Abstract
Corrosion at elevated temperature is a serious problem in running thermal power plants because of the use of low-grade fuels that contain substantial amounts of sulphur, vanadium, sodium etc. This article reports the high-temperature corrosion of weld metal and heat-affected zone (HAZ) of shielded metal arc-welding (SMAW) weldment in GrA1 steel in a molten salt (Na2SO4–60% V2O5) environment at 900°C under cyclic conditions. The thermogravimetric technique was used to observe the kinetics of corrosion. The corrosion products formed on weld metal and HAZ of SMAW welded steel were characterized by scanning electron microscopy with energy dispersive X-ray analysis (EDX) and X-ray diffraction pattern. Weld metal was found to oxidize at a higher rate than those of HAZ due to the presence of sodium and sulphur in the inner oxide scale as confirmed by EDX, and this leads to high corrosion rate (in terms of weight gain).
1 Introduction
Thermal power plants are one of the significant industries suffering from severe corrosion problems resulting in substantial losses [1]. Ferritic steels are broadly used in these power plants in several forms especially as boiler tubes; these steels have a good combination of mechanical properties particularly high thermal and creep resistance, weldability and protection from corrosion at elevated temperature [2,3]. A large number of welds were found in a typical steam generating system, and it has been discovered that heat-affected zone (HAZ) from welding may severally degrade from prolonged services [4]. High-temperature corrosion became known with the failure of boiler tubes, and later with the serious assault of gas turbine airfoil materials [5]. High-temperature corrosion happens whenever salt or ash deposits accumulate on the surfaces of alloys and alter the environment–alloy reactions that would have happened if the deposit had not been present [6]. The remains of ashes have high concentrations of vanadium, sodium and sulphur, mainly as Na2SO4–V2O5 complex and sodium–vanadate mixtures. Vanadium, sulphur and sodium are frequently present as impurities in residual oils used as fuel [7,8]. Molten sulphate–vandate deposits, resulting from the condensation of combustion products of residual fuels, are extremely corrosive to high-temperature materials in combustion systems [9]. The use of sulphur- and vanadium-containing fuels exceeds the design limit of temperature and pressure during operation, which have a detrimental effect on the performance of boiler tubes [10]. The present study has been carried out to evaluate the high-temperature corrosion behaviour of different regions of SMAW welded steel in a molten salt environment at 900°C. The X-ray diffraction (XRD) and scanning electron microscopy with energy-dispersive X-ray analysis (SEM/EDX) have been used to characterize the corrosion product after high-temperature corrosion at 900°C.
2 Experimental procedure
2.1 Preparation of weldment and specimen
ASTM SA 210-GrA1 (GrA1) steel was acquired from the thermal power plant at Bhatinda (India). The chemical compositions are shown in Table 1. Steel tubes (5 mm thickness × 33 mm diameter) after machining with a bevel angle of 37.5° of V-groove, root face of 1 mm and root gap of 1 mm were welded together by a shielded metal arc-welding (SMAW) process using basic-coated electrode AWS A5.1 E7018 at a constant arc current of 95 A and a voltage of 12 V. The welding parameters were published in the earlier paper by Kumar et al. [11]. A cross-sectional portion of the weldment was cut and polished along the long transverse area and etched in 2% nital for 20 s before they were analysed by optical microscopy. A diamond wafering saw was used to cut the specimens 15 × 5 × 2 mm3 of HAZ and weld metal from the weldment along the longitudinal direction. The specimens were polished with silicon carbide paper and then with emery paper after which the cloth-wheel polished before being corroded.
Chemical composition (wt%) for GrA1 boiler tube steel
| Type of steel | ASTM code | Composition | C | Mn | Si | S | P | Fe |
|---|---|---|---|---|---|---|---|---|
| GrA1 | SA210 Grade A1 | Nominal | 0.27 | 0.93 | 0.1 | 0.058 | 0.048 | Bal. |
| Actual | 0.2952 | 0.5977 | 0.2873 | 0.0056 | 0.0089 | Bal. |
2.2 High-temperature corrosion test
High-temperature corrosion studies were performed in a molten salt (Na2SO4–60% V2O5) environment for 50 cycles. Each cycle consisted of 1 h heating at 900°C in a silicon carbide tube furnace taken after 20 min of cooling at room temperature. Moreover, the cyclic conditions also resemble the actual industrial conditions. A coating of uniform thickness with 3–5 mg/cm2 of Na2SO4–60% V2O5 was applied with a camel hairbrush on the preheated specimens (250°C). The weight change measurements were taken towards the end of each cycle with the facility of an electronic balance of model 06120 (Contech), Mumbai, India, with a sensitivity of 1 mg. The spalled scale, assuming any, was also included at the time of measurements to determine the overall rate of corrosion. The exposed specimens were analysed using SEM/EDX and XRD for surface analysis of their scales.
3 Results and discussion
3.1 Visual examination and thermogravimetric data
The macromorphology of the oxide scale for different regions of SMAW weldment after high-temperature corrosion in Na2SO4–60% V2O5 at 900°C for 50 cycles is presented in Figure 1. For weld metal, dark colour scale showed up in the general surface from the first cycle itself, spalling was seen during the 22nd cycle, and cracks were observed. Likewise, the appearance of blackish colour in the oxide scale of HAZ was noticed from the first cycle, small spalling of the oxide scale in HAZ has showed up around the 15th cycle and significant cracks were found in the oxide scale of HAZ from the 42nd cycle onwards. Weight change expressed in mg/cm2 is plotted in Figure 2 as a function of time expressed in the number of cycles for the weld metal and HAZ regions of SMAW weldment in GrA1 steel. Total weight gain after 50 cycles of high temperature corrosion of weld metal is almost 1.2 times more than the total weight gain value of HAZ. The (weight gain/unit area)2 plot against the number of cycles shown in Figure 3 further confirmed that the parabolic law is followed by weld metal and HAZ. The values of parabolic rate constant Kp (10−8 g2 cm−4 s−1) are 6.055 and 4.148 for weld metal and HAZ, respectively.
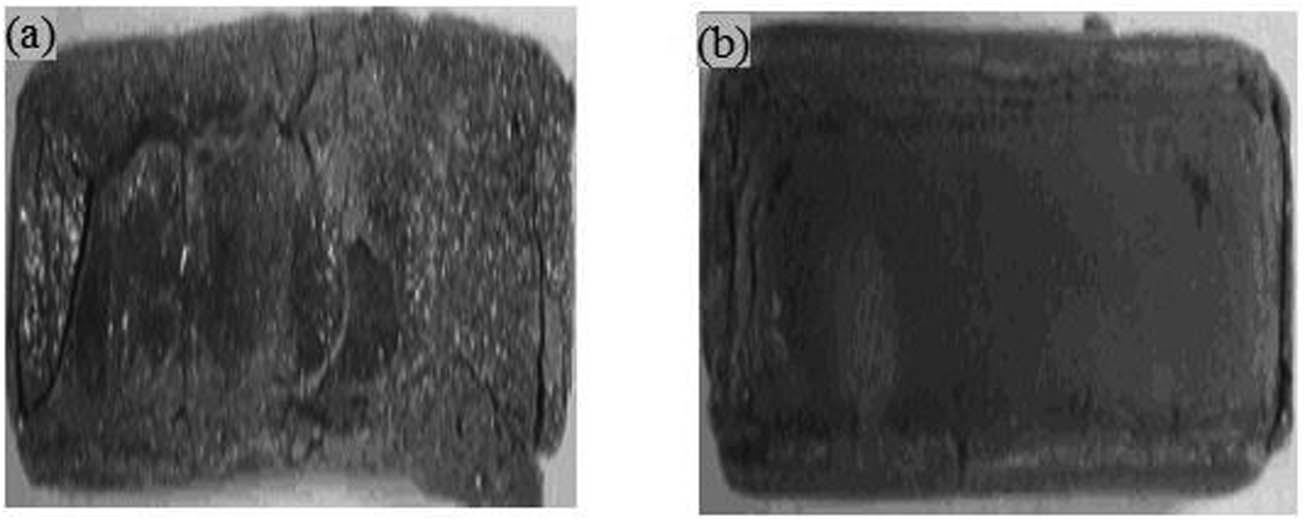
Macrographs of different regions of SMAW weldment in GrA1 steel subjected to high-temperature corrosion in Na2SO4–60% V2O5 at 900°C for 50 cycles: (a) weld metal and (b) HAZ.

Weight gain plot for different regions of SMAW weldment in GrA1 steel exposed to Na2SO4–60% V2O5 at 900°C for 50 cycles.
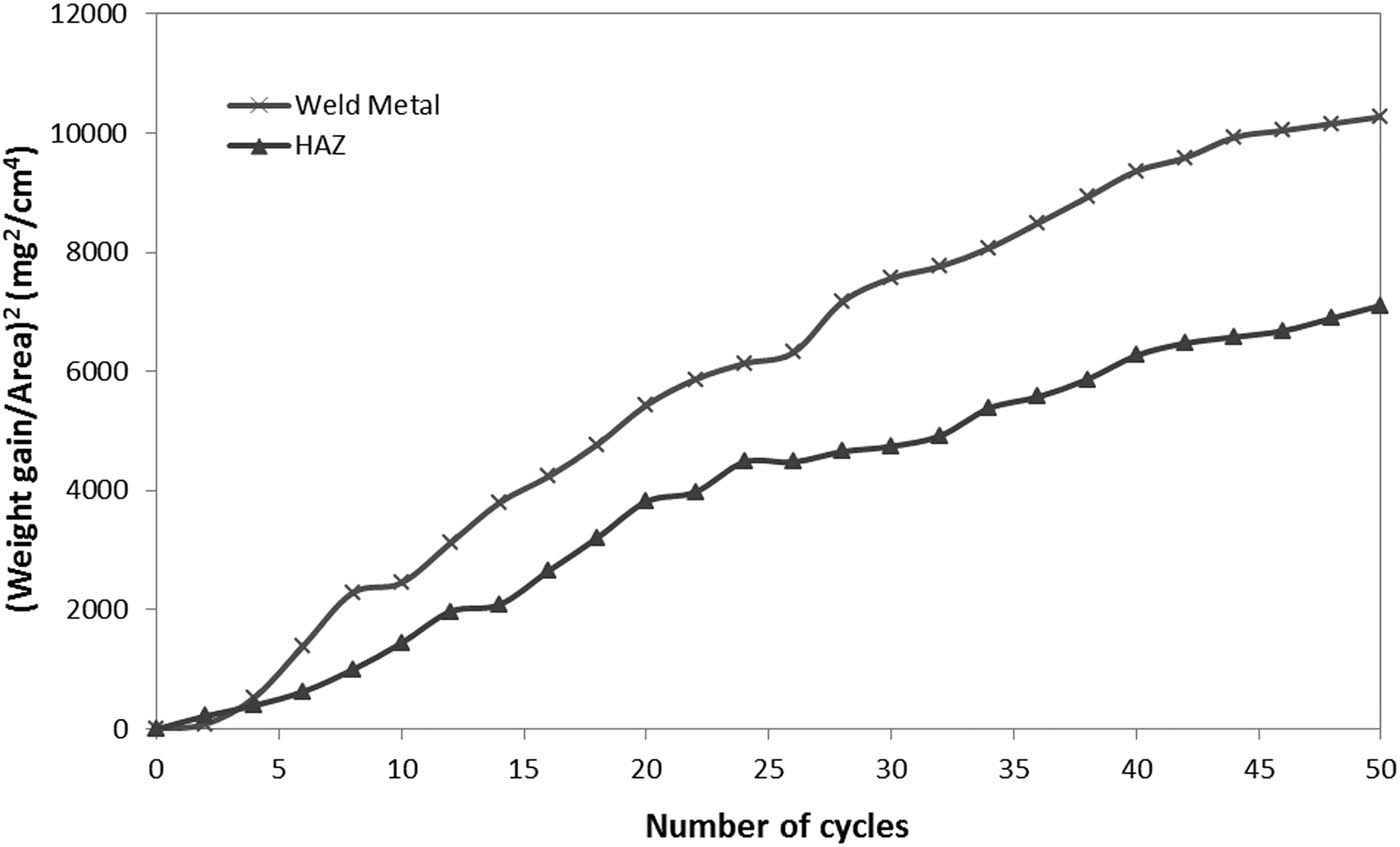
Weight gain square (mg2/cm4) plot for different regions of SMAW weldment in GrA1 steel exposed to cyclic hot corrosion in Na2SO4–60% V2O5 at 900°C for 50 cycles.
3.2 XRD Analysis of the exposed specimens
XRD analysis for corroded weld metal and HAZ is shown in Figure 4, and these diffractograms have relatively comparable phases for every one of the regions. As evident from the composition, all the regions have revealed the formation of iron oxide (Fe2O3), though intense peaks of Fe2O3 are shown in the oxide scale of the weld metal.

XRD profiles for different regions of SMAW weldment in GrA1 steel exposed to Na2SO4–60% V2O5 at 900°C for 50 cycles.
3.3 SEM/EDX analysis of the exposed specimens
SEM morphology and EDX analyses for different regions of SMAW weldment in GrA1 steel after cyclic corrosion are shown in Figure 5. The oxide scale of weld metal contains nodules both large and small in size as shown in Figure 5(a). The top oxide scale is mainly composed of Fe (96.41%) and Mn (3.28%), whereas the inner oxide scale is composed of Fe (91.34%) with Na (2.60%), Mn (2.82%), Si (1.30%) and S (1.12%). The SEM micrograph shown in Figure 5(b) for HAZ in GrA1 steel also demonstrates the development of comparable kinds of oxides comprising Fe (97.11%) with a small amount of Mn and Na at point 2. The grain boundaries are rich in the oxide of Fe (96.98%) and Mn (2.31%) at point 1.

Surface morphology and EDX analysis for different regions of SMAW weldment in GrA1 steel exposed to Na2SO4–60% V2O5 at 900°C for 50 cycles: (a) weld metal, 1,000× and (b) HAZ, 1,000×.
The weld metal indicated a higher rate of corrosion in contrast with its counterpart HAZ (Figure 2). The primary reason was discovered that the cracking and spalling of oxide scale were because of the difference in thermal coefficients of oxides in the scale from the metal as observed by Niranatlumpong et al. [12], Khanna [2] and Young [3]. Cracks in oxide were started by uneven expansion and contraction; through these cracks, the corrosive gases can enter to the metal and permit significant grain boundary corrosion attack as such penetration of sodium and sulphur was discovered in the internal oxide scale of weld metal, which was confirmed by EDX; this leads to a higher corrosion rate as reported by Kumar et al. [13]. HAZ oxidized at a lower rate due to the formation of lower extents of cracking, resulting in lesser corrosion rate difference with weld metal. Fe2O3 was seen to be the main phase (Figure 5) in the oxide scale and detected to be nonprotective in nature as suggested by Kolta et al. [14], Raman and Muddle [15] and Lai [16]. Most of these phase formations have also been reported by Sidhu and Prakash [17], while a study was being conducted in the same molten salt environment at the same temperature for base steel.
4 Conclusions
In view of thermogravimetric studies, the following points have been concluded:
Weld metal showed the formation of high intensity of Fe2O3 as revealed by XRD investigation. Weight gain of weld metal was more, which was due to the formation of cracks in the oxide scale. The HAZ indicates less weight gain.
The weight gain of weld metal and HAZ specimens follows the parabolic law in molten salt which specifies that the corrosion rate is diffusion controlled. The susceptibility to high-temperature corrosion of different regions of SMAW weldment in GrA1 steel has been observed to be in the following order: HAZ < weld metal.
References
[1] Uustilo, M. A., P. M. J. Vuoristo, and T. A. Mantyla. High temperature corrosion of coatings and boiler steels in oxidizing chlorine-containing atmosphere. Materials Science and Engineering: A, Vol. 346, 2003, pp. 168–177.10.1016/S0921-5093(02)00537-3Search in Google Scholar
[2] Khanna, A. S. Introduction to High Temperature Oxidation and Corrosion. American Society for Metals, Materials Park, OH, 2002.Search in Google Scholar
[3] Young, D. J. High Temperature Oxidation and Corrosion of Metals. Elsevier Science, Vol. 1, 1st edn, 2008.10.1016/S1875-9491(08)00001-XSearch in Google Scholar
[4] Cary, H. B. Modern Welding Technology. Prentice Hall, Inc., Englewood Cliffs, NJ, 1979.Search in Google Scholar
[5] Rapp, R. A., and Y. S. Zhang. Hot corrosion of materials: fundamental studies. JOM, Vol. 46, 1994, pp. 47–55.10.1007/BF03222665Search in Google Scholar
[6] Pettit, F. S., and C. S. Giggins. Hot corrosion, Ch. 12. In Superalloys II, Sims, C. T., N. S. Stollof, and W. C. Hagel, Eds. Wiley, New York, 1987.Search in Google Scholar
[7] Harada, Y., and T. Kawamura. Control of gas side corrosion in oil fired boiler. Mitsubishi Heavy Industries Technical Review, Vol. 17, 1980, p. 139.Search in Google Scholar
[8] Harada, Y., S. Naito, T. Tsuchiya, and Y. Nakajima. Problems of low grade oil firing boilers and their solutions. Mitsubishi Heavy Industries Technical Review, Vol. 18, 1981, pp. 85–95.Search in Google Scholar
[9] Natesan, K. Corrosion–erosion behavior of materials in a coal-gasification environment. Corrosion, Vol. 32, 1976, pp. 364–370.10.5006/0010-9312-32.9.364Search in Google Scholar
[10] Asrar, N., A. U. Malik, S. Ahmed, D. Al-Subaii, and I. M. Said. Overheating and fuel ash corrosion failure of boiler tubes in SWCC power plants - some case studies. Presented in the Second Acquired Experience Symposium on Desalination Plants O&M, September 29 – October 3, 1997, SWCC, Al-Jubail, 1997.Search in Google Scholar
[11] Kumar, R., V. K. Tewari, and S Prakash. Oxidation behaviour of welded ASTM-SA210 GrA1 boiler tube steels under cyclic conditions at 900°C in air. High Temperature Materials and Processes, Vol. 38, 2019, pp. 326–331.10.1515/htmp-2018-0017Search in Google Scholar
[12] Niranatlumpong, P., C. B. Ponton, and H. E. Evans. The failure of protective oxides on plasma-sprayed NiCrAlY overlay coatinhs. Oxidation of Metals, Vol. 53, 2000, pp. 241–258.10.1023/A:1004549219013Search in Google Scholar
[13] Kumar, R., V. K. Tewari, and S. Prakash. Characterization of hot corrosion behavior of different regions of tungsten inert gas weldment in ASTM SA 210GrA1 boiler tube steel. Materials Performance and Characterization, Vol. 7, 2018, pp. 414–422.10.1520/MPC20180099Search in Google Scholar
[14] Kolta, G. A., I. F. Hewaidy, and N. S. Felix. Reactions between sodium sulphate and vanadium pentoxide. Thermochimica Acta, Vol. 4, 1972, pp. 151–164.10.1016/S0040-6031(72)80029-7Search in Google Scholar
[15] Raman, R. K. S., and B. C. Muddle. Role of high temperature corrosion in life assessment and microstructural degradation of Cr–Mo steel weldments. International Journal of Pressure Vessels and Piping, Vol. 77, 2000, pp. 117–123.10.1016/S0308-0161(99)00092-7Search in Google Scholar
[16] Lai, G. Y. High Temperature Corrosion of Engineering Alloys, ASM International, Materials Park, OH, 1990.Search in Google Scholar
[17] Sidhu, B. S., and S. Prakash. Evaluation of the corrosion behaviour of plasma-sprayed Ni3Al coatings on steel in oxidation and molten salt environment at 900°C. Surface and Coatings Technology, Vol. 166, 2003, pp. 89–100.10.1016/S0257-8972(02)00772-7Search in Google Scholar
© 2020 Ravindra Kumar et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process
Articles in the same Issue
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process

