Abstract
To improve the wear resistance, toughness, and hardness of alloy, the quenching and partitioning (Q&P) technology was applied in the VC particulate (VCp) reinforced wear-resistant alloys, which were prepared by adding different Mn contents (2–5 wt%). The effects of partitioning time on the distribution of alloying elements shown by EDX mapping, retained austenite fraction, microstructure, macro-hardness, and impact toughness were investigated. The results showed that the effect of carbon partitioning time on the hard phase of the wear-resistant alloy was not significant. However, the carbon partitioning time greatly affected the microstructure and the mechanical properties of alloys, such as retained austenite, hardness, and impact toughness, and there was also a strong correlation with the Mn content. When the Mn content was lower (2.51 wt%), the retained austenite content increased with the carbon partitioning time, which resulted in decreased hardness and increased impact toughness. However, when the Mn content was higher (4.52 wt%), the opposite results occurred. This study provided an application of the Q&P technology in a VCp-reinforced wear-resistant alloy.
1 Introduction
Wear-resistant materials have been widely used in modern machinery manufacturing, and conventional wear-resistant alloys were mainly included high manganese steel [1], nickel-hard cast iron [2], and high-chromium cast iron [3]. Recently, the high-vanadium high-speed alloy has been given much more attention as a result of the increased requirement of increased wear resistance [4]. Cai et al. [5] proposed that the hardness of the VC-reinforced phase was up to 2,600–2,800 Hv, and its particle morphology was superior to the Cr3C7 phase in high-chromium cast iron. To improve the wear resistance of alloys [6,7,8], the common method was the addition of a large amount of carbon to alloys. However, this method inevitably led to low toughness and plasticity of alloy.
The quenching and partitioning (Q&P) technology [9,10,11,12] has been applied to improve the toughness and plasticity of medium and low carbon steels by stabilizing austenite during the martensitic transformation process. This process takes place by the diffusion of carbon from supersaturated martensite obtained at the temperature ranging from Ms to Mf by quenching transformation [13,14]. The concentration of carbon in austenite reduces the Ms temperature and stabilizes untransformed austenite through the proper Q&P process. Thus, the steels treated with the Q&P technology exhibited the excellent toughness and plasticity at the room temperature condition due to the stress and impact energy being partly absorbed during martensite transformation. Choi et al. [15] dealt with the micro-alloyed bar steel using the Q&P technology. The volume fraction of austenite retained at room temperature was mainly dependent on the quenching temperature, partitioning temperature, and partitioning time. Wendler et al. [16] studied Q&P processing with a subzero cooling step and a partitioning step at 450°C. After the Q&P process, the stainless steel exhibited outstanding mechanical properties including yield strength of 1,050 ± 12 MPa, an ultimate tensile strength of 1,550 ± 10 MPa, and a tensile elongation of 22 ± 0.3% at room temperature. Huang et al. [17] analyzed the Fe–Cr–C, Fe–Cr–C–Co and Fe–Cr–C–N stainless steels with the Q&P technology. The results showed that about 12% of the total contained carbon in the martensite partitioned into the austenite between 20 and 200°C, and the formation of tempered carbides was one of the main causes of carbon segregation.
In general, the matrix of the high-vanadium high-speed wear-resistant alloy is mainly martensite [18,19] and includes the slight austenite content. The austenite will be easily transformed to martensite after subsequently tempering. Due to the austenite content being low, the TRIP effect cannot be achieved giving to insufficient toughness. Therefore, the Q&P technology has become a feasible approach for improving the plasticity and toughness of the high-vanadium high-speed wear-resistant alloys. In this study, the high-vanadium high-speed steel wear-resistant alloys with different Mn contents were prepared. To provide a basis for the application of Q&P heat treatment technology in the VCp-reinforced wear-resistant alloy, the effects of Q&P technology on the microstructures, as shown by EDX mapping of alloying elements, retained austenite content, and hardness and impact toughness were investigated.
2 Material and methods
2.1 Preparation of experimental materials
Two types of the VCp-reinforced wear-resistant alloy were prepared by adding 3 and 5 wt% Mn, respectively. However, as the Mn element is easy to volatilize and its yield is not stable, the contents of Mn in the final samples are 2.51 and 4.52 wt%. The detailed sample preparation steps are as follows: first, the scrap and pig iron were added in a 50 kg medium-frequency induction furnace for melting, and the slag generated in the melting process was removed. Then, manganese, ferrotitanium, chromium iron, and molybdenum iron were added to the melt, and the aluminum wire was added for deoxygenation when the temperature reached 1,500°C. Later, the vanadium iron alloy was added into the mixed melt. The tapping temperature was controlled at 1,520–1,570°C, and the casting temperature was 1,450–1,480°C. A keel block was cast in the sand mold and was removed after cooling. The ingot samples were cut into several specimens of 11 mm × 11 mm × 56 mm in dimensions, and the metallographic specimens of size 10 mm × 10 mm × 15 mm were cut using a wire cutting machine. The compositions of the two types of wear-resistant alloys are presented in Table 1 (the balance was Fe).
Chemical compositions of experimental alloys (wt%) (Fe balance)
| Sample | C | Si | Mn | V | Mo | Cr | Ti | Al | S | P |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 2.83 | 0.80 | 2.51 | 6.20 | 1.52 | 1.83 | 1.12 | 0.12 | 0.05 | 0.04 |
| A2 | 2.75 | 0.79 | 4.52 | 6.50 | 1.47 | 1.90 | 1.26 | 0.14 | 0.04 | 0.06 |
2.2 The Q&P heat treatment experiment
The effects of the Q&P heat treatment on the structures and properties of two types of wear-resistant alloys were investigated. Figure 1 presented a schematic sketch of the Q&P process, which consisted of isothermal quenching and isothermal carbon partition experiments. For each Mn content alloy (2.51 wt% or 4.52 wt%), six specimens were prepared and put into the furnace together for carrying out the experiment. The quenching temperature was set at 200°C. As the temperature of the current size specimens can reach uniform distribution within 5 min, the holding time was set at 5 min. In the carbon partition process, the temperature was set at 350°C, and six specimens have been taken out in turn for considering the effect of carbon partition time, which were set at 10, 60, 300, 600, 1,800, and 5,400 s.
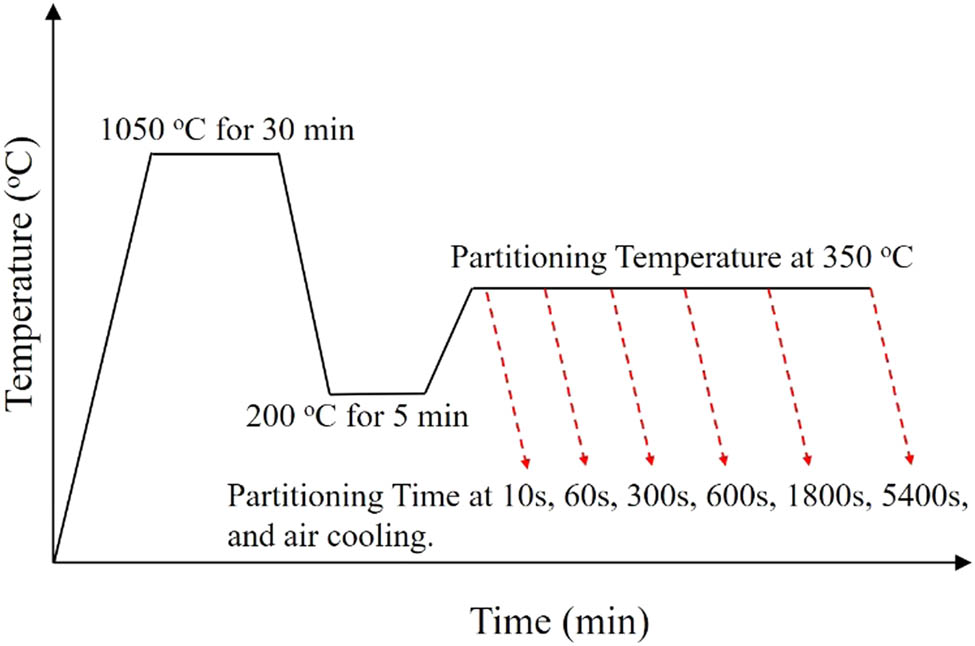
Schematic sketch of the Q&P process.
2.3 Mechanical property test and microstructure analysis of specimen
After the Q&P heat treatment, the metallographic structure of metallographic specimens were obtained by lycra microscope (DM2700M, Germany) after etched for 20 min at 60°C in a mixture of hydrochloric acid and sulfuric acid. Ingot samples were processed by wire electrical discharge machining into the standard dimensions of 10 mm × 10 mm × 55 mm, and tested for impact toughness in the fall-hammer impact testing machine (JB-100B). Subsequently, the macro hardness values of the samples were measured in a macroscopic Rockwell Hardness Tester (Tester HRS-150) five times, and the averaged value was treated as the final hardness value. In addition, the microstructure of sample was obtained by scanning electron microscope (SEM; SIGMA 300, Germany) operating at 30 kV, and its retained austenite fraction was measured by X ray diffraction (XRD; Philips-X’Pert Pro MPD, Holland) with Cu kα1 radiation (1.540598 Å). After determining the position of Bragg peaks observed over the range of 2θ = 5–90°, the phases were identified using databases of the International Centre for Diffraction Data, and the austenite proportion was calculated by [20,21]:
where
3 Results and discussion
3.1 Effects of the Q&P technology on the microstructure and elements mapping
The microstructures of two types of specimens with different Mn contents were shown in Figure 2. The black particles presented the hard phase (VCP), and the gray area around the hard phase was matrix. Figure 2(a) shows that the distribution of VCP was nonuniform when the Mn content was low (2.51 wt%). The distribution of VCP in red frame was sparse, whereas its distribution in the blue frame was denser. However, when the Mn content was high (4.52 wt%), the distribution of VCP tended to be more uniform, as shown in Figure 2(b).

SEM-BSE images showing microstructures of as-cast samples: (a) 2.51 wt% Mn and (b) 4.52 wt% Mn, and black particles are the precipitates of VC.
Figures 3(a), 4(a), and 5(a) were SEM-BSE images. The gray parts of the alloy were matrix, and the black parts present the hard phase. Figures 3(b–f), 4(b–f), and 5(b–f) show the element mapping of the A1 alloy at different partition times. All of V formed the carbide hard phase at different partition times. Ti was also a main element in the hard phase, and it was enriched in the center of the hard phase. However, with an increase in the partition time, a very slight amount of Ti diffused to the matrix. Compared with V and Ti, the amounts of Cr and Mo, which were enriched in hard phase, were smaller, and more of Cr and Mo diffused from the hard phase to the matrix with the increased partition time. When the partition time was 5,400 s, the diffusion phenomenon of Mo was significant because V, Ti, Cr, and Mo have different stabilities in carbides. The stability of carbides in alloy depends on the number of electrons in the d-layer of the element [22,23]. The lower the number of d-layer electrons of the alloy element, the more stable the precipitated carbides. The order of d-layer electrons number is Ti(2) < V(3) < Cr(4) < Mo(5) [23]. Therefore, compared with Cr and Mo, V and Ti more tended to form carbides, and their distributions in the hard phase were more stable. With the increased partition time, the slight diffusion of Ti from the hard phase to the matrix may be caused by the combination of Ti and N in the matrix [24]. However, Mn was mainly distributed in the matrix. This was because the carbide of Mn was unstable, and Mn has a strong binding force with Fe [23]. It ensured that the hardness of the hard phase did not greatly change during the Q&P process.

Elemental maps of A1 alloy for 60 s partitioning time: (a)-SEM-BSE image and EDX map for (b) V, (c) Ti, (d) Cr, (e) Mo, and (f) Mn.

Elemental maps of A1 alloy for 1,800 s partitioning time: (a)-SEM-BSE image and EDX map for (b) V, (c) Ti, (d) Cr, (e) Mo, and (f) Mn.

Elemental maps of A1 alloy for 5,400 s partitioning time: (a)-SEM-BSE image and EDX map for (b) V, (c) Ti, (d) Cr, (e) Mo, and (f) Mn.
3.2 Effect of partition time on retained austenite
The results of XRD with different Mn contents are shown in Figure 6, and the mass fraction of austenite calculated using equation (1) was presented in Table 2. The retained austenite content in alloy A1 increased from 9.3 to 15.6 wt% with the increased partition time. As the Mn content increased, the retained austenite content in alloy A2 was high at the 60 s partition time, but it decreased to 34.10 wt% with the longer partition time. This was due to the retained austenite decomposing with the increasing of partition time [25,26]. However, the final retained austenite content was higher for the larger Mn addition.

Retained austenite spectrum by XRD.
Retained austenite fraction of wear-resistant alloys (volume, %)
| Alloys | Mn (wt%) | Partition time (s) | Austenite fraction |
|---|---|---|---|
| A1 | 2.51 | 60 | 9.3 |
| 2.51 | 5,400 | 15.6 | |
| A2 | 4.52 | 60 | 85.8 |
| 4.52 | 5,400 | 34.1 |
3.3 Effect of Q&P technology on hardness and impact toughness
Figure 7(a) shows the hardness changes of alloy A1 and alloy A2 with different carbon partition times, and Figure 7(b) shows the microstructures of two alloys at different carbon partition times, where points (a), (b), (c), and (d) corresponded to the points on Figure 7(a). Figure 7(a) shows the hardness changes of these two alloys had the opposite trends. When the Mn content was 2.51 wt% (alloy A1), the initial hardness of alloy A1 was high. This was due to the martenite content in alloy A1 being relatively high, which can be clearly seen in point (a) of Figure 7(b). Due to the martenite changing to austenite point (b) of Figure 7(b)), the hardness of alloy A1 decreased sharply from (66 ± 0.32) HRC to (56 ± 0.41) HRC with the increased carbon partition time. For the alloy A2 (Mn = 4.52 wt%), the initial hardness was lower than that of alloy A1. This was because the martenite was low before 600 s, and the austenite was the main phase, as shown in point (c) of Figure 7(b). However, the hardness of alloy A2 increased with the increased partition time, especially at point (d). This was because some of austenite changed to the martenite during the later carbon partition process.

Hardness and microstructures of alloys A1 and A2 with different carbon partition times. (a) Hardness change of alloys A1 and A2 with different carbon partition times. (b) Metallographic structure of alloys A1 and A2 with different carbon partition times. In this figure, the particles present the hard phase, the black areas around particles were martenite, and the gray areas around particles were austenite.
Figure 8 shows the impact toughness changes of alloys A1 and A2 with different carbon partition times. The Q&P technology had different effects for the different Mn contents. The impact toughness of alloy A1 had an overall increase with the increased the carbon partition time, whereas the trend of alloy A2 was decreased. The impact toughness of alloy was affected by the austenite content, and the results matched well with the aforementioned analyses.

Impact toughness changes of alloys A1 and A2 with different carbon partition times.
4 Conclusions
In this study, two types of VCp-reinforced wear-resistant alloys with different Mn contents were used to investigate the effects of Q&P heat treatment technology on the alloy performances. The main conclusions are as follows:
Compared to the lower Mn content condition (2.51 wt%), the distribution of VCP had uniform distribution when the Mn content was higher (4.52 wt%).
The hard phases were carbides of Ti, V, Cr, and Mo, while the Mn element was mainly distributed in the matrix. Therefore, the hardness of the hard phase had no great change during the Q&P process.
The carbon partitioning time had a significant effect on the retained austenite fraction of alloy, and its effect varied with the Mn content. In general, the element of Mn increased the austenite content in alloy, resulting in the higher impact toughness of alloy.
Acknowledgments
The authors gratefully acknowledge the financial support for this work from the Specialized Research Fund for the National Natural Science Foundation of China (No. 51504090) and the Natural Science Foundation of Hunan Province, China (No. 2019JJ60062).
Data availability statement: The data used to support the findings of this study are available from the corresponding author upon request.
References
[1] Zhang, X. Z., F. Ni, S. Z. Wei, R. Long, and Y. P. Ji. The effects of potassium salt multi-modification on carbide morphologies and distribution of high vanadium high speed steel. Foundry, Vol. 56, 2007, pp. 290–293.Search in Google Scholar
[2] Li, P., Z. Z. Du, H. G. Fu, and Z. J. Feng. Effect of heat treatment temperature on microstructure and hardness of high vanadium high speed steel. Materials for Mechanical Engineering, Vol. 33, 2009, pp. 93–95.Search in Google Scholar
[3] Xu, L., Z. Yan, and Y. Liu. Microstructure evolution and martensitic transformation behaviors of 9Cr–1.8W–0.3Mo ferritic heat-resistant steel during quenching and partitioning treatment. The Journal of Materials Research, Vol. 28, 2013, pp. 2835–2843.10.1557/jmr.2013.267Search in Google Scholar
[4] Seo, E. J., L. Cho, and Y. Estrin. Microstructure-mechanical properties relationships for quenching and partitioning (Q&P) processed steel. Acta Materialia, Vol. 113, 2016, pp. 124–139.10.1016/j.actamat.2016.04.048Search in Google Scholar
[5] Cai, B., Y. F. Tan, H. E. Long, H. Tan, and X. L. Wang. Tribological behavior and mechanisms of graphite/CaF2/TiC/Ni-base alloy composite coatings. Transactions of Nonferrous Metals Society of China, Vol. 23, 2013, pp. 392–399.10.1016/S1003-6326(13)62475-9Search in Google Scholar
[6] Lee, S., S. J. Lee, and B. C. De Cooman. Austenite stability of ultrafine-grained transformation-induced plasticity steel with Mn partitioning. Scripta Materialia, Vol. 65, 2011, pp. 225–228.10.1016/j.scriptamat.2011.04.010Search in Google Scholar
[7] Liu, G., S. G. Zhang, Q. G. Meng, J. Wang, and J. Li. Integrated treatment of GCP and slag quenching water of LD steel making unit. Ironmaking & Steelmaking, Vol. 44, 2017, pp. 1–8.10.1080/03019233.2016.1246846Search in Google Scholar
[8] Speer, J., D. K. Matlock, and B. C. D. Cooman. Carbon partitioning into austenite after martensite transformation. Acta Materialia, Vol. 51, 2003, pp. 2611–2622.10.1016/S1359-6454(03)00059-4Search in Google Scholar
[9] Speer, J. G., D. V. Edmonds, and F. C. Rizzo. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Current Opinion in Solid State & Materials Science, Vol. 8, 2004, pp. 219–237.10.1016/j.cossms.2004.09.003Search in Google Scholar
[10] He, B., J. Li, C. B. Shi, and H. Wang. Effect of cooling intensity on carbides in Mg-containing H13 steel during the electroslag remelting process. Chinese Journal of Engineering, Vol. 38, 2016, pp. 1720–1727.Search in Google Scholar
[11] Zhong, N., Q. Wu, and Y. Yin. Microstructual evolution of amedium carbon advanced high strength steel heat-treated by Quenching-Partitioning Process. Steel Research International, Vol. 86, 2015, pp. 252–256.10.1002/srin.201400064Search in Google Scholar
[12] Liu, H. F., Y. H. Liu, and S. R. Yu. Investigation of the wear resistance of high carbon high vanadium high speed steel. Tribology, Vol. 20, 2000, pp. 401–406.Search in Google Scholar
[13] Matlock, D. K., and J. G. Speer. Third Generation of AHSS: Microstructure Design Concepts. Springer, London, 2009, pp. 185–205.10.1007/978-1-84882-454-6_11Search in Google Scholar
[14] Xu, L., Z. Yan, and Y. Liu. Microstructure evolution and martensitic transformation behaviors of 9Cr–1.8W–0.3Mo ferritic heat-resistant steel during quenching and partitioning treatment. The Journal of Materials Research, Vol. 28 , 2013, pp. 2835–2843.10.1557/jmr.2013.267Search in Google Scholar
[15] Choi, I., D. M. Bruce, D. K. Matlock, and J. G. Speer. The high speed deformation behavior of TRIP steels. Metals & Materials International, Vol. 14, 2008, pp. 139–146.10.3365/met.mat.2008.04.139Search in Google Scholar
[16] Wendler, M., C. Ullrich, M. Hauser, L. Krüger, O. Volkova, A. Weiß, et al. Quenching and partitioning (Q&P) processing of fully austenitic stainless steels. Acta Materialia, Vol. 133, 2017, pp. 346–355.10.1016/j.actamat.2017.05.049Search in Google Scholar
[17] Huang, Q., C. Schröder, H. Biermann, O. Volkova, and J. Mola. Influence of Martensite Fraction on Tensile Properties of Quenched and Partitioned (Q&P) Martensitic Stainless Steels. Steel Research International, Vol. 87, 2016, pp. 1082–1094.10.1002/srin.201500472Search in Google Scholar
[18] Clarke, A. J., J. G. Speer, and M. K. Miller. Carbon partitioning to austenite from martensite or bainite during the quench and partition (Q&P) process: A critical assessment. Acta Materialia, Vol. 56, 2008, pp. 16–22.10.1016/j.actamat.2007.08.051Search in Google Scholar
[19] Clarke, A. J., J. G. Speer, and D. K. Matlok. Influence of carbon partitioning kinetics on final austenite fraction during quenching and partitioning. Scripta Materialia, Vol. 61, 2009, pp. 149–152.10.1016/j.scriptamat.2009.03.021Search in Google Scholar
[20] Edmonds, D. V., K. He, and F. C. Rizzo. Quenching and partitioning martensite—A novel steel heat treatment. Materials Science and Engineering: A, Vol. 25, 2006, pp. 25–34.10.1016/j.msea.2006.02.133Search in Google Scholar
[21] Cho, L., E. J. Seo, and B. C. De Cooman. Near-Ac3 austenitized ultra-fine-grained quenching and partitioning (Q&P) steel. Scripta Materialia, Vol. 123, 2016, pp. 69–72.10.1016/j.scriptamat.2016.06.003Search in Google Scholar
[22] Sluiter, M. Phase Stability of Carbides and Nitrides in Steel. Materials Research Society Proceedings, Vol. 979, 2006, pp. 3–8.10.1557/PROC-979-0979-HH14-03Search in Google Scholar
[23] Yu, X. X., C. R. Weinberger, and G. B. Thompson. Ab initio investigations of the phase stability in group IVB and VB transition metal carbides. Computational Materials Science, Vol. 112, 2016, pp. 18-326.10.1016/j.commatsci.2015.10.038Search in Google Scholar
[24] Yugeswaran, S., A. Kobayashi, K. Suresh, and B. Subramanian. Characterizationof gas tunnel type plasma sprayed TiN reinforced Fe-basedmetallic glass coatings. Journal of Alloys and Compounds, Vol. 551, 2013, pp. 168–175.10.1016/j.jallcom.2012.09.111Search in Google Scholar
[25] Zhu, S., S. Kuang, and Y. Jiang. Effect of quenching and partitioning process on microstructure and mechanical properties of Q&P steel. Jinshu Rechuli/Heat Treatment of Metals, Vol. 38, 2013, pp. 1–4.Search in Google Scholar
[26] Zhou, Y., and G. Zhang. Study on electronic theory of the interaction between rare earth elements and impurities at grain boundaries in Ni-base superalloy. Rare Metal Materials & Engineering, Vol. 36, 2007, pp. 2160–2162.Search in Google Scholar
© 2020 Bo Zhang and Song-Sheng Zeng, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process
Articles in the same Issue
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process

