Abstract
The liquidus surface projection and isothermal section at 1,800°C in the Mo–Ti–C ternary system are examined using arc-melted alloys. A ternary transition peritectic reaction (L + Mo2C → Mo + TiC) takes place during solidification, which is apparently different from the ternary eutectic reaction (L → Mo + TiC + Mo2C) observed in a previous report. Since the composition of the eutectic reaction (L → Mo + TiC) shifts toward the Mo–Ti binary line with increasing Ti concentration, the volume fraction of the Mo phase and the interlamellar spacing of the Mo and TiC phases increase in the eutectic microstructure. At 1,800°C, the TiC phase in equilibrium with the Mo phase can contain more than 28 at% Mo and a Mo/TiC/Mo2C three-phase region exists at around Mo–15Ti–10C.
1 Introduction
The efficiency and performance of jet engines and gas turbines have steadily improved as their operating temperature has increased [1]. While Ni-based superalloys are commonly used in such engines, the engine operating temperatures often exceed the superalloy melting points [2]. Consequently, high-pressure turbine blades made of superalloys usually need to be protected by ceramic thermal barrier coatings and by the passage of cooling air, which reduces efficiency. To overcome this problem, new refractory-metal-based materials with higher melting points, such as molybdenum- and niobium-based alloys reinforced with silicides and/or ceramics, have been widely studied [1,3,4,5,6,7].
Cermets are a type of composites composed of ceramic and metal phases that are generally fabricated by sintering. Ceramics such as TiC, ZrC and HfC are reinforcement phases and have the property of being hard but brittle (KIC = 2–5 MPa(m)1/2) [8]. The metal phases are, in contrast, ductile and tough. Therefore, cermets exhibit high hardness, stiffness and wear resistance, and their fracture toughness is better than that of ceramics [9,10]. Among them, TiC-based cermets are attractive for high-temperature applications because of their relatively good oxidation resistance in addition to their low density (4.93 g/cm3) and high melting point (3,067°C) of TiC [10]. However, the melting point of the metal phases in TiC-based cermets, which include Ni (1,455°C) and Co (1,495°C) phases, limits their high-temperature applications [11]. Additionally, cermets have small grain sizes because of their sintering production process, which may degrade creep strength [12].
In this study, molybdenum, which has a high melting point (2,623°C), is focused on as a metal phase and the microstructure formed during solidification is carefully investigated [13]. It has been well known that molybdenum has an eutectic reaction during solidification with group IV element carbides such as TiC, ZrC and HfC [14,15,16]. Kurisita et al. reported the eutectic microstructure and the high-temperature strength of Mo–TiC alloys produced by arc-melting [17,18]. The volume fractions of Mo and TiC in the eutectic areas of the literature look similar though the eutectic point in the pseudo binary phase diagram is closer to the Mo solid solution than to TiC [19]. Suzuki et al. systematically studied the mechanical properties of Mo–TiC, ZrC and HfC [20,21,22,23].
On the other hand, the material properties of the TiC phase should be varied due to off-stoichiometry. In the fractography of an MoSiBTiC alloy, river patterns can be observed in both the Mo solid solution and TiC phases, suggesting that the TiC phase may be toughened by off-stoichiometry and solid solute Mo [24]; however, the fracture toughness of stoichiometric TiC is only 3 MPa(m)1/2 [25]. The TiC phase in the MoSiBTiC alloy is in equilibrium with Mo solid solution and contains more than 20 at% of Mo, which might also affect the properties of the TiC phase, such as its elastic modulus and deformability [26]. Considering this background, the Mo–Ti–C ternary system was investigated in this study.
To understand the solidification pathways and off-stoichiometry of the TiC phase in equilibrium with Mo solid solution, knowledge about phase equilibria in the Mo–Ti–C ternary system is necessary. According to the literature on the liquidus surface projection of the Mo–Ti–C system, a ternary eutectic reaction [L → Mo + TiC + Mo2C] occurs at around Mo–18Ti–20C [14]. However, the microstructural observations in the previous study [14] were only conducted by optical microscopy, and thus a more detailed microstructural analysis is required. In fact, the results obtained in our preliminary research do not show any ternary eutectic reaction. Eremenco and Ya Velikanova investigated the isothermal section of Mo–Ti–C at 1,400, 1,700, 2,000 and 2,200°C [19]. Rudy investigated the isothermal section at 1,500, 1,750, 2,500 and 2,750°C [14]. However, the changes in the Mo/TiC two-phase regions with temperature did not agree with each other, as the shape of the phase boundary and the gradient of the tie line were completely different.
Therefore, the objective of this study was to reconsider and understand the details of the microstructural evolution of Mo solid solution and TiC phases during solidification, and the off-stoichiometry of the TiC phase in equilibrium with the Mo phase. In this paper, we determine the liquidus surface projection and isothermal section at 1,800°C in the Mo–Ti–C ternary system.
2 Experimental
The composition of the alloys used in this study ranged from Mo–(5 to 90)Ti–(10 to 20)C in atomic percent. These alloys were prepared as 9–10 cm3 ingots by an arc-melting technique from pure Mo (99.99 wt%), Ti (99.9 wt%), Mo2C (99 wt%) and TiC (99 wt%) in an argon (Ar) atmosphere. In the rest of this paper, all compositions are expressed in atomic percentages unless otherwise noted. Each ingot was melted five times and was turned over each time to avoid segregation. Heat treatment was carried out at 1,800°C for 72 h in an Ar atmosphere to ensure phase equilibria. The microstructure was examined by field-emission (FE) scanning electron microscopy. Constituent phases were identified by X-ray diffractometry (XRD). Phase compositions were analyzed using a FE electron-probe microanalyzer (EPMA) equipped with a wavelength-dispersive X-ray spectroscope operated at 10 kV and 5.0 × 10−8 A. The composition results were obtained from more than five sets of data, calibrated by the ZAF correction method. The standard samples for each element were as follows: pure Mo for Mo in the Mo solid solution and the TiC phase; pure Ti for Ti in the Mo solid solution; pure TiC fabricated by spark plasma sintering (SPS) at 1,650°C and 65 MPa for 10 min for Ti and C in the TiC phase, C in Mo solid solution and Ti in the Mo2C phase; and Mo2C fabricated by SPS at 1,650°C and 65 MPa for 10 min for Mo and C in the Mo2C phase.
3 Results and discussion
3.1 Microstructure
Figure 1 shows backscatter electron images (BEIs) of the as-cast Mo–Ti–C alloys. Mo–5Ti–15C is composed of a primary phase with bright contrast and a eutectic phase with bright and medium contrast (Figure 1(a)). In Mo–10Ti–20C, the primary phase changes to a medium-contrast phase, and a eutectic phase with bright and medium contrast is also observed. Besides, a finer eutectic phase is also observed in the finally solidified areas, especially around the medium-contrast phase (Figure 1(b)). In Mo–10Ti–15C, the primary phase is a bright-contrast phase and it is apparent that there are two types of eutectic phases, as observed in Mo–10Ti–20C (Figure 1(c)). The finer eutectic phase shows a lamellar microstructure of bright- and dark-contrast phases, and the eutectic lamellae have interfaces with the bright- and medium-contrast phases (Figure 1(d)). These suggest that the finer eutectic microstructure in the finally solidified areas is formed by a ternary transition peritectic reaction. In Mo–15Ti–15C, the primary phase is a bright-contrast phase, and a eutectic phase composed of bright- and dark-contrast phases is observed (Figure 1(e)). The finer eutectic phase is not observed in the alloy. In Mo–30Ti–15C, the primary phase changes to a dark-contrast phase (Figure 1(f)). The volume fraction of the bright-contrast phase and the interlamellar spacing of the bright- and dark-contrast phases increase with increasing Ti concentration in the eutectic microstructure.

BEIs of as-cast alloys: (a) Mo–5Ti–15C, (b) Mo–10Ti–20C, (c and d) Mo–10Ti–15C, (e) Mo–15Ti–15C and (f) Mo–30Ti–15C.
Figure 2 shows the BEIs of the microstructures after heat treatment at 1,800°C. For Mo–5Ti–15C, it is observed that the coarse primary phase with bright contrast contains an amount of small precipitates, and coarsened lamellae with bright- and medium-contrast phases are also evident (Figure 2(a)). The interlamellar spacing has changed to approximately 5 µm, which is much larger than that in the as-cast microstructure shown in Figure 1(a). In Mo–10Ti–20C, three phases are observed with bright, medium and dark contrast (Figure 2(b)). Volume fraction is the highest for the medium-contrast phase and the lowest for the dark-contrast phase. In Mo–10Ti–15C also, three phases are observed, which are similar to those in Mo–10Ti–20Ti (Figure 2(c)). In this alloy, the bright-contrast phase has the highest volume fraction among the three phases. Only bright- and dark-contrast phases are observed in Mo–15Ti–15C even after heat treatment (Figure 2(d)).
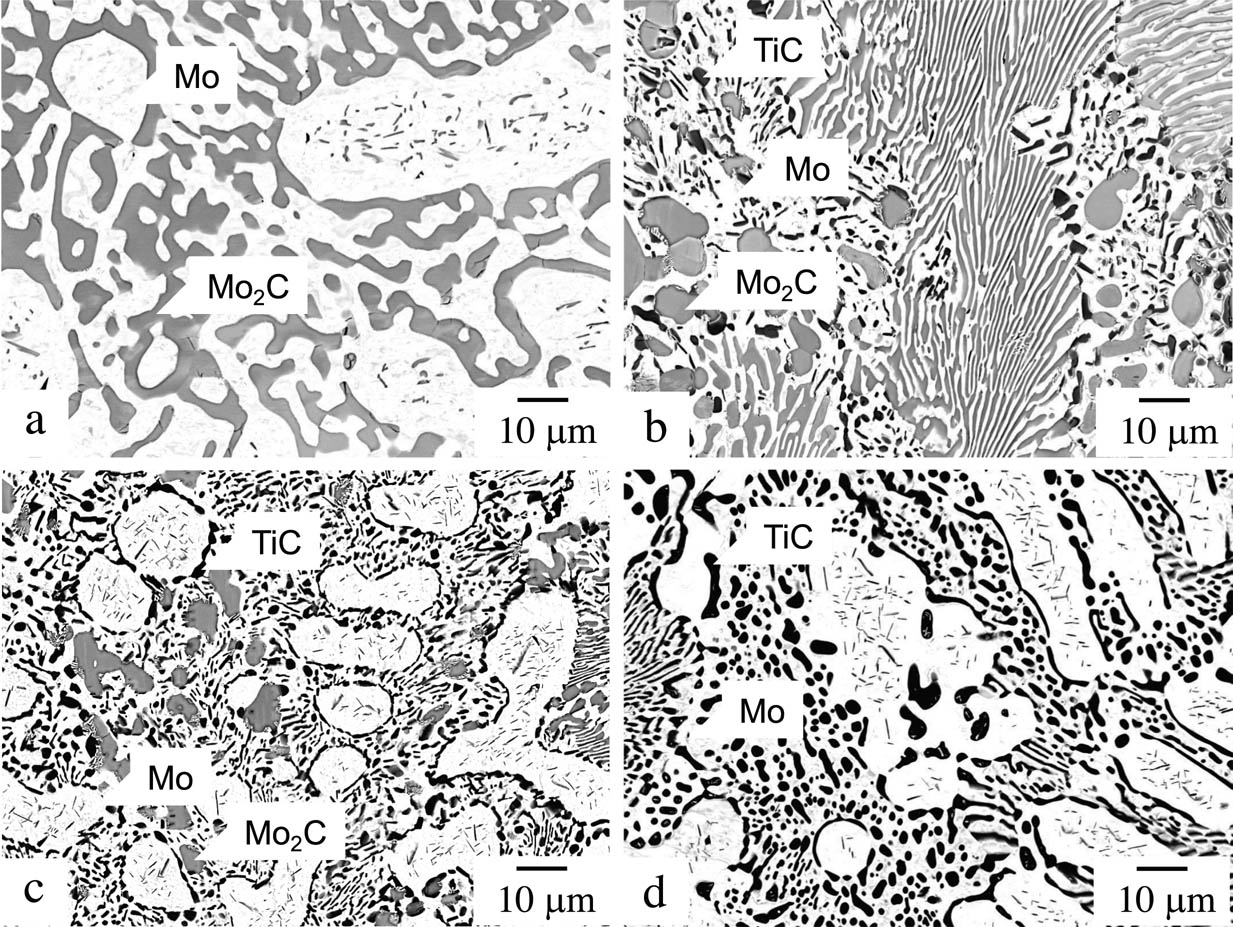
BEIs of microstructures after heat treatment at 1,800°C: (a) Mo–5Ti–15C, (b) Mo–10Ti–20C, (c) Mo–10Ti–15C and (d) Mo–15Ti–15C.
Figure 3 shows the results of phase identification by XRD for the as-cast alloys and the heat-treated alloys at 1,800°C. Simulated patterns for pure Mo, TiC and Mo2C phases are also shown in Figure 3 [27,28,29]. For Mo–5Ti–15C, since the peaks of the Mo and Mo2C phases are detected in both the as-cast and heat-treated alloys (Figure 3(a) and (b)), the constituent phases with bright and medium contrasts are determined to be Mo solid solution (Moss) and Mo2C phases, respectively. Mo–10Ti–15C shows peaks of the TiC phase as well as Moss and Mo2C phases on both the as-cast and heat-treated alloys (Figure 3(c) and (d)) and, thus, the dark-contrast phase is determined to be the TiC phase. On the other hand, the peaks of the Mo and TiC phases are detected on the XRD profiles in both the as-cast and heat-treated alloys of Mo–15Ti–15C (Figure 3(e) and (f)), indicating that the bright- and dark-contrast phases are Moss and TiC phases. It should be noted that the experimental peaks of the TiC phase are slightly shifted to the high-angle side, indicating that the TiC phase with the NaCl structure is slightly contracted by the solute atom of Mo.
![Figure 3 XRD patterns of samples used in this work and comparison with simulated ones: (a) Mo–5Ti–15C, as-cast; (b) Mo–5Ti–15C, 1,800°C/72 h; (c) Mo–10Ti–15C, as-cast; (d) Mo–10Ti–15C, 1,800°C/72 h; (e) Mo–15Ti–15C, as-cast; (f) Mo–15Ti–15C, 1,800°C/72 h; (g) Mo (Im3̅\bar{3}m) [27]; (h) TiC (Fm3̅\bar{3}m) [28]; and (i) Mo2C (P63/mmc) [29].](/document/doi/10.1515/htmp-2020-0053/asset/graphic/j_htmp-2020-0053_fig_003.jpg)
XRD patterns of samples used in this work and comparison with simulated ones: (a) Mo–5Ti–15C, as-cast; (b) Mo–5Ti–15C, 1,800°C/72 h; (c) Mo–10Ti–15C, as-cast; (d) Mo–10Ti–15C, 1,800°C/72 h; (e) Mo–15Ti–15C, as-cast; (f) Mo–15Ti–15C, 1,800°C/72 h; (g) Mo (Im
Mo solute atoms would have a small effect on the lattice parameter of TiC near the stoichiometric composition, but Tsurekawa et al. [30] pointed out that the size misfit between Ti and Mo is too small to consider the decrease in the lattice parameter. On the other hand, off-stoichiometry in TiC rather than the effect of Mo solute atom is likely to cause the decrease in the lattice parameter [31]. To consider both the effect of off-stoichiometry and Mo solute atom, the lattice parameter of the TiC phase was compared at almost the same C/(Mo + Ti) (0.72–0.75) ratios but with different Ti/(Ti + Mo) ratios. Figure 4 shows the change in the lattice parameter of the TiC phase as a function of Ti/(Ti + Mo) atomic ratio at the C/(Mo + Ti) ratio of 0.72–0.75 for the heat-treated alloys at 1,800°C. It is clarified that the lattice parameter increases continuously with the increase in the Ti/(Ti + Mo) atomic ratio. This strongly suggests that the lattice parameter of the C-poor off-stoichiometric TiC phase with the NaCl structure is slightly contracted by solute elements such as Mo.

Lattice parameter of TiC as a function of Ti/(Ti + Mo) atomic ratio in Mo–(10, 15, 30)Ti–15C and Mo–10Ti–20C heat-treated at 1,800°C/72 h.
3.2 Liquidus projection and isothermal section at 1,800°C
Figure 5 shows the liquidus surface projection for the Mo–Ti–C ternary system. The lines are drawn based on the microstructural observations and phase identifications performed in this study with the binary data [14,32,33]. As mentioned before, it is found that a ternary transition peritectic reaction of L + Mo2C → Mo + TiC takes place in the Mo-rich region, which was not shown in the ternary liquidus projection previously reported [14]. Furthermore, the mono-variant eutectic reaction composition of L → Mo + TiC shifts toward the Mo–Ti binary line with increasing Ti concentration.
The isothermal section of the Mo–Ti–C ternary system shown in Figure 6 is also drawn based on the microstructural observations, phase identifications and compositional analyses of the constituent phases. The compositions of the constituent phases analyzed by EPMA are shown in Table 1. The broken line is drawn based on the results reported in the literature [14,30,31]. It is realized that the TiC phase in equilibrium with Moss contains more than 28 at% Mo. A Mo/TiC/Mo2C three-phase region should exist around Mo–15Ti–10C.
Analyzed compositions of the constituent phases present in the alloys of the Mo–Ti–C ternary system at 1,800°C
| Bulk alloy composition | Constituent phase | Composition (at%) | ||||
|---|---|---|---|---|---|---|
| Mo | Ti | C | Mo | Ti | C | |
| Bal. | 5 | 15 | Mo | 97.4 | 1.6 | 0.9 |
| Mo2C | 59.6 | 10.8 | 29.5 | |||
| Bal. | 10 | 15 | Mo | 96.5 | 2.7 | 0.7 |
| Mo2C | 52.3 | 14.3 | 33.4 | |||
| TiC | 28.4 | 29.6 | 42.1 | |||
| Bal. | 10 | 20 | Mo | 96.5 | 2.6 | 0.9 |
| Mo2C | 52.7 | 14.3 | 32.9 | |||
| TiC | 28.1 | 30.1 | 41.8 | |||
| Bal. | 15 | 15 | Mo | 94.5 | 4.8 | 0.6 |
| TiC | 20.2 | 37.0 | 42.7 | |||
| Bal. | 30 | 15 | Mo | 78.7 | 20.3 | 1.1 |
| TiC | 6.2 | 50.9 | 42.8 | |||
| Bal. | 50 | 15 | Mo | 53.6 | 45.6 | 0.8 |
| TiC | 2.3 | 57.8 | 39.8 | |||
3.3 Comparison with previous reports
This study clarifies that there is a ternary transition peritectic reaction of L + Mo2C → Mo + TiC in the Mo–Ti–C ternary system. Therefore, even if the bulk composition is close to the Mo2C primary region, for example, in Mo–15Ti–15C, the Mo2C phase cannot remain during solidification and the lamellar microstructure of the Moss and TiC phases develops around the Mo2C phase, as shown in Figure 1(b) and (d). This microstructural development apparently differs from that reported by Eremenco and Velikanova [19] and Rudy [14], who suggested that a ternary eutectic reaction of L → Mo + TiC + Mo2C takes place.
At 1,800°C, the TiC phase in equilibrium with the Moss phase contains more than 28 at% Mo, as shown in Table 1. This value is close to the value previously reported. However, the compositional region of Mo2C shown in Figure 6 is much larger than that reported previously [14,19], which makes the Mo/TiC/Mo2C three-phase region slightly small.
4 Conclusions
The liquidus surface projection and isothermal section at 1,800°C for the Mo–Ti–C ternary system were examined using arc-melted ternary alloys. The conclusions can be summarized as follows.
A ternary transition peritectic reaction (L + Mo2C → Mo + TiC) takes place, which is apparently different from the ternary eutectic reaction (L → Mo + TiC + Mo2C) previously reported.
Since the composition of the eutectic reaction (L → Mo + TiC) shifts toward the Mo–Ti binary line with increasing Ti concentration, the volume fraction of the Mo phase and the interlamellar spacing of the Mo and TiC phases increase in the eutectic microstructure.
At 1,800°C, the TiC phase in equilibrium with the Mo phase can contain more than 28 at% Mo, and a Mo/TiC/Mo2C three-phase region exists at around Mo–10Ti–15C.
The lattice parameter of the C-poor off-stoichiometric TiC phase is slightly contracted by the solute atom of Mo.
Acknowledgements
This work was supported by the Advanced Low Carbon Technology Research and Development Program (ALCA) of the Japan Science and Technology Agency (JST) (No. JPMJAL1303).
References
[1] Perepezko, J. H. Materials science. The hotter the engine, the better. Science, Vol. 326, No. 5956, Nov. 20, 2009, pp. 1068–1069.Search in Google Scholar
[2] Reed, R. C. The Superalloys, Cambridge University Press, Cambridge, 2006.10.1017/CBO9780511541285Search in Google Scholar
[3] Bewlay, B. P., M. R. Jackson, J.-C. Zhao, P. R. Subramanian, M. G. Mendiratta, and J. J. Lewandowski. Ultrahigh-temperature Nb-silicide-based composites. MRS Bulletin, Vol. 28, No. 9, 2003, pp. 646–653.10.1557/mrs2003.192Search in Google Scholar
[4] Ma, C. L., J. G. Li, Y. Tan, R. Tanaka, and S. Hanada. Microstructure and mechanical properties of Nb/Nb5Si3 in situ composites in Nb–Mo–Si and Nb–W–Si systems. Materials Science and Engineering A, Vol. 386, No. 1–2, 2004, pp. 375–383.10.1016/S0921-5093(04)01013-5Search in Google Scholar
[5] Ito, K., K. Ihara, K. Tanaka, M. Fujikura, and M. Yamaguchi. Physical and mechanical properties of single crystals of the T2 phase in the Mo–Si–B system. Intermetallics, Vol. 9, No. 7, 2001, pp. 591–602.10.1016/S0966-9795(01)00049-8Search in Google Scholar
[6] Yoshimi, K., S. Nakatani, T. Suda, S. Hanada, and H. Habazaki. Oxidation behavior of Mo5SiB2-based alloy at elevated temperatures. Intermetallics, Vol. 10, 2002, pp. 407–414.10.1016/S0966-9795(02)00013-4Search in Google Scholar
[7] Mitra, R. Mechanical behaviour and oxidation resistance of structural silicides. International Materials Reviews, Vol. 51, No. 1, 2006, pp. 13–64.10.1179/174328006X79454Search in Google Scholar
[8] Flem, M. L., A. Allemand, S. Urvoy, D. Cédat, and C. Rey. Microstructure and thermal conductivity of Mo–TiC cermets processed by hot isostatic pressing. Journal of Nuclear Materials, Vol. 380, 2008, pp. 85–92.10.1016/j.jnucmat.2008.01.033Search in Google Scholar
[9] Compton, B. G., and F. W. Zok. Impact resistance of TiC-based cermets. International Journal of Impact Engineering, Vol. 62, 2013, pp. 75–87.10.1016/j.ijimpeng.2013.06.008Search in Google Scholar
[10] Rajabi, A., M. J. Ghazali, and A. R. Daud. Chemical composition, microstructure and sintering temperature modifications on mechanical properties of TiC-based cermet – A review. Materials and Design, Vol. 67, 2015, pp. 95–106.10.1016/j.matdes.2014.10.081Search in Google Scholar
[11] Nishizawa, T., and K. Ishida. The Co–Ni (cobalt–nickel) system. Bulletin of Alloy Phase Diagrams, Vol. 4, No. 4, 1983, pp. 390–395.10.1007/BF02868090Search in Google Scholar
[12] Evans, R. W., and B. Wilshire. Introduction to Creep, Institute of Materials, London, 1993.Search in Google Scholar
[13] Massalski, T. B., ed. Binary Alloy Phase Diagrams, ASM International, Materials Park, OH, 1990, pp. 861–862.Search in Google Scholar
[14] Rudy, E. Part V in Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems, USAF 33(615)-1249 and 33(615)-67-C-1513, Air Force Materials Laboratory, Wright-Patterson Air Force Base, 1969.Search in Google Scholar
[15] Trefilov, V. I., O. M. Barabash, V. A. Zakharkin, V. P. Krashenko, V. F. Moiseyev, and E. P. Pechkovskiy. Russian Metallurgy (Metally), Vol. 6, 1977, pp. 110–116.Search in Google Scholar
[16] Villars, P., A. Prince, and H. Okamoto. Handbook of Ternary Alloys Phase Diagrams, ASM International, Materials Park, OH, Vol. 6, pp. 6939–6950.Search in Google Scholar
[17] Kurishita, H., H. Yoshinaga, F. Takao, and S. Goto. Mechanical properties of Mo-TiC eutectic composite. Journal of the Japan Institute of Metals, Vol. 44, No. 4, 1980, pp. 395–403.10.2320/jinstmet1952.44.4_395Search in Google Scholar
[18] Kurishita, H., J. Shiraishi, R. Matsubara, and H. Yoshinaga. Measurement and analysis of the strength of Mo-TiC composites in the temperature range 285 K–2270 K. Journal of the Japan Institute of Metals and Materials, Vol. 49, No. 11, 1985, pp. 963–971.10.2320/matertrans1960.28.20Search in Google Scholar
[19] Eremenco, V. N., and T. Ya. Velikanova. Handbook of Ternary Alloy Phase Diagrams, ASM International, Metals Park, OH, USA, Vol. 6, 1995, pp. 7085–7096.Search in Google Scholar
[20] Suzuki, T., H. Matsumoto, N. Nomura, and S. Hanada. Transactions of the Materials Research Society of Japan, Vol. 26, 2001, pp. 307–310.Search in Google Scholar
[21] Suzuki, T., H. Matsumoto, N. Nomura, and S. Hanada. Microstructures and fracture toughness of directionally solidified Mo-ZrC eutectic composites. Science and Technology of Advanced Materials, Vol. 3, No. 2, 2002, pp. 137–143.10.1016/S1468-6996(02)00009-8Search in Google Scholar
[22] Suzuki, T., N. Nomura, K. Yoshimi, and S. Hanada. High-temperature strength and room-temperature fracture toughness of Mo-ZrC in-situ composites with hyper-eutectic structure. Journal of the Japan Institute of Metals, Vol. 64, No. 11, 2000, pp. 1082–1088.10.2320/jinstmet1952.64.11_1082Search in Google Scholar
[23] Suzuki, T., N. Nomura, K. Yoshimi, and S. Hanada. Microstructure and creep of Mo–ZrC in-situ composite. Materials Transactions, Vol. 41, No. 9, 2000, pp. 1164–1167.10.2320/matertrans1989.41.1164Search in Google Scholar
[24] Moriyama, T., K. Yoshimi, M. Zhao, T. Masnou, T. Yokoyama, J. Nakamura, et al. Room-temperature fracture toughness of MoSiBTiC alloys. Intermetallics, Vol. 84, 2017, pp. 92–102.10.1016/j.intermet.2017.01.004Search in Google Scholar
[25] Endo, H., M. Ueki, and H. Kubo. Microstructure and mechanical properties of hot-pressed SiC-TiC composites. Journal of Materials Science, Vol. 26, No. 14, 1991, 3769–3774.10.1007/BF01184969Search in Google Scholar
[26] Uemura, S., T. Yamamuro, J. W. Kim, Y. Morizono, S. Tsurekawa, and K. Yoshimi. Quantitative evaluation of microstructure in Mo-Si-B-TiC alloy produced by melting and tilt casting methods. Materials Transactions, Vol. 59, No. 1, 2018, pp. 136–145.10.2320/matertrans.M2017194Search in Google Scholar
[27] Trojko, R., Ž. Blažina, and Z. Ban. The effect of silicon, aluminium and germanium on the stabilization of the C14 polymorph of HfMo2. Journal of the Less Common Metals, Vol. 92, No. 1, 1983, pp. 67–74.10.1016/0022-5088(83)90225-4Search in Google Scholar
[28] Dubrovinskaia, N. A., L. S. Dubrovinsky, S. K. Saxena, R. Ahuja, and B. Johansson. High-pressure study of titanium carbide. Journal of Alloys and Compounds, Vol. 289, No. 1–2, 1999, pp. 24–27.10.1016/S0925-8388(99)00159-0Search in Google Scholar
[29] Lönnberg, B. Thermal expansion studies on the subcarbides of group V and VI transition metals. Journal of the Less Common Metals, Vol. 120, No. 1, 1986, pp. 135–146.10.1016/0022-5088(86)90635-1Search in Google Scholar
[30] Tsurekawa, S., M. Nakashima, A. Murata, H. Kurishita, and H. Yoshinaga. Solid solution hardening of titanium carbide by niobium and zirconium at high-temperatures. Journal of the Japan Institute of Metals, Vol. 55, No. 54, 1991, pp. 390–397.10.2320/jinstmet1952.55.4_390Search in Google Scholar
[31] Storms, E. K. The Refractory Carbides, Academic Press, 1967.10.1016/B978-1-4832-3070-2.50004-5Search in Google Scholar
[32] Seifert, H. J., H. L. Lukas, and G. Petzow. Thermodynamic optimization of the Ti-C system. Journal of Phase Equilibria, Vol. 17, No. 1, 1996, pp. 24–35.10.1007/BF02648366Search in Google Scholar
[33] Massalski, T. B., ed. Binary Alloy Phase Diagrams, 2nd edn, ASM International, Materials Park, OH, 1990, pp. 2675–2678.Search in Google Scholar
© 2020 Shuntaro Ida et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process
Articles in the same Issue
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process

![Figure 5 Liquidus surface projection of the Mo–Ti–C ternary system [14,30,31].](/document/doi/10.1515/htmp-2020-0053/asset/graphic/j_htmp-2020-0053_fig_005.jpg)
![Figure 6 Isothermal section of the Mo–Ti–C ternary system at 1,800°C [14,32,33].](/document/doi/10.1515/htmp-2020-0053/asset/graphic/j_htmp-2020-0053_fig_006.jpg)
