear in the uveal area and leptomeninges [1]. Major histopathological forms of melanoma are the superficial, nodular, lentigo maligna and acral lentiginous [2]. Melanoma constitutes 5.5% of all cancers. The incidence of cutaneous melanoma has increased from 14.1 to 22.7 cases per 1,00,000 individuals during 1992 to 2016 [3]. During the fourth decade of age, the incidence of melanoma is found to be higher in females, but by the age of 75 years, melanoma incidence is reported thrice in males than in females [4]. Melanoma-related mortality rates are relatively higher in fair-skinned population especially for those who live in lower latitudes [1].
Melanoma is an immunogenic tumor, and therefore, targeting immunological pathways for the development of efficacious treatments is essential [5]. For many decades, the most common treatment regimens for metastatic melanoma were the systemic immuno-stimulating cytokines such as interleukin-2 (IL-2) and interferon-alpha (IFN-α). However, metastatic melanoma poorly responds to cytokines, and the cure rate remains less than 10% [6]. With the use of immune checkpoint inhibitors, immunotherapy for melanoma has substantially improved. Ipilimumab, the first fully humanized immunoglobulin (Ig) G1 monoclonal antibody, was approved for metastatic melanoma. Ipilimumab blocks the cytotoxic T-lymphocyte antigen (CTLA)-4 to produce anticancer effects [7].
Later, the use of antibodies against the programmed cell death 1 protein (PD1) further improved the survival of melanoma patients [8]. Blockade of PD1–PDL1 interactions has been found to produce good antitumor response. Pembrolizumab is a high-affinity humanized immunoglobulin G4 monoclonal antibody against the immune checkpoint protein, PD1, on activated T cells. One of its ligands, the PD ligand 1 (PD-L1), which is expressed on tumor cells, macrophages, and dendritic cells, triggers tolerance to immune system and thence promotes tumor proliferation [9].
Many authors have reported the outcomes of the efficacy and safety of pembrolizumab either alone or in combination with other therapies in advanced melanoma patients, but outcomes vary considerably in these studies, which provides impetus for a systematic review seeking a refined evidence of pembrolizumab’s therapeutic potentials. The aim of this study is to conduct a meta-analysis of response and survival rates of advanced melanoma patients who were treated with pembrolizumab either alone or with other therapies to gain an up-to-date evidence of its efficacy and safety and to identify the factors affecting the efficacy.
2 Methods
2.1 Inclusion and exclusion criteria
Inclusion criteria for the current meta-analysis are as follows: a study that (a) investigated the efficacy and safety of pembrolizumab either alone or in combination with other related therapies for the management of advanced/metastatic melanoma patients and (b) reported the efficacy indices including objective response rate (ORR), progression-free survival (PFS), and overall survival (OS). Exclusion criteria are as follows: a study that reported (a) the outcomes of more than one anti-PD1 drugs without distinction, (b) pharmacokinetic or pharmacodynamic investigation, (c) in vitro, molecular, or experimental investigations, or (d) qualitative information.
2.2 Literature search
Google Scholar, PubMed, and Science Direct electronic databases were searched by using specific keywords and medical subject headings. Primarily, pembrolizumab–melanoma efficacy combination was used, which was then used with several other words including programmed cell death, PD1, ligand, PD-L1, response, survival, tumor, node, metastasis, TNM, B-Raf proto-oncogene (BRAF), safety, tolerability, adverse events, toxicity, and trial. Search encompassed research articles published before September 2019 in English. In addition, the bibliographies of important related papers were also screened.
2.3 Data and analyses
Baseline demographic, clinical, oncological, and genetical data; and study design, methodological, analytical, and outcome data of the included studies were obtained from respective research articles and were organized in datasheets. Quality assessment of the included studies was performed with New Castle–Ottawa Scale for the Quality Assessment of Cohort studies.
Response and survival rates reported by the individual studies were pooled under the random-effects model to achieve an overall effect size of each endpoint as an inverse variance weighted average of the individual study effect sizes. Statistical heterogeneity was estimated with I2 index. Subgroup analyses were performed with regards to the combinational use of pembrolizumab and the percentage of patients with prior systemic therapy.
In meta-regression analyses, the ORR was used as a dependent variable to seek its relationships with several independent variables including follow-up duration, age, gender, tumor/node/metastasis (TNM) status, Eastern Cooperative Oncology Group performance status (ECOG PS), PD1 ligand status, BRAF status, percentage of patients with high lactic dehydrogenase (LDH) levels, and prior systemic therapy. The restricted maximum likelihood method was used for meta-regression analyses. All statistical analyses were performed using Stata software (Stata Corporation, Texas, USA).
3 Results
Twenty studies fulfilled the eligibility criteria and thence were included in the meta-analysis (Figure 1). These studies were published in 25 research articles [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. In these studies, 2,909 patients with advanced/metastatic melanoma were treated with pembrolizumab either alone (n = 2,139) or in combination with other therapies (n = 770). Characteristics of the included studies are presented in Tables S1a and b. The average age of these patients was 62.5 years [95% confidence interval (CI): 60.3, 64.8]. The percentage of females in this population was 39% [95% CI: 36, 40]. The quality of the included studies was moderate to high in general (Table S2).

A flowchart of study screening and selection process.
The average median follow-up duration in these studies was 25.6 months [95% CI: 20.4, 30.8]. Of all patients, 41.3% [95% CI: 40.6, 40.0] had prior systemic treatment for melanoma. The percentage of patients with BRAF mutation was 33% [95% CI: 25, 41], whereas 42.7% [95% CI: 34.8, 50.5] patients were PD-L1 positive. Percentages of patients with M0, M1a, M1b, and M1c TNM stages were 5.0% [95% CI: 4.2, 5.8], 10.5% [95% CI: 9.0, 12.0], 17.9% [95% CI: 15.4, 20.4], and 69.4% [95% CI: 66.3, 72.6], respectively. Among these patients, 21.8% [95% CI: 19.9, 23.8] had metastases in the brain. Percentages of patients with ECOG PS 0 and ECOG PS 1 were 65.6% [95% CI: 60.1, 71.1] and 28.7% [95% CI: 26.1, 31.3], respectively.
3.1 Response rate
Response was achieved in 12.1 weeks [95% CI: 12.0, 12.2], and the response duration was not reached within the follow-up durations of most studies. The overall ORR was 34.2% [95% CI: 30.4, 38.0]. However, ORR differed with respect to the history of prior systemic therapy. The ORR was substantially lower in studies with over 50% patients with prior systemic therapy (25.5% [95% CI: 22.4, 28.5]) than in studies with less than 50% patients with prior systemic therapy (40.1% [95% CI: 34.1, 46.1; Figure 2a). The ORR was higher for pembrolizumab monotherapy (32.9% [95% CI: 28.1, 37.7]) than for pembrolizumab–ipilimumab combination (27.6% [95% CI: 24.0, 31.2]; Figure 2b).

(a) A forest graph showing the pooled effect sizes of ORR (ES; effect size with 95% CI) with regards to the percentage of patients with prior therapy. (b) A forest graph showing the pooled effect sizes of ORR (ES; effect size with 95% CI) with regards to the combination of pembrolizumab treatment.
The complete remission (CR) and partial remission (PR) rates were also lower in studies with over 50% patients with prior systemic therapy than in studies with less than 50% patients with prior systemic therapy (Figures S1a and b). The CR rate was slightly higher for pembrolizumab monotherapy than pembrolizumab–ipilimumab combination (Figure S2a), whereas the PR rate was similar for pembrolizumab monotherapy and pembrolizumab–ipilimumab combination (Figure S2b). The stable disease (SD) and progressive disease (PD) rates were higher in studies with over 50% patients with prior systemic therapy than in studies with less than 50% patients with prior systemic therapy (Figures S3a and b). The SD rate was lower with pembrolizumab monotherapy than with pembrolizumab–ipilimumab combination (Figure S4a), whereas the PD rate was slightly higher with pembrolizumab monotherapy than with pembrolizumab–ipilimumab combination (Figure S4b).
3.2 Factors affecting the objective response rate
In the meta-regression analyses, independently, the overall ORR was significantly positively associated with the percentage of patients with TNM M1a stage, TNM M1b stage, and ECOG PS 0 but was significantly inversely associated with the percentage of patients with TNM M1c stage, high LDH levels, and prior systemic therapy (Table 1). In multivariate metaregression analyses with TNM M1c, high LDH levels, and prior therapy as covariates, only TNM M1c was significantly inversely associated with the overall ORR. Moreover, in multivariate analyses with TNM M1a, TNM M1b, and ECOG PS 0 as covariates, only TNM M1b was significantly positively associated with the overall ORR.
Independent relationships of ORR with explanatory variables
| Explanatory variable | Metaregression coefficient [95% CI] | P | Datasets |
|---|---|---|---|
| Age (years) | 0.87 [−0.641, 2.390] | = 0.246 | 29 |
| Females (%) | −0.241 [−1.078, 0.597] | = 0.561 | 32 |
| Follow-up duration (months) | −0.037 [−0.735, 0.661] | = 0.914 | 29 |
| % patients with TNM M0 | 0.018 [−0.276, 0.314] | = 0.892 | 14 |
| % patients with TNM M1a | 1.201 [0.464, 1.938] | = 0.004 | 14 |
| % patients with TNM M1b | 1.259 [0.414, 2.104] | = 0.007 | 14 |
| % patients with TNM M1c | −0.857 [−1.251, −0.463] | <0.00001 | 19 |
| % patients with brain metastasis | 0.043 [−0.219, 0.306] | = 0.736 | 23 |
| % patients with 0 ECOG PS | 0.596 [0.133, 1.060] | = 0.014 | 23 |
| % patients with 1 ECOG PS | −0.180 [−0.969, 0.610] | = 0.644 | 28 |
| % patients with BRAF mutations | −0.110 [−0.565, 0.345] | = 0.622 | 26 |
| % patients with high LDH levels | −0.355 [−0.777, 0.066] | = 0.094 | 26 |
| % patients with PD-L1 positivity | 0.119 [−0.137, 0.375] | = 0.326 | 12 |
| % patients with prior therapy | −0.240 [−0.392, −0.088] | = 0.003 | 32 |
3.3 Survival
The PFS of melanoma patients treated with pembrolizumab either alone or in combination with other therapies was 5.73 months [95% CI: 4.72, 6.74]. However, it was lower in studies with over 50% patients with prior therapy (3.92 months [95% CI: 2.83, 5.01]) than in studies with under 50% patients with prior therapy (6.95 months [95% CI: 5.34, 8.55]; Figure 3). The 12-, 24-, and 60-month PFS rates of patients treated with pembrolizumab either alone or in combination with other therapies were 44.22% [95% CI: 37.56, 50.89], 27.45% [95% CI: 21.98, 32.93], and 24.92% [95% CI: 22.69, 27.16], respectively (Figure 4).

A forest graph showing the pooled PFS (ES; effect size with 95% CI) with regards to the percentage of patients with prior therapy.

A forest graph showing the pooled 12-, 24-, and 60-month PFS (ES; effect size with 95% CI).
The OS was not achieved within the follow-up durations of many studies. For the remaining of the studies (n = 6), the OS was 20.16 months [95% CI: 16.04, 24.27], which was lower in studies with over 50% patients with prior systemic therapy (15.15 months [95% CI: 11.97, 18.34]) than in studies with under 50% patients with prior systemic therapy (25.58 months [95% CI: 19.23, 31.92]; Figure S5). The 12-, 24-, and 60-month OS rates of patients treated with pembrolizumab either alone or in combination with other therapies were 64.57% [95% CI: 60.11, 69.03], 50.24% [95% CI: 42.90, 57.59], and 40.90% [95% CI: 37.76, 44.03], respectively (Figure 5).
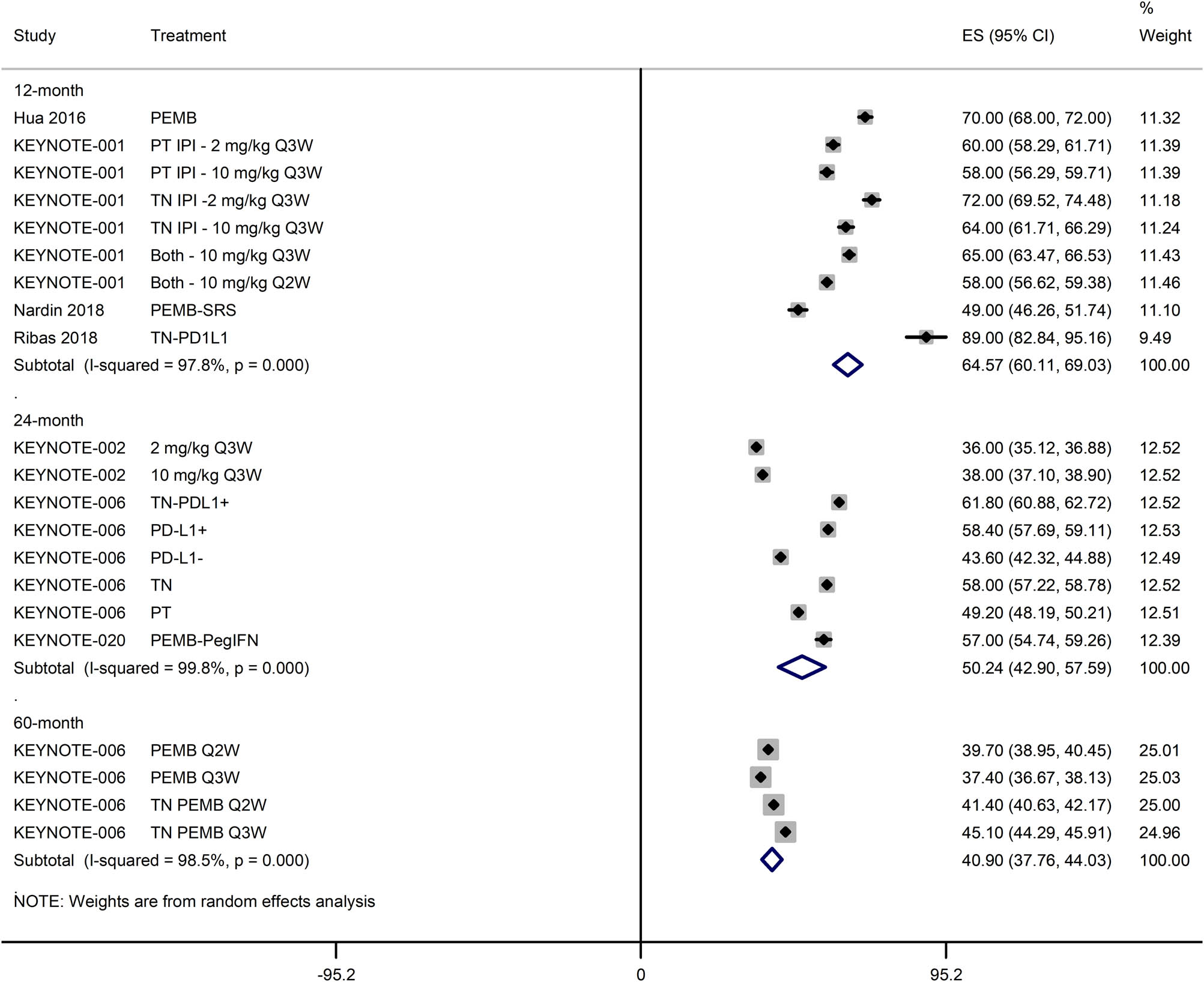
A forest graph showing the pooled 12-, 24-, and 60-month OS (ES; effect size with 95% CI).
3.4 Safety analysis
Major adverse events observed by the included studies are summarized in Table 2. The percentage of AEs that led to discontinuation of treatment was 13.0% [95% CI: 10.5, 15.6]. Fatigue, headache, pruritis, rash, nausea/vomiting, diarrhea, vitiligo, and arthralgia were the most frequent AEs that were observed in two or more studies. AEs reported by less than two studies included abdominal pain, alopecia, asthenia, constipation, dyspnea, eczema, high amylase, high lipase, hypersensitivity, hypoalbunemia, hypocalcemia, hyponatremia, hypophosphatemia, leukopenia, malaise, perilesional edema, seizures, and thrombosis.
Adverse events observed during pembrolizumab treatment reported by the included studies
| Adverse event | Incidence (weighted, averaged percentage) | Number of studies |
|---|---|---|
| AEs leading to discontinuation | 13.02 [10.49, 15.55] | 7 |
| Anemia | 4.79 [3.46, 6.12] | 4 |
| Anorexia | 14.48 [9.43, 19.53] | 3 |
| Arthralgia | 11.28 [9.32, 13.25] | 7 |
| Colitis | 5.16 [3.62, 6.70] | 4 |
| Diarrhea | 15.69 [12.58, 18.80] | 5 |
| Fatigue | 29.92 [23.43, 36.41] | 5 |
| Headache | 29.18 [21.31, 37.04] | 3 |
| High ALT | 7.94 [5.42, 10.46] | 2 |
| High AST | 7.74 [5.82, 9.66] | 2 |
| Hyperthyroidism | 5.66 [1.95, 9.37] | 2 |
| Hypophosphatemia | 5.70 [3.45, 7.96] | 2 |
| Hypothyroidism | 8.42 [5.60, 11.23] | 7 |
| Lymphopenia | 7.55 [5.44, 9.67] | 2 |
| Myalgia | 7.43 [5.74, 9.12] | 4 |
| Nausea/vomiting | 16.36 [12.85, 19.87] | 7 |
| Neutropenia | 3.37 [2.49, 4.25] | 3 |
| Pneumonitis | 2.00 [1.18, 2.82] | 2 |
| Pruritis | 22.91 [19.23, 26.58] | 7 |
| Rash | 18.17 [15.49, 20.85] | 7 |
| Renal | 0.47 [0.21, 0.72] | 3 |
| Thrombocytopenia | 0.42 [0.31, 0.53] | 2 |
| Vitiligo | 11.82 [10.68, 12.97] | 4 |
Abbreviations: AEs, adverse events; ALT, alanine transaminase; AST, aspartate transaminase.
4 Discussion
In this meta-analysis, we have found that the pembrolizumab treatment either alone or in combination with other therapies led to the ORR, PFS, and OS of approximately 34%, 5.7 months, and 20.3 months, respectively, which were lower in previously treated patients than in naïve patients. The overall ORR was higher for pembrolizumab monotherapy than pembrolizumab–ipilimumab combination. Independently, the overall ORR was significantly inversely associated with TNM M1c and the percentage of patients with prior therapy but was positively associated with ECOG PS 0 score. Two-year OS rate of pembrolizumab either alone or in combination with other therapies was approximately 50%.
The immune-checkpoint blockade is a type of passive immunotherapy to enhance innate antitumor response by blocking interactions between T-lymphocytes and neoplasm. Pembrolizumab blocks the interaction between PD1 and PD-L1 to make melanoma cells vulnerable to the T-lymphocyte attack. Because PD-L1 is highly expressed in at least 50% of melanomas, targeting PD1–PDL1 pathway is now foreseen as a promising therapeutic target [35].
A combined therapy with ipilimumab and nivolumab resulted in better response and survival outcomes than their monotherapies; however, this was associated with higher toxicity [36,37]. In the present study, we have found that the response rate of pembrolizumab monotherapy was higher than the response rate of pembrolizumab–ipilimumab combination, which shows that the superiority of pembrolizumab monotherapy over its combinational use with ipilimumab is promising for melanoma patients. KEYNOTE-006 authors have supported the use of pembrolizumab monotherapy as the standard of care for advanced melanoma based on their study findings [19].
Mechanistically, anti-PD1 and anti-CTLA4 monotherapies manifest many distinct effects, which differ also from their combinational use. In vivo studies have shown that CTLA4 blockade leads to T cell proliferation and PD1 blockade induces several genes involved in cytolysis and natural killer cell function [38]. The anti-PD1 activity makes T cells and myeloid-derived suppressor cells more available in tumors. Such a pronounced effect is observed in CD8(+) effector memory T-cell expansion in biopsies of patients who responded to therapy [39].
In a study in which patients were treated with pembrolizumab when they progressed on ipilimumab, ipilimumab PFS was related to pembrolizumab outcomes, so that the patients with prolonged PFS on ipilimumab also had higher response, PFS, and OS rates with pembrolizumab and patients who progressed earlier on ipilimumab also exhibited a worse response to pembrolizumab. The authors suggested that this may indicate the presence of “immune-responsive” and “immune-resistant” phenotypes in melanoma patients, which may require targeting each category separately with appropriate therapies [32]. It has been suggested that trials with longer follow-up are important to determine whether there exists a “plateau effect” in overall survival after pembrolizumab treatment [22]. In the present study, we have noticed that although 24-month and 60-month survival differed more from 12-month survival, the difference was less between 24-month and 60-month survival, which may support the notion of the existence of a “plateau effect” in the survival of melanoma patients after pembrolizumab therapy.
We have found that although TNM M1a and M1b stages were positively associated with the ORR, TNM M1c was inversely associated with the ORR. Because these stages represent metastases in various anatomical sites, i.e., distant skin, subcutaneous, or nodal metastases (M1a), pulmonary metastases (M1b), and visceral metastases (M1c), our results suggest that visceral metastases lead to poor prognosis. However, in the population of the present study, percentages of patients with M0, M1a, M1b, and M1c were 5.0%, 10.5%, 17.9%, and 69.4%, respectively. This imbalanced distribution might have affected the overall analysis.
Some limitations of the present study are important to mention. High statistical heterogeneity in most analyses is an important factor. Variations in designs, combinational use of pembrolizumab, tumor stage, PDL1 +/− status, and prior treatment history across the included studies could have played roles in contributing heterogeneity. Thus, such factors might have caused high I2 values. Another factor was that combinational use could be studied with considerable power only in pembrolizumab–ipilimumab. Thus, the outcomes presented herein are majorly derived from pembrolizumab monotherapy.
In conclusion, a population of advanced/metastatic melanoma patients, of whom 33% had BRAF mutation, 43% were PD-L1 positive, and 41% had prior systemic therapy, were followed up for approximately for 26 months, and pembrolizumab treatment either alone or in combination with other agents led to the ORR, PFS, and OS of approximately 34%, 5.7 months, and 20.3 months, respectively, all of which were higher in treatment in naïve patients. The response rates were higher for pembrolizumab monotherapy than pembrolizumab–ipilimumab combination. Two-year OS rate was approximately 50% in this population. These results suggest that the superiority of pembrolizumab monotherapy over its combinational use with ipilimumab is promising for melanoma patients.
Funding: None.
Conflict of interest: None.
References
[1] Matthews NH, Li WQ, Qureshi AA, Weinstock MA, Cho E. Epidemiology of Melanoma, In: Cutaneous melanoma: etiology and therapy, Ward WH, Farma JM, editors. Brisbane (AU): Codon Publications; 2017.10.15586/codon.cutaneousmelanoma.2017.ch1Suche in Google Scholar PubMed
[2] Situm M, Buljan M, Kolic M, Vucic M. Melanoma—clinical, dermatoscopical, and histopathological morphological characteristics. Acta Dermatovenerol Croat. 2014;22(1):1–12.Suche in Google Scholar
[3] National Institutes of Health. National Cancer Institute. Cancer stat facts: melanoma of the skin. https://seer.cancer.gov/statfacts/html/melan.html. Accessed on September 17, 2019.Suche in Google Scholar
[4] Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In vivo (Athens, Greece). 2014;28(6):1005–11.Suche in Google Scholar
[5] Hegde UP, Chakraborty N, Kerr P, Grant-Kels JM. Melanoma in the elderly patient: relevance of the aging immune system. Clin Dermatol. 2009;27(6):537–44.10.1016/j.clindermatol.2008.09.012Suche in Google Scholar PubMed
[6] Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16.10.1200/JCO.1999.17.7.2105Suche in Google Scholar PubMed
[7] Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37(4):764–82.10.1016/j.clinthera.2015.02.018Suche in Google Scholar PubMed PubMed Central
[8] Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2:Cd011123.10.1002/14651858.CD011123Suche in Google Scholar
[9] du Rusquec P, de Calbiac O, Robert M, Campone M, Frenel JS. Clinical utility of pembrolizumab in the management of advanced solid tumors: an evidence-based review on the emerging new data. Cancer Manag Res. 2019;11:4297–312.10.2147/CMAR.S151023Suche in Google Scholar PubMed PubMed Central
[10] Anderson ES, Postow MA, Wolchok JD, Young RJ, Ballangrud A, Chan TA, et al. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer. 2017;5(1):76.10.1186/s40425-017-0282-xSuche in Google Scholar PubMed PubMed Central
[11] Du Four S, Janssen Y, Michotte A, Van Binst AM, Van den Begin R, Duerinck J, et al. Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med. 2018;7(10):4870–9.10.1002/cam4.1726Suche in Google Scholar PubMed PubMed Central
[12] Heppt MV, Heinzerling L, Kahler KC, Forschner A, Kirchberger MC, Loquai C, et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer. 2017;82:56–65.10.1016/j.ejca.2017.05.038Suche in Google Scholar PubMed
[13] Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51.10.1001/jamadermatol.2015.2707Suche in Google Scholar PubMed
[14] Hwang SJ, Carlos G, Wakade D, Byth K, Kong BY, Chou S, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74(3):455–61.e1.10.1016/j.jaad.2015.10.029Suche in Google Scholar
[15] Ibrahim T, Mateus C, Baz M, Robert C. Older melanoma patients aged 75 and above retain responsiveness to anti-PD1 therapy: results of a retrospective single-institution cohort study. Cancer Immunol Immunother. 2018;67(10):1571–8.10.1007/s00262-018-2219-8Suche in Google Scholar
[16] Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA. 2016;315(15):1600–9.10.1001/jama.2016.4059Suche in Google Scholar
[17] Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37–45.10.1016/j.ejca.2017.07.022Suche in Google Scholar
[18] Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–18.10.1016/S1470-2045(15)00083-2Suche in Google Scholar
[19] Carlino MS, Long GV, Schadendorf D, Robert C, Ribas A, Richtig E, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: A randomised clinical trial. Eur J Cancer. 2018;101:236–43.10.1016/j.ejca.2018.06.034Suche in Google Scholar
[20] Petrella TM, Robert C, Richtig E, Miller Jr. WH, Masucci GV, Walpole E, et al. Patient-reported outcomes in KEYNOTE-006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur J Cancer. 86, 2017:115–24.10.1016/j.ejca.2017.08.032Suche in Google Scholar
[21] Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32.10.1056/NEJMoa1503093Suche in Google Scholar
[22] Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–51.10.1016/S1470-2045(19)30388-2Suche in Google Scholar
[23] Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–62.10.1016/S0140-6736(17)31601-XSuche in Google Scholar
[24] Davar D, Wang H, Chauvin JM, Pagliano O, Fourcade JJ, Ka M, et al. Phase Ib/II study of pembrolizumab and pegylated-interferon alfa-2b in advanced melanoma. J Clin Oncol. 2018;Jco1800632.10.1200/JCO.18.00632Suche in Google Scholar
[25] Long GV, Atkinson V, Cebon JS, Jameson MB, Fitzharris BM, McNeil CM, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18(9):1202–10.10.1016/S1470-2045(17)30428-XSuche in Google Scholar
[26] Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20(8):1083–97.10.1016/S1470-2045(19)30274-8Suche in Google Scholar
[27] Kirchberger MC, Hauschild A, Schuler G, Heinzerling L. Combined low-dose ipilimumab and pembrolizumab after sequential ipilimumab and pembrolizumab failure in advanced melanoma. Eur J Cancer. 2016;65:182–4.10.1016/j.ejca.2016.07.003Suche in Google Scholar PubMed
[28] Kirchberger MC, Moreira A, Erdmann M, Schuler G, Heinzerling L. Real world experience in low-dose ipilimumab in combination with PD-1 blockade in advanced melanoma patients. Oncotarget. 2018;9(48):28903–9.10.18632/oncotarget.25627Suche in Google Scholar PubMed PubMed Central
[29] Moya-Plana A, Herrera Gomez RG, Rossoni C, Dercle L, Ammari S, Girault I, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immun. 2019;68(7):1171–8.10.1007/s00262-019-02351-7Suche in Google Scholar PubMed
[30] Nardin C, Mateus C, Texier M, Lanoy E, Hibat-Allah S, Ammari S, et al. Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res. 2018;28(2):111–9.10.1097/CMR.0000000000000413Suche in Google Scholar PubMed
[31] Ribas A, Medina T, Kummar S, Amin A, Kalbasi A, Drabick JJ, et al. SD-101 in combination with pembrolizumab in advanced melanoma: results of a phase Ib, multicenter study. Cancer Discov. 2018;8(10):1250–7.10.1158/2159-8290.CD-18-0280Suche in Google Scholar PubMed PubMed Central
[32] Shreders A, Joseph R, Peng C, Ye F, Zhao S, Puzanov I, et al. Prolonged benefit from ipilimumab correlates with improved outcomes from subsequent pembrolizumab. Cancer Immunol Res. 2016;4(7):569–73.10.1158/2326-6066.CIR-15-0281Suche in Google Scholar PubMed PubMed Central
[33] Taquin H, Fontas E, Massol O, Chevallier P, Balloti R, Beranger G, et al. Efficacy and safety data for checkpoint inhibitors in advanced melanoma under real-life conditions: a monocentric study conducted in Nice from 2010 to 2016. Ann Dermatol Venereol. 2018;145(11):649–58.10.1016/j.annder.2018.06.008Suche in Google Scholar PubMed
[34] Wei KZ, Baxter M, Casasola R. Hypophysitis induced by immune checkpoint inhibitors in a Scottish melanoma population. Melanoma Manag. 2019;6:Mmt13.10.2217/mmt-2018-0009Suche in Google Scholar PubMed PubMed Central
[35] Spain L, Younger E, Hatipoglu E, Larkin J. Pembrolizumab in the management of metastatic melanoma. Melanoma Manag. 2015;2(4):315–25.10.2217/mmt.15.33Suche in Google Scholar PubMed PubMed Central
[36] Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.10.1056/NEJMoa1504030Suche in Google Scholar PubMed PubMed Central
[37] Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17.10.1056/NEJMoa1414428Suche in Google Scholar PubMed PubMed Central
[38] Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194(3):950–9.10.4049/jimmunol.1401686Suche in Google Scholar PubMed PubMed Central
[39] Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. 2016;4(3):194–203.10.1158/2326-6066.CIR-15-0210Suche in Google Scholar PubMed PubMed Central
© 2020 Qi Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Article
- MicroRNA-451b participates in coronary heart disease by targeting VEGFA
- Case Report
- A combination therapy for Kawasaki disease with severe complications: a case report
- Vitamin E for prevention of biofilm-caused Healthcare-associated infections
- Research Article
- Differential diagnosis: retroperitoneal fibrosis and oncological diseases
- Optimization of the Convolutional Neural Networks for Automatic Detection of Skin Cancer
- NEAT1 promotes LPS-induced inflammatory injury in macrophages by regulating miR-17-5p/TLR4
- Plasma matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 as prognostic biomarkers in critically ill patients
- Effects of extracorporeal magnetic stimulation in fecal incontinence
- Case Report
- Mixed germ cell tumor of the endometrium: a case report and literature review
- Bowel perforation after ventriculoperitoneal-shunt placement: case report and review of the literature
- Research Article
- Prognostic value of lncRNA HOTAIR in colorectal cancer : a meta-analysis
- Case Report
- Treatment of insulinomas by laparoscopic radiofrequency ablation: case reports and literature review
- Research Article
- The characteristics and nomogram for primary lung papillary adenocarcinoma
- Undiagnosed pheochromocytoma presenting as a pancreatic tumor: A case report
- Bioinformatics Analysis of the Expression of ATP binding cassette subfamily C member 3 (ABCC3) in Human Glioma
- Diagnostic value of recombinant heparin-binding hemagglutinin adhesin protein in spinal tuberculosis
- Primary cutaneous DLBCL non-GCB type: challenges of a rare case
- LINC00152 knock-down suppresses esophageal cancer by EGFR signaling pathway
- Case Report
- Life-threatening anaemia in patient with hereditary haemorrhagic telangiectasia (Rendu-Osler-Weber syndrome)
- Research Article
- QTc interval predicts disturbed circadian blood pressure variation
- Shoulder ultrasound in the diagnosis of the suprascapular neuropathy in athletes
- The number of negative lymph nodes is positively associated with survival in esophageal squamous cell carcinoma patients in China
- Differentiation of pontine infarction by size
- RAF1 expression is correlated with HAF, a parameter of liver computed tomographic perfusion, and may predict the early therapeutic response to sorafenib in advanced hepatocellular carcinoma patients
- LncRNA ZEB1-AS1 regulates colorectal cancer cells by miR-205/YAP1 axis
- Tissue coagulation in laser hemorrhoidoplasty – an experimental study
- Classification of pathological types of lung cancer from CT images by deep residual neural networks with transfer learning strategy
- Enhanced Recovery after Surgery for Lung Cancer Patients
- Case Report
- Streptococcus pneumoniae-associated thrombotic microangiopathy in an immunosuppressed adult
- Research Article
- The characterization of Enterococcus genus: resistance mechanisms and inflammatory bowel disease
- Case Report
- Inflammatory fibroid polyp: an unusual cause of abdominal pain in the upper gastrointestinal tract A case report
- Research Article
- microRNA-204-5p participates in atherosclerosis via targeting MMP-9
- LncRNA LINC00152 promotes laryngeal cancer progression by sponging miR-613
- Can keratin scaffolds be used for creating three-dimensional cell cultures?
- miRNA-186 improves sepsis induced renal injury via PTEN/PI3K/AKT/P53 pathway
- Case Report
- Delayed bowel perforation after routine distal loopogram prior to ileostomy closure
- Research Article
- Diagnostic accuracy of MALDI-TOF mass spectrometry for the direct identification of clinical pathogens from urine
- The R219K polymorphism of the ATP binding cassette subfamily A member 1 gene and susceptibility to ischemic stroke in Chinese population
- miR-92 regulates the proliferation, migration, invasion and apoptosis of glioma cells by targeting neogenin
- Clinicopathological features of programmed cell death-ligand 1 expression in patients with oral squamous cell carcinoma
- NF2 inhibits proliferation and cancer stemness in breast cancer
- Body composition indices and cardiovascular risk in type 2 diabetes. CV biomarkers are not related to body composition
- S100A6 promotes proliferation and migration of HepG2 cells via increased ubiquitin-dependent degradation of p53
- Review Article
- Focus on localized laryngeal amyloidosis: management of five cases
- Research Article
- NEAT1 aggravates sepsis-induced acute kidney injury by sponging miR-22-3p
- Pericentric inversion in chromosome 1 and male infertility
- Increased atherogenic index in the general hearing loss population
- Prognostic role of SIRT6 in gastrointestinal cancers: a meta-analysis
- The complexity of molecular processes in osteoarthritis of the knee joint
- Interleukin-6 gene −572 G > C polymorphism and myocardial infarction risk
- Case Report
- Severe anaphylactic reaction to cisatracurium during anesthesia with cross-reactivity to atracurium
- Research Article
- Rehabilitation training improves nerve injuries by affecting Notch1 and SYN
- Case Report
- Myocardial amyloidosis following multiple myeloma in a 38-year-old female patient: A case report
- Research Article
- Identification of the hub genes RUNX2 and FN1 in gastric cancer
- miR-101-3p sensitizes non-small cell lung cancer cells to irradiation
- Distinct functions and prognostic values of RORs in gastric cancer
- Clinical impact of post-mortem genetic testing in cardiac death and cardiomyopathy
- Efficacy of pembrolizumab for advanced/metastatic melanoma: a meta-analysis
- Review Article
- The role of osteoprotegerin in the development, progression and management of abdominal aortic aneurysms
- Research Article
- Identification of key microRNAs of plasma extracellular vesicles and their diagnostic and prognostic significance in melanoma
- miR-30a-3p participates in the development of asthma by targeting CCR3
- microRNA-491-5p protects against atherosclerosis by targeting matrix metallopeptidase-9
- Bladder-embedded ectopic intrauterine device with calculus
- Case Report
- Mycobacterial identification on homogenised biopsy facilitates the early diagnosis and treatment of laryngeal tuberculosis
- Research Article
- The will of young minors in the terminal stage of sickness: A case report
- Extended perfusion protocol for MS lesion quantification
- Identification of four genes associated with cutaneous metastatic melanoma
- Case Report
- Thalidomide-induced serious RR interval prolongation (longest interval >5.0 s) in multiple myeloma patient with rectal cancer: A case report
- Research Article
- Voluntary exercise and cardiac remodeling in a myocardial infarction model
- Electromyography as an intraoperative test to assess the quality of nerve anastomosis – experimental study on rats
- Case Report
- CT findings of severe novel coronavirus disease (COVID-19): A case report of Heilongjiang Province, China
- Commentary
- Directed differentiation into insulin-producing cells using microRNA manipulation
- Research Article
- Culture-negative infective endocarditis (CNIE): impact on postoperative mortality
- Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome
- Plasma microRNAs in human left ventricular reverse remodelling
- Bevacizumab for non-small cell lung cancer patients with brain metastasis: A meta-analysis
- Risk factors for cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage
- Problems and solutions of personal protective equipment doffing in COVID-19
- Evaluation of COVID-19 based on ACE2 expression in normal and cancer patients
- Review Article
- Gastroenterological complications in kidney transplant patients
- Research Article
- CXCL13 concentration in latent syphilis patients with treatment failure
- A novel age-biomarker-clinical history prognostic index for heart failure with reduced left ventricular ejection fraction
- Case Report
- Clinicopathological analysis of composite lymphoma: A two-case report and literature review
- Trastuzumab-induced thrombocytopenia after eight cycles of trastuzumab treatment
- Research Article
- Inhibition of vitamin D analog eldecalcitol on hepatoma in vitro and in vivo
- CCTs as new biomarkers for the prognosis of head and neck squamous cancer
- Effect of glucagon-like peptide-1 receptor agonists on adipokine level of nonalcoholic fatty liver disease in rats fed high-fat diet
- 72 hour Holter monitoring, 7 day Holter monitoring, and 30 day intermittent patient-activated heart rhythm recording in detecting arrhythmias in cryptogenic stroke patients free from arrhythmia in a screening 24 h Holter
- FOXK2 downregulation suppresses EMT in hepatocellular carcinoma
- Case Report
- Total parenteral nutrition-induced Wernicke’s encephalopathy after oncologic gastrointestinal surgery
- Research Article
- Clinical prediction for outcomes of patients with acute-on-chronic liver failure associated with HBV infection: A new model establishment
- Case Report
- Combination of chest CT and clinical features for diagnosis of 2019 novel coronavirus pneumonia
- Research Article
- Clinical significance and potential mechanisms of miR-223-3p and miR-204-5p in squamous cell carcinoma of head and neck: a study based on TCGA and GEO
- Review Article
- Hemoperitoneum caused by spontaneous rupture of hepatocellular carcinoma in noncirrhotic liver. A case report and systematic review
- Research Article
- Voltage-dependent anion channels mediated apoptosis in refractory epilepsy
- Prognostic factors in stage I gastric cancer: A retrospective analysis
- Circulating irisin is linked to bone mineral density in geriatric Chinese men
- Case Report
- A family study of congenital dysfibrinogenemia caused by a novel mutation in the FGA gene: A case report
- Research Article
- CBCT for estimation of the cemento-enamel junction and crestal bone of anterior teeth
- Case Report
- Successful de-escalation antibiotic therapy using cephamycins for sepsis caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae bacteremia: A sequential 25-case series
- Research Article
- Influence factors of extra-articular manifestations in rheumatoid arthritis
- Assessment of knowledge of use of electronic cigarette and its harmful effects among young adults
- Predictive factors of progression to severe COVID-19
- Procedural sedation and analgesia for percutaneous trans-hepatic biliary drainage: Randomized clinical trial for comparison of two different concepts
- Acute chemoradiotherapy toxicity in cervical cancer patients
- IGF-1 regulates the growth of fibroblasts and extracellular matrix deposition in pelvic organ prolapse
- NANOG regulates the proliferation of PCSCs via the TGF-β1/SMAD pathway
- An immune-relevant signature of nine genes as a prognostic biomarker in patients with gastric carcinoma
- Computer-aided diagnosis of skin cancer based on soft computing techniques
- MiR-1225-5p acts as tumor suppressor in glioblastoma via targeting FNDC3B
- miR-300/FA2H affects gastric cancer cell proliferation and apoptosis
- Hybrid treatment of fibroadipose vascular anomaly: A case report
- Surgical treatment for common hepatic aneurysm. Original one-step technique
- Neuropsychiatric symptoms, quality of life and caregivers’ burden in dementia
- Predictor of postoperative dyspnea for Pierre Robin Sequence infants
- Long non-coding RNA FOXD2-AS1 promotes cell proliferation, metastasis and EMT in glioma by sponging miR-506-5p
- Analysis of expression and prognosis of KLK7 in ovarian cancer
- Circular RNA circ_SETD2 represses breast cancer progression via modulating the miR-155-5p/SCUBE2 axis
- Glial cell induced neural differentiation of bone marrow stromal cells
- Case Report
- Moraxella lacunata infection accompanied by acute glomerulonephritis
- Research Article
- Diagnosis of complication in lung transplantation by TBLB + ROSE + mNGS
- Case Report
- Endometrial cancer in a renal transplant recipient: A case report
- Research Article
- Downregulation of lncRNA FGF12-AS2 suppresses the tumorigenesis of NSCLC via sponging miR-188-3p
- Case Report
- Splenic abscess caused by Streptococcus anginosus bacteremia secondary to urinary tract infection: a case report and literature review
- Research Article
- Advances in the role of miRNAs in the occurrence and development of osteosarcoma
- Rheumatoid arthritis increases the risk of pleural empyema
- Effect of miRNA-200b on the proliferation and apoptosis of cervical cancer cells by targeting RhoA
- LncRNA NEAT1 promotes gastric cancer progression via miR-1294/AKT1 axis
- Key pathways in prostate cancer with SPOP mutation identified by bioinformatic analysis
- Comparison of low-molecular-weight heparins in thromboprophylaxis of major orthopaedic surgery – randomized, prospective pilot study
- Case Report
- A case of SLE with COVID-19 and multiple infections
- Research Article
- Circular RNA hsa_circ_0007121 regulates proliferation, migration, invasion, and epithelial–mesenchymal transition of trophoblast cells by miR-182-5p/PGF axis in preeclampsia
- SRPX2 boosts pancreatic cancer chemoresistance by activating PI3K/AKT axis
- Case Report
- A case report of cervical pregnancy after in vitro fertilization complicated by tuberculosis and a literature review
- Review Article
- Serrated lesions of the colon and rectum: Emergent epidemiological data and molecular pathways
- Research Article
- Biological properties and therapeutic effects of plant-derived nanovesicles
- Case Report
- Clinical characterization of chromosome 5q21.1–21.3 microduplication: A case report
- Research Article
- Serum calcium levels correlates with coronary artery disease outcomes
- Rapunzel syndrome with cholangitis and pancreatitis – A rare case report
- Review Article
- A review of current progress in triple-negative breast cancer therapy
- Case Report
- Peritoneal-cutaneous fistula successfully treated at home: A case report and literature review
- Research Article
- Trim24 prompts tumor progression via inducing EMT in renal cell carcinoma
- Degradation of connexin 50 protein causes waterclefts in human lens
- GABRD promotes progression and predicts poor prognosis in colorectal cancer
- The lncRNA UBE2R2-AS1 suppresses cervical cancer cell growth in vitro
- LncRNA FOXD3-AS1/miR-135a-5p function in nasopharyngeal carcinoma cells
- MicroRNA-182-5p relieves murine allergic rhinitis via TLR4/NF-κB pathway
Artikel in diesem Heft
- Research Article
- MicroRNA-451b participates in coronary heart disease by targeting VEGFA
- Case Report
- A combination therapy for Kawasaki disease with severe complications: a case report
- Vitamin E for prevention of biofilm-caused Healthcare-associated infections
- Research Article
- Differential diagnosis: retroperitoneal fibrosis and oncological diseases
- Optimization of the Convolutional Neural Networks for Automatic Detection of Skin Cancer
- NEAT1 promotes LPS-induced inflammatory injury in macrophages by regulating miR-17-5p/TLR4
- Plasma matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 as prognostic biomarkers in critically ill patients
- Effects of extracorporeal magnetic stimulation in fecal incontinence
- Case Report
- Mixed germ cell tumor of the endometrium: a case report and literature review
- Bowel perforation after ventriculoperitoneal-shunt placement: case report and review of the literature
- Research Article
- Prognostic value of lncRNA HOTAIR in colorectal cancer : a meta-analysis
- Case Report
- Treatment of insulinomas by laparoscopic radiofrequency ablation: case reports and literature review
- Research Article
- The characteristics and nomogram for primary lung papillary adenocarcinoma
- Undiagnosed pheochromocytoma presenting as a pancreatic tumor: A case report
- Bioinformatics Analysis of the Expression of ATP binding cassette subfamily C member 3 (ABCC3) in Human Glioma
- Diagnostic value of recombinant heparin-binding hemagglutinin adhesin protein in spinal tuberculosis
- Primary cutaneous DLBCL non-GCB type: challenges of a rare case
- LINC00152 knock-down suppresses esophageal cancer by EGFR signaling pathway
- Case Report
- Life-threatening anaemia in patient with hereditary haemorrhagic telangiectasia (Rendu-Osler-Weber syndrome)
- Research Article
- QTc interval predicts disturbed circadian blood pressure variation
- Shoulder ultrasound in the diagnosis of the suprascapular neuropathy in athletes
- The number of negative lymph nodes is positively associated with survival in esophageal squamous cell carcinoma patients in China
- Differentiation of pontine infarction by size
- RAF1 expression is correlated with HAF, a parameter of liver computed tomographic perfusion, and may predict the early therapeutic response to sorafenib in advanced hepatocellular carcinoma patients
- LncRNA ZEB1-AS1 regulates colorectal cancer cells by miR-205/YAP1 axis
- Tissue coagulation in laser hemorrhoidoplasty – an experimental study
- Classification of pathological types of lung cancer from CT images by deep residual neural networks with transfer learning strategy
- Enhanced Recovery after Surgery for Lung Cancer Patients
- Case Report
- Streptococcus pneumoniae-associated thrombotic microangiopathy in an immunosuppressed adult
- Research Article
- The characterization of Enterococcus genus: resistance mechanisms and inflammatory bowel disease
- Case Report
- Inflammatory fibroid polyp: an unusual cause of abdominal pain in the upper gastrointestinal tract A case report
- Research Article
- microRNA-204-5p participates in atherosclerosis via targeting MMP-9
- LncRNA LINC00152 promotes laryngeal cancer progression by sponging miR-613
- Can keratin scaffolds be used for creating three-dimensional cell cultures?
- miRNA-186 improves sepsis induced renal injury via PTEN/PI3K/AKT/P53 pathway
- Case Report
- Delayed bowel perforation after routine distal loopogram prior to ileostomy closure
- Research Article
- Diagnostic accuracy of MALDI-TOF mass spectrometry for the direct identification of clinical pathogens from urine
- The R219K polymorphism of the ATP binding cassette subfamily A member 1 gene and susceptibility to ischemic stroke in Chinese population
- miR-92 regulates the proliferation, migration, invasion and apoptosis of glioma cells by targeting neogenin
- Clinicopathological features of programmed cell death-ligand 1 expression in patients with oral squamous cell carcinoma
- NF2 inhibits proliferation and cancer stemness in breast cancer
- Body composition indices and cardiovascular risk in type 2 diabetes. CV biomarkers are not related to body composition
- S100A6 promotes proliferation and migration of HepG2 cells via increased ubiquitin-dependent degradation of p53
- Review Article
- Focus on localized laryngeal amyloidosis: management of five cases
- Research Article
- NEAT1 aggravates sepsis-induced acute kidney injury by sponging miR-22-3p
- Pericentric inversion in chromosome 1 and male infertility
- Increased atherogenic index in the general hearing loss population
- Prognostic role of SIRT6 in gastrointestinal cancers: a meta-analysis
- The complexity of molecular processes in osteoarthritis of the knee joint
- Interleukin-6 gene −572 G > C polymorphism and myocardial infarction risk
- Case Report
- Severe anaphylactic reaction to cisatracurium during anesthesia with cross-reactivity to atracurium
- Research Article
- Rehabilitation training improves nerve injuries by affecting Notch1 and SYN
- Case Report
- Myocardial amyloidosis following multiple myeloma in a 38-year-old female patient: A case report
- Research Article
- Identification of the hub genes RUNX2 and FN1 in gastric cancer
- miR-101-3p sensitizes non-small cell lung cancer cells to irradiation
- Distinct functions and prognostic values of RORs in gastric cancer
- Clinical impact of post-mortem genetic testing in cardiac death and cardiomyopathy
- Efficacy of pembrolizumab for advanced/metastatic melanoma: a meta-analysis
- Review Article
- The role of osteoprotegerin in the development, progression and management of abdominal aortic aneurysms
- Research Article
- Identification of key microRNAs of plasma extracellular vesicles and their diagnostic and prognostic significance in melanoma
- miR-30a-3p participates in the development of asthma by targeting CCR3
- microRNA-491-5p protects against atherosclerosis by targeting matrix metallopeptidase-9
- Bladder-embedded ectopic intrauterine device with calculus
- Case Report
- Mycobacterial identification on homogenised biopsy facilitates the early diagnosis and treatment of laryngeal tuberculosis
- Research Article
- The will of young minors in the terminal stage of sickness: A case report
- Extended perfusion protocol for MS lesion quantification
- Identification of four genes associated with cutaneous metastatic melanoma
- Case Report
- Thalidomide-induced serious RR interval prolongation (longest interval >5.0 s) in multiple myeloma patient with rectal cancer: A case report
- Research Article
- Voluntary exercise and cardiac remodeling in a myocardial infarction model
- Electromyography as an intraoperative test to assess the quality of nerve anastomosis – experimental study on rats
- Case Report
- CT findings of severe novel coronavirus disease (COVID-19): A case report of Heilongjiang Province, China
- Commentary
- Directed differentiation into insulin-producing cells using microRNA manipulation
- Research Article
- Culture-negative infective endocarditis (CNIE): impact on postoperative mortality
- Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome
- Plasma microRNAs in human left ventricular reverse remodelling
- Bevacizumab for non-small cell lung cancer patients with brain metastasis: A meta-analysis
- Risk factors for cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage
- Problems and solutions of personal protective equipment doffing in COVID-19
- Evaluation of COVID-19 based on ACE2 expression in normal and cancer patients
- Review Article
- Gastroenterological complications in kidney transplant patients
- Research Article
- CXCL13 concentration in latent syphilis patients with treatment failure
- A novel age-biomarker-clinical history prognostic index for heart failure with reduced left ventricular ejection fraction
- Case Report
- Clinicopathological analysis of composite lymphoma: A two-case report and literature review
- Trastuzumab-induced thrombocytopenia after eight cycles of trastuzumab treatment
- Research Article
- Inhibition of vitamin D analog eldecalcitol on hepatoma in vitro and in vivo
- CCTs as new biomarkers for the prognosis of head and neck squamous cancer
- Effect of glucagon-like peptide-1 receptor agonists on adipokine level of nonalcoholic fatty liver disease in rats fed high-fat diet
- 72 hour Holter monitoring, 7 day Holter monitoring, and 30 day intermittent patient-activated heart rhythm recording in detecting arrhythmias in cryptogenic stroke patients free from arrhythmia in a screening 24 h Holter
- FOXK2 downregulation suppresses EMT in hepatocellular carcinoma
- Case Report
- Total parenteral nutrition-induced Wernicke’s encephalopathy after oncologic gastrointestinal surgery
- Research Article
- Clinical prediction for outcomes of patients with acute-on-chronic liver failure associated with HBV infection: A new model establishment
- Case Report
- Combination of chest CT and clinical features for diagnosis of 2019 novel coronavirus pneumonia
- Research Article
- Clinical significance and potential mechanisms of miR-223-3p and miR-204-5p in squamous cell carcinoma of head and neck: a study based on TCGA and GEO
- Review Article
- Hemoperitoneum caused by spontaneous rupture of hepatocellular carcinoma in noncirrhotic liver. A case report and systematic review
- Research Article
- Voltage-dependent anion channels mediated apoptosis in refractory epilepsy
- Prognostic factors in stage I gastric cancer: A retrospective analysis
- Circulating irisin is linked to bone mineral density in geriatric Chinese men
- Case Report
- A family study of congenital dysfibrinogenemia caused by a novel mutation in the FGA gene: A case report
- Research Article
- CBCT for estimation of the cemento-enamel junction and crestal bone of anterior teeth
- Case Report
- Successful de-escalation antibiotic therapy using cephamycins for sepsis caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae bacteremia: A sequential 25-case series
- Research Article
- Influence factors of extra-articular manifestations in rheumatoid arthritis
- Assessment of knowledge of use of electronic cigarette and its harmful effects among young adults
- Predictive factors of progression to severe COVID-19
- Procedural sedation and analgesia for percutaneous trans-hepatic biliary drainage: Randomized clinical trial for comparison of two different concepts
- Acute chemoradiotherapy toxicity in cervical cancer patients
- IGF-1 regulates the growth of fibroblasts and extracellular matrix deposition in pelvic organ prolapse
- NANOG regulates the proliferation of PCSCs via the TGF-β1/SMAD pathway
- An immune-relevant signature of nine genes as a prognostic biomarker in patients with gastric carcinoma
- Computer-aided diagnosis of skin cancer based on soft computing techniques
- MiR-1225-5p acts as tumor suppressor in glioblastoma via targeting FNDC3B
- miR-300/FA2H affects gastric cancer cell proliferation and apoptosis
- Hybrid treatment of fibroadipose vascular anomaly: A case report
- Surgical treatment for common hepatic aneurysm. Original one-step technique
- Neuropsychiatric symptoms, quality of life and caregivers’ burden in dementia
- Predictor of postoperative dyspnea for Pierre Robin Sequence infants
- Long non-coding RNA FOXD2-AS1 promotes cell proliferation, metastasis and EMT in glioma by sponging miR-506-5p
- Analysis of expression and prognosis of KLK7 in ovarian cancer
- Circular RNA circ_SETD2 represses breast cancer progression via modulating the miR-155-5p/SCUBE2 axis
- Glial cell induced neural differentiation of bone marrow stromal cells
- Case Report
- Moraxella lacunata infection accompanied by acute glomerulonephritis
- Research Article
- Diagnosis of complication in lung transplantation by TBLB + ROSE + mNGS
- Case Report
- Endometrial cancer in a renal transplant recipient: A case report
- Research Article
- Downregulation of lncRNA FGF12-AS2 suppresses the tumorigenesis of NSCLC via sponging miR-188-3p
- Case Report
- Splenic abscess caused by Streptococcus anginosus bacteremia secondary to urinary tract infection: a case report and literature review
- Research Article
- Advances in the role of miRNAs in the occurrence and development of osteosarcoma
- Rheumatoid arthritis increases the risk of pleural empyema
- Effect of miRNA-200b on the proliferation and apoptosis of cervical cancer cells by targeting RhoA
- LncRNA NEAT1 promotes gastric cancer progression via miR-1294/AKT1 axis
- Key pathways in prostate cancer with SPOP mutation identified by bioinformatic analysis
- Comparison of low-molecular-weight heparins in thromboprophylaxis of major orthopaedic surgery – randomized, prospective pilot study
- Case Report
- A case of SLE with COVID-19 and multiple infections
- Research Article
- Circular RNA hsa_circ_0007121 regulates proliferation, migration, invasion, and epithelial–mesenchymal transition of trophoblast cells by miR-182-5p/PGF axis in preeclampsia
- SRPX2 boosts pancreatic cancer chemoresistance by activating PI3K/AKT axis
- Case Report
- A case report of cervical pregnancy after in vitro fertilization complicated by tuberculosis and a literature review
- Review Article
- Serrated lesions of the colon and rectum: Emergent epidemiological data and molecular pathways
- Research Article
- Biological properties and therapeutic effects of plant-derived nanovesicles
- Case Report
- Clinical characterization of chromosome 5q21.1–21.3 microduplication: A case report
- Research Article
- Serum calcium levels correlates with coronary artery disease outcomes
- Rapunzel syndrome with cholangitis and pancreatitis – A rare case report
- Review Article
- A review of current progress in triple-negative breast cancer therapy
- Case Report
- Peritoneal-cutaneous fistula successfully treated at home: A case report and literature review
- Research Article
- Trim24 prompts tumor progression via inducing EMT in renal cell carcinoma
- Degradation of connexin 50 protein causes waterclefts in human lens
- GABRD promotes progression and predicts poor prognosis in colorectal cancer
- The lncRNA UBE2R2-AS1 suppresses cervical cancer cell growth in vitro
- LncRNA FOXD3-AS1/miR-135a-5p function in nasopharyngeal carcinoma cells
- MicroRNA-182-5p relieves murine allergic rhinitis via TLR4/NF-κB pathway

