Abstract
In this work, the influence of pulse frequency on the corrosion resistance of Cu–Zn binary alloy has been investigated by using electrochemical workstation, scanning electron microscope and X-ray diffraction. The results have shown that after the electric pulse treatment, the average grain size in the microstructure of high-zinc binary brass decreased and the corrosion resistance increased. The thickness of dezincification layer decreased from 49.8 to 30.8 µm. The diameter of capacitive reactance arc increased from 741.1 to 2898.0 µm. The corrosion potential increased from −0.2719 to −0.2378 V, and the corrosion current density decreased from 6.3147 × 10−6 to 4.6971 × 10−7 A cm−2 by one magnitude order.
1 Introduction
Due to the excellent cold and hot workability, cutting performance, conductivity and thermal conductivity, brass has been widely used in automobile production, aircraft manufacturing and electric component [1,2,3]. The service life of workpieces has been reduced by the dezincification of brass especially in the aqueous solution with the gas of O2, CO2, H2S, SO2 and NH3. It has attracted much attention from the researchers in the world on how to further eliminate the dezincification and to improve the service life of workpieces [4,5,6]. Literature have expressed that the corrosion resistance of brass can be improved by the trace elements of Boron and Arsenic. But the solid solubility of B in brass has been restricted, and excessive B has precipitated and concentrated distribution as the form of boride. On the other hand, due to the corrosion, brass resistance reduced, workpieces failed prematurely and the environment could be polluted by the element of As [7,8].
The electric pulse treatment (EPT) has the characteristics of simple equipment, easy to operate and no pollution to the environment. The progress of metal solidification could be controlled and the solidification structure of metals could be refined by EPT; therefore, the comprehensive performance of alloys improved [9,10]. The literature [11] has expressed that after EPT, the corrosion potential of silicon brass increased, the corrosion current density decreased and the corrosion resistance increased. In this work, the influence of pulse frequency on the corrosion resistance of Cu–Zn binary alloy has been examined by the method of adjusting the pulse frequency, based on the cluster theory and the existing research results of the solidification process of liquid metal.
2 Experiment materials and methods
Brass alloy (Zn: 52 wt%, Cu bar) was used as the experiment material for the present study. First, the graphite crucible was preheated to 800°C, and the furnace with silicon carbide rods was heated to 1,150°C. When Cu and Zn were melted, the temperature was held for 5 min. The melt was then poured into a permanent mold. After solidification, the original samples were obtained. After EPT with different pulse frequencies, the residual melt was poured, and the experimental samples were obtained. The graphite electrode was inserted vertically into the alloy melt of 3 cm depth. The electric pulse frequencies were 3, 8 and 15 Hz. The pulse voltage was 500 V, and the treatment time was 30 s. When the samples solidified and cooled, the samples were sealed by epoxy resin for metallography, and corrosion tests were prepared.
3 Results and discussions
The metallographic microstructure of brass alloy with and without EPT was observed by a scanning electron microscope and ZEISS Axiovert2000MAT metallurgical microscope, and the results are presented in Figure 1. The average grain size of brass alloy with and without EPT is presented in Table 1.

Microstructure of brass alloy with and without EPT (a) original sample, (b) 3 Hz, (c) 8 Hz and (d) 15 Hz.
The average grain size of brass alloy with and without EPT
| Pulse frequency/Hz | 0 | 3 | 8 | 15 |
| Grain size/µm | 576.4 | 362.5 | 504.7 | 525.6 |
Based on Figure 1 and Table 1, the average grain size of original samples was 576.4 µm. The γ phase was star shaped and distributed aggregately on the grain boundary. The grain size of samples with EPT and the γ phase decreased and dispersed, and the brittleness of brass alloy also decreased. The average grain size of sample (b) with 3 Hz EPT was 362.5 µm, which is 62% of the original samples and decreased more obviously. The size of γ phase has changed smaller and the amount has changed less and distributed uniformity. The average grain size of sample (c) with 8 Hz EPT and sample (d) with 15 Hz EPT increased, which were 88% and 91% of the original samples, and the γ phase became fish bone shape and increased.
The polarization curve and the AC impedance spectroscopy of the samples with and without EPT were tested in 3.5% NaCl solution by an electrochemical workstation corrosion resistance test system (IVIUM Stat.XRi). The platinum was used as the auxiliary electrode of 1 × 1 cm in size. The reference electrode was the standard calomel electrode. The scan speed was 0.5 mV/s and the open circuit potential ranged from −250 to 1,000 mV in the polarization curve tests. The sine-wave amplitude modulation was 10 mV and the frequency was 100 kHz–0.01 Hz in EIS AC impedance spectroscopy testing. The results are presented in Table 2.
The free corrosion potential and corrosion current density of brass alloy with 500 V EPT
| Samples | Free corrosion potential (V) | Corrosion current density (A cm−2) |
|---|---|---|
| Original | −0.2719 | 6.3147 × 10−6 |
| 3 Hz | −0.2378 | 4.6971 × 10−7 |
| 8 Hz | −0.2505 | 2.7545 × 10−6 |
| 15 Hz | −0.2525 | 1.3559 × 10−6 |
The steady-state polarization curves of samples with and without EPT are given in Figure 2. It can be seen that the polarization curve of the cathode was smooth, while the polarization curve of anode was flat, suggesting the smaller electrode reaction and resistance of the polarizability. The two characteristic regions, the active dissolution region and the transition region of active deactivation, were presented in the polarization curve of cathode of all the four samples. The free corrosion potential of samples with EPT was higher than those without EPT, and the corrosion current density of samples with EPT was lower, implying that the ability of passivation of the samples with EPT obviously improved.
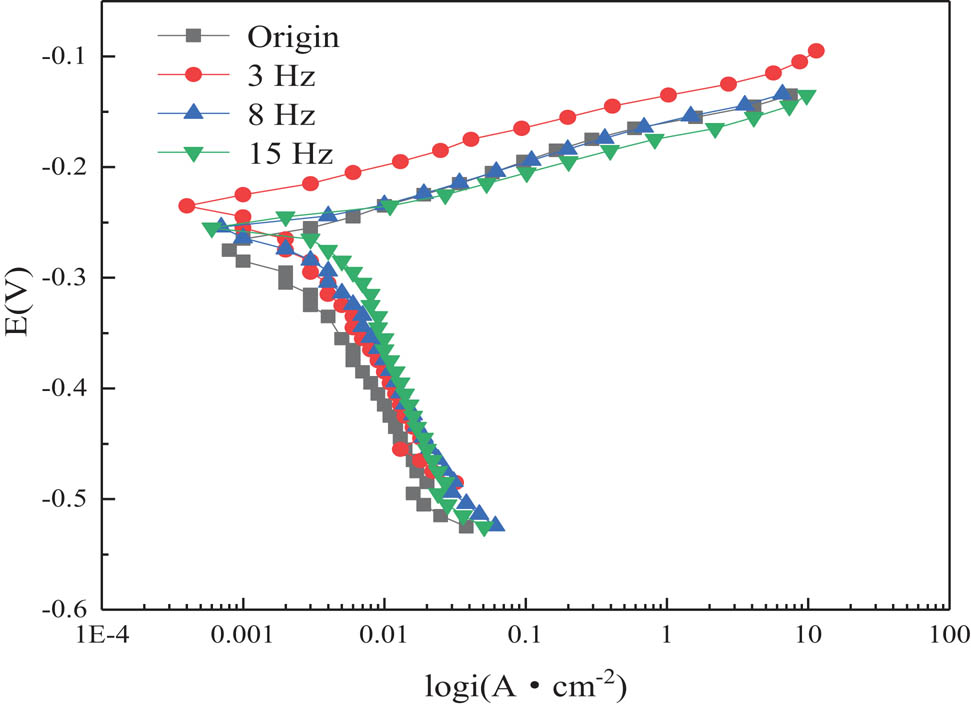
Polarization curves of brass alloy with and without EPT.
Based on Figure 2 and Table 2, the free corrosion potential and corrosion current density of samples with EPT increased and decreased, respectively. After EPT, the free corrosion potential of brass alloy samples with 3 Hz increased by 3.41%, and the corrosion current density decreased by one magnitude order. Therefore, it can then be concluded that the corrosion resistance of brass alloy has significantly increased. In this experiment, the corrosion resistance of 3 Hz brass alloy sample is the best.
The corrosion resistance of samples in corrosion medium can be indicated by the diameter of the capacitive reactance arc in EIS. A bigger diameter of the capacitive reactance arc was usually related to a better corrosion resistance of the samples. The EIS of brass alloy samples is shown in Figure 3. It can be seen that after EPT, the diameter of capacitive reactance arc increased by four times from 741.1 to 2898.0 µm, suggesting the enhanced corrosion resistance of the aluminum brass with EPT.
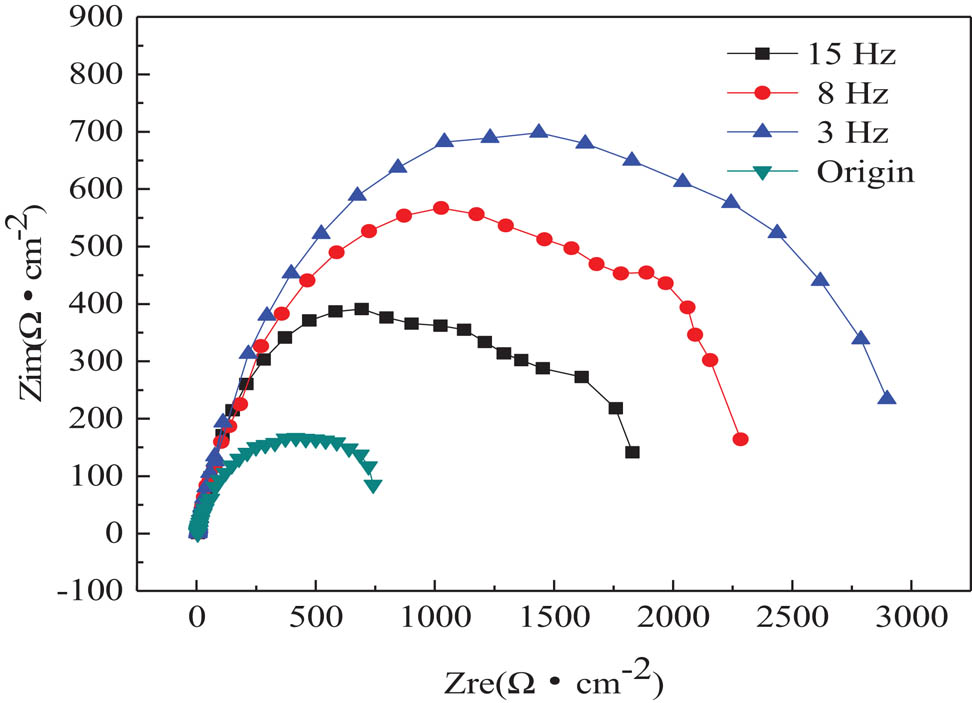
Impedance spectroscopy of brass alloy with and without EPT.
According to the literature, three mechanisms describe the dezincification corrosion, namely, the preferential dissolution and resolution deposit mechanism, the double-space mechanism and the seepage mechanism. To further explore the internal relations between the changes in the corrosion resistance and phase structures, the X-ray diffraction tests were carried out, and the results are shown in Figure 4. It can be concluded from Figure 4 that the β phase (CuZn) and γ phase (Cu5Zn8) were found in the samples without corrosion. The β phase disappeared, the γ phase decreased and the Cu phase precipitated with corrosion.
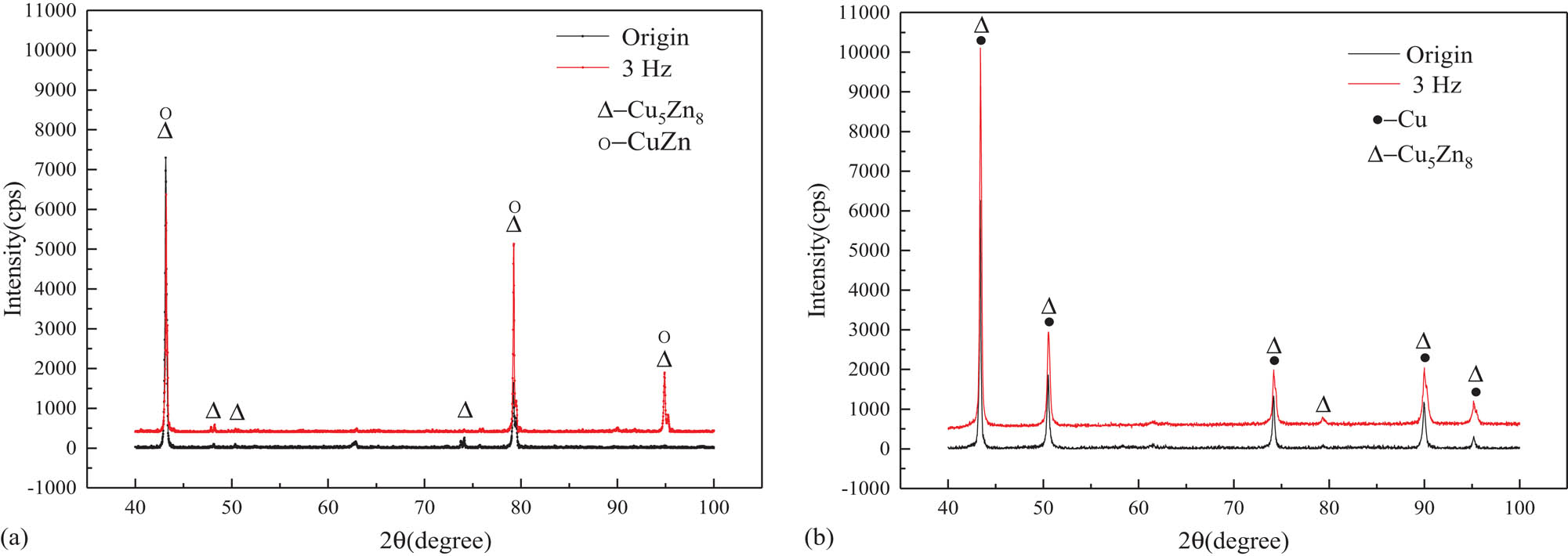
Phase analysis of brass alloy with and without EPT (a) without corrosion and (b) with corrosion.
The distribution of elements on electrochemical corrosion surface of brass samples was detected and the results are shown in Figure 5. It can be seen that the Zn content decreased and the Cu contest was constant. In summary, dezincification of the original brass alloy sample was in progress. The findings show that Zn dissolved first as the anode in the corrosion progressed and double space occurred. The double space then diffused into the brass alloy under the concentration gradient in brass alloy, and Zn diffused to the surface of brass alloy, therefore, leading to the preferential dissolution of Zn.
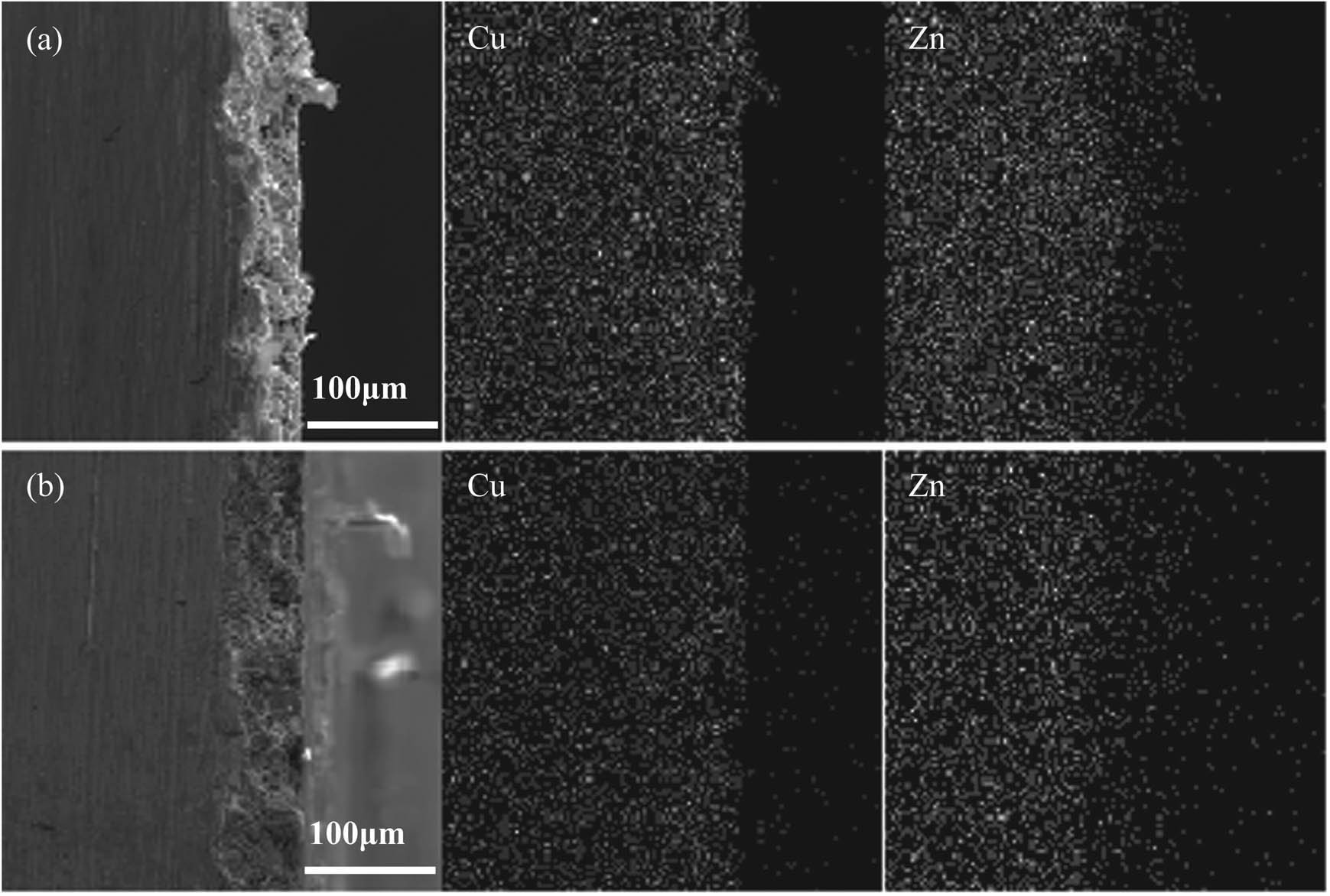
Element distribution analysis of brass alloy with and without corrosion (a) original sample and (b) with corrosion.
The profile morphology of corrosion layer in brass alloy with electrochemical corrosion is shown in Figure 6. It can be seen that the dezincification layer of the original brass samples was porous (Figure 6a); and for the samples with 3 Hz EPT, the surface of matrix was covered tightly and uniformly.
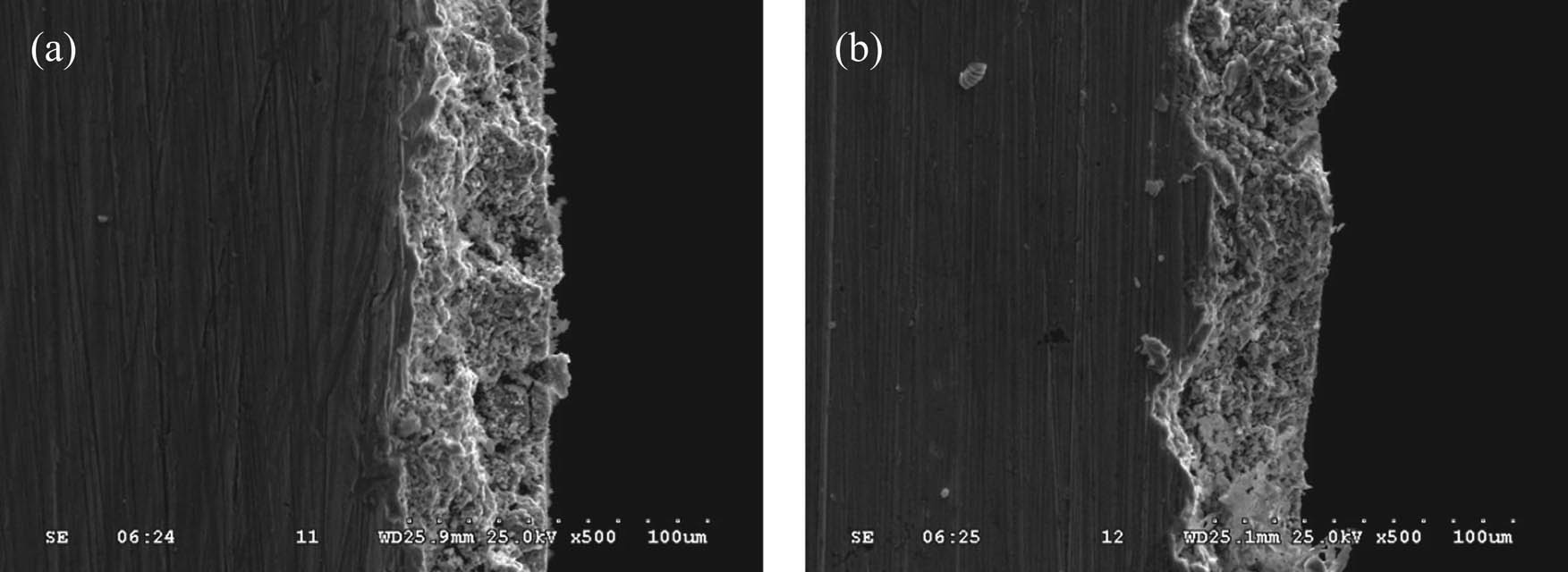
Profile morphology of brass alloy with and without EPT (a) original sample and (b) 3 Hz.
The findings show that dezincification occurred on the boundary between β and γ phases during the corrosion, and the β phase was then corroded as anode. The corrosion of the γ phase did not occur until the porous shape was formed on the corrosion layer, and the corrosion rate was related to the quantity of the galvanic couple [12]. The nucleation rate of the brass alloy melt obviously improved by EPT and the quantity of galvanic couple decreased, therefore, efficiently inhibiting the dezincification progress. The undercooling of brass alloy melt improved by EPT, thus the size of atomic cluster decreased and the quantity increased. Additionally, the critical dimension of nucleation decreased during the nucleation process. The morphology, size and distribution of the γ phase can be ameliorated by EPT, and the rate of dezincification obviously decreased.
The profile morphology of corrosion layer in brass alloy was contrasted and analyzed, and the results are given in Figure 7. The thickness of corrosion layer in brass alloy is given in Table 3. It can be seen that the average thickness of corrosion layer (the deep gray in surface) in the original samples was 49.8 µm, and in the sample (b) with 3 Hz EPT it was 30.8 µm, which was 61.8% of the original samples. Therefore, the thickness of corrosion layer decreased obviously by 3 Hz EPT, and the corrosion resistance of brass efficiently improved.
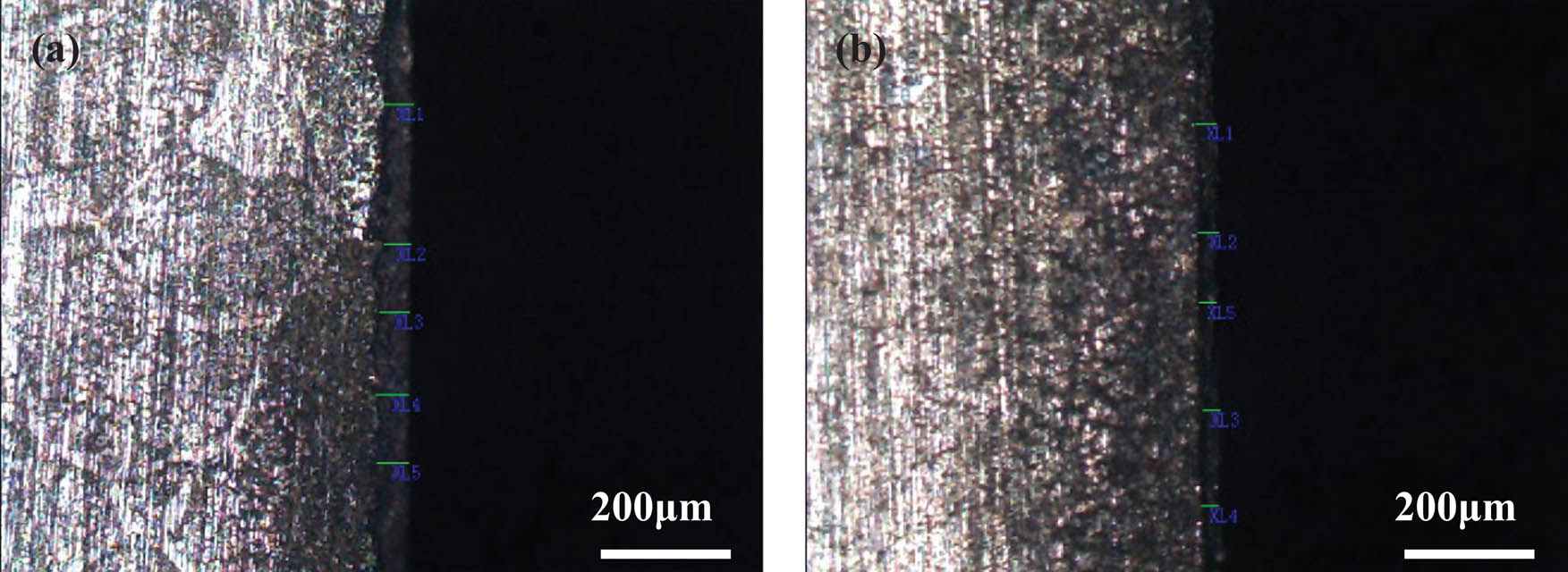
The morphology of corrosion in brass alloy with and without EPT (a) original sample and (b) 3 Hz.
The thickness of the corrosion layer in brass alloy with and without EPT (µm)
| Corrosion layer | 1 | 2 | 3 | 4 | 5 | Average thickness |
|---|---|---|---|---|---|---|
| Origin | 48.7 | 46.9 | 49.6 | 52.1 | 51.7 | 49.8 |
| 3 Hz | 34.8 | 32.5 | 27.7 | 27.2 | 26.5 | 30.8 |
According to the structure model of liquid metallic clusters which was proffered by Bing [13], for the most stable cluster r0 in molten metal, its existence probability was bigger than others which were correspond by other magic numbers. But the cluster, which was embryo in the progress of liquid metal solidification, depends on the relationship between cluster size and undercooling. Only the cluster that corresponds to the size r0max of minimum undercooling can change into the critical nucleus [14,15]. For this solidification system of brass in this experiment, the outer shell of Zn atom cluster has distorted repeatedly in brass alloy sample with EPT. Thus, the potential of the other outer shells decreased and the connection with surrounding atoms increased, and the barrier between atom and clusters decreased. Therefore, the combination of the surrounding atoms and clusters is promoted, and the nucleation rate increased. The crystal structure of brass alloy samples is distributed uniformly; thus, Zn atom is distributed uniformly. The quantity of galvanic couple decreased, and the progress of dezincification efficiently inhibited.
The electromagnetic force occurred in the brass alloy melt with EPT. With the increasing temperature of brass alloy melt with EPT and with the effect of electromagnetic force, the non-dendrite and dendritic microstructures were produced. The non-dendrite and dendritic microstructures then grow as the particles of nucleation; thus, increasing the nucleation rate. Meanwhile, the brass alloy melts were compressed and shocked by the electromagnetic force. The fresh dendrites dropped from the cavity into the brass alloy melt, and fresh crystal nucleus was produced in the cavity. The nucleation rate of brass alloy melt increased and the solidification structure thinned in the repetitive progress. In conclusion, the Zn atom cluster was distributed uniformly in the brass solidification system with EPT. The γ phase decreased and dispersed. The dezincification rate decreased, and the corrosion resistance of brass alloy increased.
4 Conclusions
The thickness of corrosion layer in brass alloy with EPT decreased. The average grain size of sample with 3 Hz EPT decreased by 62% to 362.5 µm, and the γ phase changed little. The surface of the matrix was covered tightly and uniformly. The average thickness decreased by 61.8% to 30.8 µm.
The corrosion resistance of brass alloy increased by 3 Hz EPT. The free corrosion potential increased by 3.41% to −0.2378 V. The free corrosion current density decreased by one magnitude order to 4.6971 × 10−7 A cm−2. The diameter of the capacitive reactance arc increased by 3 times to 2898.0 µm.
References
[1] Li, Y., and Y. L. Zhu. Advances in researches of dezincification mechanism of brass. Corrosion & Protection, Vol. 27, No. 5, 2006, pp. 222–225, 262.Suche in Google Scholar
[2] Ke, G. The present situation and development of easy-cutting lead-free brass alloy material. Shanghai Nonferrous Metals, Vol. 34, No. 3, 2013, pp. 134–137.Suche in Google Scholar
[3] Yang, S. L., and Y. Wu. Advances in researches of dezincification mechanism of brass. World Nonferrous Metals, No. 4, 2013, pp. 50–53.Suche in Google Scholar
[4] Bingxuan, C., S. Jing, and Z. Jianhua. Research of the corrosion-resistant and wear-resisting performance and environmental brass. Foundry, Vol. 55, No. 5, 2006, pp. 516–518.Suche in Google Scholar
[5] Yan, J., S.-Y. Tang, J. Wang, M.-D. Tao, Z. Zhao, Z.-L. He, et al. Effects of processing technology on corrosion resistance of unleaded brass. Special Casting & Nonferrous Alloys, Vol. 26, No. 11, 2006, pp. 733–735.Suche in Google Scholar
[6] Mingjun, J., G. Xiaochuan, and Y. Jun. The characteristics and protective methods of brass dezincification corrosion. Journal of Logistical Engineering University, 2008, Vol. 24, No. 4, pp. 35–38, 61.Suche in Google Scholar
[7] Juan, Z. Effects of Trace Element on Corrosion Resistance of Aluminum Brass. Doctoral dissertation. Central South University, Changsha, 2008.Suche in Google Scholar
[8] Jihui, W., J. Xiaoxia, and L. Shizhuo. Chinese Journal of Materials Research, Vol. 13, No. 1, 1999, pp. 1–8.Suche in Google Scholar
[9] Zhao, Z.-F., Qi, J.-G., Wang, J.-Z., Liu, X.-J., Wang, J.-Y. Effects of electric pulse treatment on corrosion resistance of silicon brass. Journal of South China University of Technology (Natural Science Edition), Vol. 43, No. 7, 2015, pp. 28–32, 67.Suche in Google Scholar
[10] Li, X., Z. F. Zhao, D. D. Shan, L. Liu, B. Y. Huo, Z. R. Mo, et al. Effect of pulse frequency on solid boriding process of Cr12MoV steel. Heat Treatment of Metals, Vol. 43, No. 4, 2018, pp. 208–212.Suche in Google Scholar
[11] Zhao, Z.-F., J.-G. Qi, and J.-Z. Wang. Effects of electric pulse treatment on γ phase in silicon brass. Foundry Technology, Vol. 34, No. 9, 2013, pp. 1108–1111.Suche in Google Scholar
[12] Zhenbin, Z. Study on the Modification Process of Pulse Electric Field on Molten Al–Cu Alloy. Doctoral dissertation. University of Science and Technology Beijing, Beijing, 2007.Suche in Google Scholar
[13] Bing, W. Research of Solidification Microstructures, Properties and Technology of Casting Al–5%Cu-based Alloy Under Electric Pulse Modification. Doctoral dissertation. University of Science and Technology Beijing, Beijing, 2008.Suche in Google Scholar
[14] Zhao, Z. F. Research on Solidification Microstructures, Properties and Mechanism of Brass Alloy under Electric Pulse Treatment. Doctoral dissertation. University of Science and Technology Beijing, Beijing, 2015.Suche in Google Scholar
[15] Zhang, W., M. L. Sui, Y. Z. Zhou, G. H. He, J. D. Guo, and D. X. Li. Electropulsing-induced evolution of microstructures in materials. Acta Metallrugica Sinica, Vol. 39, No. 10, 2003, pp. 1009–1018.10.1016/S0968-4328(03)00025-8Suche in Google Scholar
© 2020 Zhao Zuofu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process
Artikel in diesem Heft
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process

