Abstract
Ce, Zr and Ce–Zr composite experimentl steel were prepared by vacuum induction furnace and 550 twin-roll reversible rolling mill. Optical microscope (OM), scanning electronic microscopy (SEM) and energy dispersive spectrometer (EDS) were used to observe the rolling microstructure of the experimental steel. The mechanical properties of the experimental steel were tested and analyzed. The effect of cerium zirconium oxide inclusions on nucleation, tensile and impact fracture mechanism of intragranular acicular ferrite (IAF) was investigated. The results show that the rolling microstructure of steel containing 0.0052% Ce and the steel under composite treatment containing 0.0053% Ce and 0.0055% Zr is refined. IAF generation can be induced by Al–Ce–O inclusion of the size of 4 µm or induced by Al–Ce–Zr–O + MnS inclusion of the size of 3 µm. The yield strength and tensile strength of the steel treated by Ce–Zr are 428 and 590 MPa, respectively, the elongation is 23.55%, the longitudinal impact energy at −60°C is 189 J, which are 31, 45, 46 J and 6.25%, respectively, higher than those of the matrix steel. The dimple of the experimental steel at the fracture surface is larger and deeper than that of the matrix steel. The small inclusions in uniform distribution contribute to the high tensile strength of the experimental steel.
1 Introduction
To improve the welding efficiency of the shipbuilding industry, high heat input welding is wildly used. However, the increase in welding heat input will lead to decrease toughness in heat affected zone (HAZ), especially in coarse grained heat affected zone (CGHAZ). Therefore, shipbuilding steel plates with high strength, toughness, plasticity and excellent weldability are required. At present, many research on nitrides, oxides and sulfides of Ti, Mg, B, Zr, Al, etc., are available, which can refine austenite grains or promote the growth of acicular ferrite in grains, so as to improve the toughness of shipbuilding steel plate [1,2,3,4,5]. However, there are only a few studies on the microstructure and properties of the high strength shipbuilding steel plate for high heat input welding under rare earth treatment.
Studies have shown that the treatment of Ce or Zr alone can produce a large number of fine second-phase inclusions in steel [6,7,8,9,10,11]. This kind of particles can effectively induce ferrite nucleation, pin austenite grain boundary, refine microstructure of HAZ and improve low-temperature-impact toughness. Yamashita et al. [12], and Yang et al. [13,14] reported that adding a small amount of rare earth Ce can convert not only the large-size, long strip and irregular rare-earth sulfide inclusions into spherical inclusions but also weaken the segregation phenomenon and obviously refine the grains. After adding a small amount of rare earth Ce, the distribution of dimples tends to be more uniform, the fracture morphology and the impact performance showed improvement. Milyuts et al. [15] reported that the original coarse and irregular inclusions in the shipbuilding steel plate treated by rare earth La and Ce changed into small and spherical rare earth oxides. The interfacial tension between liquid and solid phase was reduced. The nucleation rate of the crystal was promoted. The primary crystal structure was refined. Thewlis [16] reported that fine and dispersed secondary phase particles such as rare earth oxides and rare earth sulfides can form a low degree of mismatch with α-Fe. The secondary phase particles can promote heterogeneous nucleation of acicular ferrite and refine microstructure even under high heat input welding conditions. Van der Eijk et al. [17] treated the alloy steel with cerium and sulfur. It is found that the dispersed tiny sulfur oxide particles in the steel were conducive to promote ferrite nucleation. Zhang et al. [18], Wang [19], and Zhao et al. [20] discussed that zirconium is a strong oxide forming element, which can be used as an inoculant to transform harmful inclusions into beneficial fine and dispersed distribution in steel. Zirconium can change the influence of inclusions on steel properties and improve the strength and toughness of steel as the phase transformation core.
In view of the fact that there is no report about the effect of Ce–Zr composite treatment on the structure and properties of shipbuilding steel plate at home and abroad, FH40 shipbuilding steel plate was studied by Ce, Zr treatment and Ce–Zr composite treatment in order to further play the role of trace elements. The casting parts were forged and rolled. The rolling microstructure was observed and analyzed. And the particle size distribution and inclusion composition of typical nonmetallic inclusions in steel were investigated. The influence of nonmetallic inclusions such as Ce, Zr on ferrite nucleation and fracture mechanism was further researched. The results established the foundation for the development of Ce–Zr composite treatment to improve the tensile strength and impact toughness of HAZ of shipbuilding steel plate for high heat input welding.
2 Experiment
The experimental FH40 shipbuilding steel plate was smelted in a 50 kg vacuum induction furnace. Part of the cold material was put into the furnace. After the material was melted for 5 min, ferroniobium was added. Carbon sheet, ferrosilicon and Mn were added successively in 1 min. Al was added as the melting is done. After all the alloys were melted, zirconium metal was added. Rare earth cerium block was wrapped with pure iron sheet and inserted into the bottom of the liquid metal. After stirring for 1 min, the rare earth block was poured. The ingot taken out was a cylinder with a circle of Φ150 mm at the bottom and a height of 170 mm. The chemical composition of the experimental steel is shown in Table 1. The mass fractions of C, Si, Mn, Ni, Nb, Ti, Al, P and S were measured by spectrum analyzer. The mass fractions of O and N were measured by TC-600 oxygen nitrogen analyzer. The mass fractions of Ce and Zr were measured by inductively coupled plasma atomic emission spectrometry.
Chemical composition of N1–N4 experimental steel (wt/%)
| No. | C | Si | Mn | P | S | Ni | Nb | Ti | Al | N | O | Ce | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | 0.063 | 0.249 | 1.59 | 0.008 | 0.01 | 0.298 | 0.032 | 0.125 | 0.032 | 0.0070 | 0.0041 | — | — |
| N2 | 0.061 | 0.252 | 1.6 | 0.009 | 0.01 | 0.295 | 0.033 | 0.123 | 0.030 | 0.0071 | 0.0041 | 0.0052 | — |
| N3 | 0.060 | 0.250 | 1.58 | 0.008 | 0.009 | 0.307 | 0.034 | 0.124 | 0.031 | 0.0069 | 0.0040 | — | 0.0056 |
| N4 | 0.059 | 0.252 | 1.6 | 0.01 | 0.11 | 0.3 | 0.031 | 0.120 | 0.030 | 0.0070 | 0.0042 | 0.0053 | 0.0055 |
The experimental samples included experimental matrix FH40 shipbuilding steel plate (N1), experimental FH40 shipbuilding steel plate (N2) with 0.0052% cerium, experimental FH40 shipbuilding steel plate (N3) with 0.0056% zirconium and composite experimental FH40 shipbuilding steel plate (N4) with 0.0053% cerium and 0.0055% zirconium. As shown in Table 2, with the help of professional image analysis software Ipp6.0, the area fraction of polygonal ferrite (PF), pearlite structures in the solidification structure of experimental steel were quantitatively analyzed.
Volume fractions of PF, EF and P in test samples
| No | PF/EF/% | P/% | Average grain size/µm |
|---|---|---|---|
| N1 | 93.3 ± 0.70 | 6.7 ± 0.70 | 9.81 ± 1.24 |
| N2 | 88.3 ± 0.42 | 11.7 ± 0.51 | 9.34 ± 1.17 |
| N3 | 81.4 ± 0.67 | 18.6 ± 0.44 | 8.26 ± 0.98 |
| N4 | 81.9 ± 0.28 | 18.1 ± 0.39 | 7.84 ± 1.08 |
The ingot was heated at 1,150°C for 30 min after removing both the ends. The ingot size was forged in shape of 100 mm × 100 mm × 300 mm and then air cooled to room temperature. The experiment was conducted according to the following rolling process: the forging was heated up to 1,200°C for 2 h. The starting rolling temperature of rough rolling was 1,150°C, and the finishing rolling temperature of rough rolling was over 1,000°C. Three processes are involved in rough rolling, while the deformation of 24.5%, 13.4% and 12.1% occurred at each process, and the total deformation was 50%. Four processes are involved in fine rolling, while the deformation of 26.2%, 20.4%, 15.1%, 8.3% occurred at each process, and the total deformation was 70%. The finish rolling temperature of the fine rolling was 890°C. The air–mist cooling was conducted at 840–450°C, and the cooling rate was 10°C/s. The final plate thickness was 16 mm.
The metallographic specimens in the size of 10 mm × 10 mm × 10 mm were cut at 1/4 of the width of the steel plate. The metallographic specimens were mechanically polished (without polishing solution) and then corroded by 4% nitric acid alcohol solution. The microstructure of the rolled experimental steel was analyzed by Axiovert200 Zeiss metallographic microscope and Quanta250 cold field SEM.
According to GB/T2975, the standard R7 tensile specimen was cut at 1/4 of the width of the steel plate along the rolling direction, and the room temperature test was carried out with INSTRON-3382 electronic universal tensile tester. The impact specimen should be cut on the cross section of the steel plate according to GB/T 229-2007, and the standard 10 mm × 10 mm × 55 mm Charpy V-Notch impact specimen was cut along the rolling direction and the vertical rolling direction, respectively, at 1/4 of the width of the steel plate. PIT452g-4 pendulum impact test machine was used for impact test at −60°C. Quanta250 cold field scanning electron microscopy was used to detect the fracture of tensile and impact specimens, and the impact fracture mechanism was analyzed.
3 Results and discussion
3.1 The rolling microstructure of FH40 shipbuilding steel plate containing cerium and zirconium
Figure 1(a) shows the rolling morphology and microstructure of FH40 matrix steel N1, and the rolling structure consists of PF, bainite (B) and a little amount of pearlite (P). Figure 1(b) shows the rolling morphology and microstructure of shipbuilding steel plate N2 containing cerium. It shows that after adding 0.0052% Ce to the matrix steel, the PF becomes equiaxed ferrite (EF), the ferrite grains become smaller, the amount of pearlite increases from 6.7% to 11.7% and granular bainite (GB) [21] is formed. The white part is ferrite, and the black part is pearlite. Figure 1(c) shows the rolling morphology and the microstructure of the rolling shipbuilding steel plate N3 containing zirconium. It can be seen that the main microstructure of the experimental steel added 0.0056% Zr is also ferrite and pearlite. The pearlite is greatly increased and distributed in chain. Figure 1(d) shows the rolling morphology and microstructure of FH40 shipbuilding steel plate N4 by Ce–Zr composite treatment. It shows that the microstructure of the experimental steel with 0.0053% Ce + 0.0055% Zr is also white ferrite, black pearlite and dispersed GB. The quantity of the black pearlite decreases. The rolling morphology and microstructure of the experimental steel with Ce and Zr treated separately are integrated, and it was found that the ferrite grain size is smaller than that of the two separate treatments [12]. After adding rare earth elements, rare earth compounds were produced. The formation of rare earth compounds, reduces the carbon content of the solid solution in austenite, inhibits the austenite grain growth and the banded structure is weakened [22]. The quantity of chain pearlite decreases,and the new size of ferrite crystal decreases (as shown in Table 2). The pearlite content of N4 is higher than that of the steel treated by Ce alone and lower than that of the steel treated by Zr. The size of bainite grain increases in irregular distribution [22,23].

N1–N4 rolled metallographic microstructure: (a) N1; (b) N2; (c) N3; (d) N4.
Figure 2 shows the rolling morphology and microstructure of N1 steel and the energy spectrum analysis results of Al–O + MnS composite phase. It can be seen from the figure that in addition to the bulk ferrite, part of GB and small GB islands are dispersed in N1 steel [21]. Generally, the GB is the product of medium-temperature transformation of undercooled austenite. These GBs have high strength and toughness [24], playing a second-phase strengthening role in steel [25]. The GB with fine dispersion distribution can refine the structure. The GB is of nearly spherical and isotropic shape, which can strengthen the oxide inclusions and improve the comprehensive mechanical properties of steel [26]. The inclusions consist of Al–O + MnS. The inclusion size as shown in Figure 2 is about 2.45 µm, without intragranular acicular ferrite (IAF) (according to the statistics of automatic inclusion analysis system of feature, the average size of the inclusions in 1 mm2 random zone is 2.48 µm).

Energy spectrum and microstructure of Al–O + MnS composite phase in N1 steel.
Figure 3 shows the rolling morphology and microstructure and energy spectrum analysis results of Al–Ce–O + MnS composite phase of shipbuilding steel plate containing Ce. The inclusion consists of an Al–Ce–O + MnS composite phase with a size of about 4 µm. The inclusion is elliptical and forms two pieces of IAF (a1,a2), with the inclusion being the core. Rare earth Ce element has certain modification effect on sulfur and oxygen inclusions, which makes the inclusions present spheroidization dispersion in the matrix [27,28]. In the process of solidification, nucleation requires the formation of nucleus which is larger than the critical size obtained from the liquid phase. High undercooling is required in the process of solidification. However, when there are suitable second-phase particles in the melt, the activation energy of heterogeneous nucleation and the required undercooling degree decreases greatly. The increase in nucleation density promotes the formation of acicular ferrite and grain refinement.

N2 steel morphology and Al–Ce–O + MnS composite phase spectrum.
Figure 4 shows the rolling morphology and microstructure and the results of energy spectrum analysis of Zr–O + MnS inclusions in FH40 shipbuilding steel plate. It can be seen from the figure that there are GB islands on the ferrite surface of Zr-treated steel. Zr–O is the core of the inclusion, and MnS is attached to the inclusion surface. The inclusion size is about 2 µm, no IAF is found around the oxide [29]. In order to further analyze the composition distribution of the inclusions, the results of surface scanning analysis of Zr–O + MnS composite phase is shown in Figure 5. It can be seen that the black area is MnS. MnS is not uniformly attached to the surface of inclusion Zr–O, is only on the left side of inclusion Zr–O.

Microstructure of N3 steel and energy spectrum of Zr–O + MnS composite phase: A is Zr–O; B is MnS.
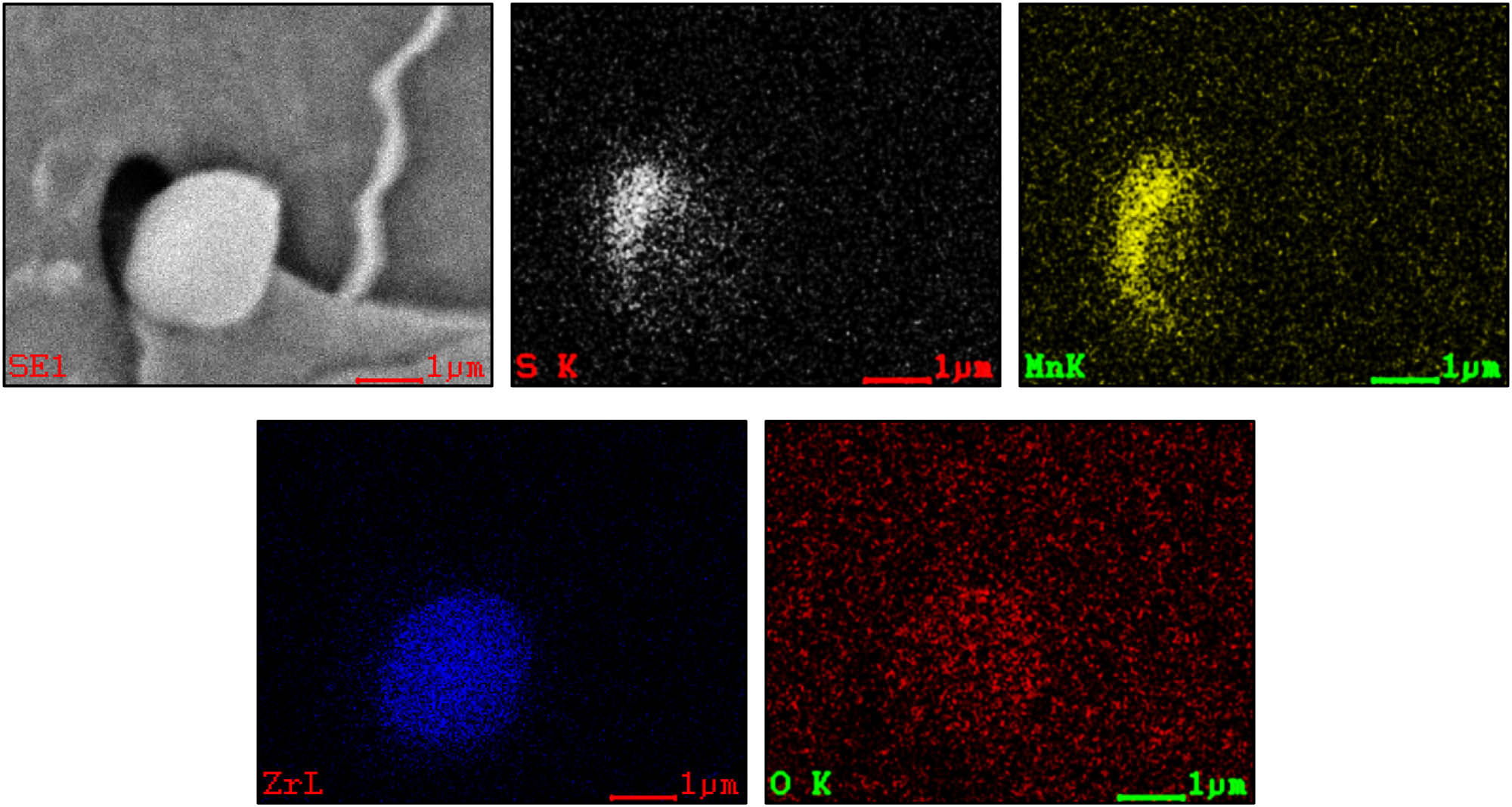
Surface scanning of Zr–O + MnS composite phase in N3 steel.
Figure 6 shows the rolling morphology and microstructure of FH40 shipbuilding steel plate with cerium zirconium and the energy spectrum analysis of Al–Ce–Zr–O + MnS composite phase. The inclusions take Al–Ce–Zr–O + MnS as the core, and the size is about 3 µm. Four pieces of IAF (a1,a2,a3,a4) were formed at 90°, which is more than two pieces of IAF produced by N2. According to the two-dimensional mismatch calculation model proposed by Bramfitt [30] and Turnbull and Vonnegut [31], the lattice mismatch degree of Ce inclusions and α-Fe phase in steel at 912°C is calculated. The smaller the mismatch degree between inclusions and α-Fe phase, the more favorable the nucleation of intragranular ferrite, and the finer the grain structure formed by the crystallization. When the mismatch degree of inclusions and α-Fe phase is less than 6%, the nucleation effect is the best. The mismatch degree between the inclusion and α-Fe phase is only 1.5%, which is far less than 6%. So it has very similar lattice matching degree. Therefore, it has good nucleation ability and can promote the formation of intragranular ferrite.

Microstructure of N4 steel and energy spectrum of Al–Ce–Zr–O + MnS composite phase.
The mismatch calculation model is shown as follows:
Here (hkl)s is the low index surface of the basement, (hkl)n is the low index surface of new crystalline phase, [uvw]s is the low index direction of (hkl)s and [uvw]n is the low index direction of (hkl)n.
3.2 Mechanical properties of FH40 shipbuilding steel plate containing cerium and zirconium
3.2.1 Tensile test
Table 3 shows the experimental results of tensile properties of N1–N4 experimental steel. It can be seen that the tensile strength and elongation of FH40 shipbuilding steel plate with cerium zirconium composite are better than those of other experimental steels. This is because the grain size of the rolling microstructure of N4 experimental steel is smaller than that of other three experimental steel (as shown in Table 2). It is generally believed that the yield strength ratio of steel should be less than 0.85, which could ensure the safety and stability of the shipbuilding steel plate [32]. The results show that the yield strength ratio of the four experimental steel is about 0.75, which has excellent plastic deformation, and is not easy to fracture.
Tensile test results of N1–N4 steel
| No. | Upper yield strength ReL, MPa | Tensile strength Rm, MPa | Yield ratio | Elongation after fracture A, % |
|---|---|---|---|---|
| N1 | 397 | 545 | 0.73 | 17.3 |
| N2 | 423 | 557 | 0.76 | 21.1 |
| N3 | 435 | 585 | 0.74 | 17.17 |
| N4 | 428 | 590 | 0.73 | 23.55 |
Figure 7 shows the comparison results of tensile properties of N1–N4 experimental steel at room temperature. Figure 7(a) shows that there are appearance of elastic stage, yield stage, strengthening stage and necking fracture stage in the N1, N2, N3 and N4 experimental steels. When the elongation of N1, N2, N3 and N4 experimental steels is 9%, 12%, 11.5% and 12.6%, the maximum tensile strength is 545, 557, 585 and 590 MPa, respectively. The tensile strength of N2–N4 experimental steel is higher than that of N1 experimental steel, which is due to not only the refinement of the rolling structure but also the formation of IAF induced by oxide inclusions in the steel. The mismatch degree between the inclusions formed by Ce–Zr and α-Fe phase is less than 6%, which is conducive to the nucleation of intragranular ferrite on the surface of inclusions [30,31]. That is to say, the original austenite grain is divided into many acicular ferrite grains and then the grain structure is refined. According to Hall Petch formula [33], the yield strength and tensile strength of steel will be improved to a certain extent by ferrite grain refinement. Figure 7(b) shows that the elastic deformation rate is slow in the stress–strain curve of N1 experimental steel. The elastic deformation stages of N2–N4 experimental steel is almost the same. N2–N4 experimental steel shows different characteristics in the stages of yield, plastic deformation and necking deformation. The yield stage of the tensile curve of FH40 shipbuilding steel plate with cerium and FH40 shipbuilding steel plate with zirconium is approximately continuous. The yield phenomenon of FH40 shipbuilding steel plate with zirconium is obvious. Figure 7(c) shows that the yield strength N3 > N4 > N2 > N1; the tensile strength N4 > N3 > N2 > N1; and the elongation N4 > N2 > N1 > N3.

Tensile properties of N1–N4 experimental steel (a) tensile engineering stress–strain curve, (b) yield stage enlarged view and (c) tensile properties.
Figure 8 is SEM photo of tensile fracture morphology of experimental steel. Generally, the crack source is preferentially located in the slip zone, grain boundary. Sometimes the crack source is located in the subgrain boundary with a large number of dislocations. For the experimental steel, the inclusion is most likely to be the origin of the crack. The reason is that the interface between inclusions and iron matrix has different elastic–plastic properties when the sample is subjected to external force. When the external force exceeds the binding force between them, they separate from each other and form microcracks [34]. Figure 8(a) shows that the fracture surface has many small round dimples. Under the action of external force, these dimples begin to connect with each other to form a fiber zone, which is formed in the center of tensile specimen. And there are a few round dimples with larger diameter and deeper depth, with nonmetallic inclusions in the dimples. Figure 8(b) shows that the dimple is directionally elongated. The dimple is shallow and some inclusions exists. Figure 8(c) shows that the dimple is larger and deeper compared with Figure 8(b). Figure 8(d) shows that the dimple is the largest and deepest among all the experimental steels. The size of inclusions in dimples is very important. When the size of inclusions is larger than the critical particle size, inclusions are the source of cracks. When the inclusion size is smaller than the critical particle size, cracks will not appear. So it has the highest tensile strength. In addition, the reason of the crack is also related to the tight binding between the inclusions in the dimple and the matrix [35]. Because of little difference in the dimple size and depth of the fracture morphology, only little difference can be observed in the tensile strength of N2–N3 experimental steel.

Experimental steel tensile fracture morphology (a) N1, (b) N2, (c) N3 and (d) N4.
3.2.2 Low temperature impact
Table 4 shows the impact toughness test results of N1–N4 experimental steel at low temperature (−60°C). It is known from the experimental results that the low-temperature-impact toughness of the experimental steel meets the requirements of the International Association of Classification Societies (IACS) [36]. The impact toughness can be used to qualitatively judge the toughness and brittleness of materials. The energy required for brittle and low plastic metal materials is less than that required for high-plastic metal materials. Generally, the high-impact energy depends on plasticity, and the low-impact energy depends on strength.
Low-temperature-impact toughness of N1–N4 experimental steel
| No. | Experimental temperature/°C | Impact energy AKV (J) | |
|---|---|---|---|
| Longitudinal | Transverse | ||
| N1 | −60 | 143 | 42.6 |
| N2 | 179 | 68.4 | |
| N3 | 151 | 38.2 | |
| N4 | 189 | 75.1 | |
Figure 9 shows the comparison results of low-temperature-impact toughness of experimental steel. The low-temperature-impact toughness of experimental FH40 shipbuilding steel plate N2 improved comparing with that of N1. The longitudinal impact energy increased by 25% from 143 to 179 J, and the transverse impact energy increased by 60% from 42.6 to 68.4 J. Compared with the N1 experimental steel, the N3 experimental steel is not significantly improved, with only 8 J increase in the longitudinal direction and 4.4 J decrease in the transverse direction. This is due to the weak effect of Zr on fine grain strengthening in steel under the conditions of this study, and other strengthening mechanisms (such as precipitation strengthening, second-phase strengthening, etc.) are dominant. It results in the increased strength and decreased toughness and plasticity. At the same time, the addition of Zr leads to the banded distribution of P structure. The banded distribution of P structure increases the anisotropy of steel and makes the properties of steel have direction. This will also affect its mechanical properties to a certain extent and result in a significant decrease in the transverse impact toughness of the steel plate [37]. Figure 9 shows that the impact energy of N4 experimental steel at low temperature increases by 32% in longitudinal direction and 76% in transverse direction. As a result of the lattice distortion caused by the solution of trace Ce into the shipbuilding steel plate, the storage energy increases, and the recrystallization driving force increases, and the recrystallization is promoted, thus improving the impact toughness [38]. Therefore, the addition of cerium and zirconium can improve the low-temperature-impact energy of the experimental steel and make the steel have good low-temperature-impact toughness.

Experimental steel low-temperature-impact performance.
4 Conclusion
The rolling microstructure of matrix FH40 steel is composed of ferrite, bainite and a little pearlite. The ferrite changed from strip shape to equiaxed shape, and the grain size becoming smaller, the pearlite increasing and the bainite shape becoming granular by 0.0052% Ce treatment. The pearlite increased greatly and distributed in chain by 0.0056% Zr treatment. The structure composes of white ferrite, black pearlite and dispersed GB by composite treatment of 0.0053% Ce + 0.0055% Zr. Ferrite grains is finer, and the size decreases from 9.81 to 7.84 µm. The pearlite content increases from 6.7% to 18.1%. Bainite grain size increases in irregular distribution.
FH40 matrix steel produced the Al–Ce–O + MnS inclusion which is about 4 µm in size. The produced inclusion can induce two pieces of IAF nucleation and refine the structure because of the adding of 0.0052% Ce treatment. Granular bainite is produced by the addition of 0.0053% Ce + 0.0055% Zr. And inclusions with the core of Al–Ce–Zr–O + MnS of 3 µm size were produced, resulting in the formation of four pieces of IAF, which is close to the pearlite.
The dimples of FH40 matrix steel were directionally elongated, and the dimples were shallow with a certain amount of nonmetallic inclusions by the treatment with 0.0052% Ce. The dimples on the cross section of the experimental steel become larger and deeper, and inclusions can be observed by 0.0053% Ce + 0.0055% Zr composite treatment.
The yield strength, tensile strength and low-temperature-impact toughness of FH40 matrix steel improved due to the addition of cerium or zirconium. The yield strength shows N3 > N4 > N2 > N1. The tensile strength shows N4 > N3 > N2 > N1 and the elongation shows N4 > N2 > N1 > N3. Compared with N1 matrix steel, the longitudinal impact energy of N2–N4 experimental steel increased by 36, 8 and 46 J, respectively. And the transverse impact energy of N2–N4 experimental steel compared with N1 matrix steel changed by 21.8, −4.2 and 32.5 J, respectively.
Acknowledgments
The present study is supported by the National Natural Science Foundation of China through grant number (51974129), and 2020 Tangshan Science and Technology Project (20130228b).
References
[1] Yan, C., D. W. Qi, and M. Q. Wu. R&D present status and progress of high strength ship plate steel at home and abroad. Special Steel, Vol. 32, 2011, pp. 26–30.Search in Google Scholar
[2] Yan, C., and Z. Li. Brief introduction of ship plate production of Japanese iron and steel enterprises. Wide and Heavy Plate, Vol. 13, 2007, pp. 44–48.Search in Google Scholar
[3] Li, X. B., C. J. Liu, and M. F. Jiang. Effect of M (M = Mg, Zr, Mg–Zr) addition on microstructure and mechanical properties in FH40 ship plates steel. Journal of Central South University (Science and Technology), Vol. 46, 2015, pp. 3586–3593.Search in Google Scholar
[4] Chen, Z. Y. Research and development of micro-alloying high-strength shipbuilding plate, Springer International Publishing, Cham, 2016, pp. 1141–1147.10.1007/978-3-319-48767-0_143Search in Google Scholar
[5] Wu, J. N., W. J. Li, Q. H. Pang, J. Guo, L. Yan, and J. J. Wang. Status and prospect of researches on effects of rare earth elements on microstructure and properties of high heat input welding steels. Journal of University of Science and Technology Liaoning, Vol. 41, 2019, pp. 13–21.Search in Google Scholar
[6] Li, C. F., and X. Z. Lin. Development of new manufacturing technology of ship building steel and the progress in baosteel. Shanghai Metals, Vol. 33, 2011, pp. 57–62.Search in Google Scholar
[7] Xi, T. H., X. Chen, and Z. X. Yuan. Progress in research on structure and properties of heat affected zone of steel for high heat input welding. Special Metals, Vol. 24, 2003, pp. 1–5.Search in Google Scholar
[8] Yang, Q. X., H. Q. Wu, X. J. Ren, and X. Guo. Study on the effect of rare earth oxides on weld inclusion and microstructure. Chinese Rare Earths, Vol. 10, 1994, pp. 151–154.Search in Google Scholar
[9] Mizoguchi, S., and J. Takamura. Process 6th International Iron and Steel Congress, ISIJ International, Nagoya, Japan, 1990, pp. 2331–2342.Search in Google Scholar
[10] Takamura, J., and S. Mizoguchi. Proceedings of the 6th International Iron and Steel Congress, ISIJ International, Nagoya, Japan, 1990, pp. 591–597.Search in Google Scholar
[11] Mizoguchi, S., and J. Takamura. Proceedings of the 6th International Iron and Steel Congress, ISIJ International, Nagoya, Japan, 1990, pp. 598–604.Search in Google Scholar
[12] Yamashita, T., J. Shimamura, K. Oi, M. Nagoshi, K. Oikawa, and K. Ishida. Grain refinement of heat affected zone in high heat input welding by liquid phase pinning of oxy-sulfide. Journal of the Iron and Steel institute of Japan, Vol. 55, 2015, pp. 2018–2026.10.2355/isijinternational.ISIJINT-2015-033Search in Google Scholar
[13] Yang, J. L., M. Z. Jiang, Z. L. Cui, and S. B. Wang. Effect of Ce addition on microstructures and properties of 65 Mn steel. Iron Steel Vanadium Titanium, Vol. 36, 2015, pp. 141–149.Search in Google Scholar
[14] Yang, J. C., H. C. Yu, and J. J. Gao. Effects of Ce on the microstructure and mechanical properties of A36 ship plate steel. Chinese Rare Earths, Vol. 36, 2015, pp. 43–48.Search in Google Scholar
[15] Milyuts, V. G., V. V. Tsukanov, O. Y. Malykhina, A. B. Nasonovskaya, A. G. Vladimirov, V. A. Golubtsov, et al. Effect of complex inoculation of a high-strength shipbuilding steel on the composition and morphology of nonmetallic inclusions. Inorganic Materials: Applied Research, Vol. 5, 2014, pp. 554–561.10.1134/S2075113314060070Search in Google Scholar
[16] Thewlis, G. Effect of cerium sulphide particle dispersions on acicular ferrite microstructure development in steels. Materials Science and Technology, Vol. 22, 2006, pp. 153–166.10.1179/026708306X81432Search in Google Scholar
[17] Van der Eijk, C., I. Lassila, O. Grong, O. Svein Kleven, and L. Holappa. Ferrite nucleation on Ce-oxysulphide inclusions in low alloyed steels. Materials Science and Technology, Vol. 34, 2004, pp. 199–206.Search in Google Scholar
[18] Zhang, Y. Q., H. Q. Zhang, S. X. Zhao, and M. W. Liu. Effects of Nb on microstructure and toughness of high-strength structural steels heat affected zone at high heat input. Transactions of the China Welding Institution, Vol. 29, 2008, pp. 96–100.Search in Google Scholar
[19] Wang, M. Effect of weld thermal cycle on second phase particles in Ti-Nb micro-alloyed steel. Hot Working Technology, Vol. 38, 2009, pp. 22–28.Search in Google Scholar
[20] Zhao, H., S. P. Hu, H. B. Wu, and T. Q. Li. Simulation study on heat affected zone of X120 pipeline steel. Hot Working Technology, Vol. 39, 2010, pp. 20–23.Search in Google Scholar
[21] Hu, Y. H. Transactions of Metal Heat Treatment, Vol. 1, 1980, pp. 15−25.Search in Google Scholar
[22] Hu, J., L. X. Du, H. Liu, G. S. Sun, H. Xie, H. L. Yi, et al. Structure-mechanical property relationship in a low-C medium-Mn ultrahigh strength heavy plate steel with austenite-martensite submicro-laminate structure. Materials Science & Engineering A, Vol. 647, 2015, pp. 144–151.10.1016/j.msea.2015.09.008Search in Google Scholar
[23] Xiao, J. G., H. J. Cheng, and F. M. Wang. Effects of rare earth in ship hull plate steel on its microstructure and low temperature toughness. Chinese Rare Earths, Vol. 31, 2010, pp. 52–58.Search in Google Scholar
[24] Li, X. B. Research of the Microstructure and Properties based on Mg or Zr Treated Ship-building Steel Plates, PhD thesis, Northeastern University, Shenyang, 2016.Search in Google Scholar
[25] Zhou, Y. L., T. Jia, X. J. Zhang, Z. Y. Liu, and R. D. K. Misra. Investigation on tempering of granular bainite in an offshore platform steel. Materials Science and Engineering: A, Vol. 626, 2015, pp. 352–361.10.1016/j.msea.2014.12.074Search in Google Scholar
[26] Kumar, A., and A. Singh. Toughness dependence of nano-bainite on phase fraction and morphology. Materials Science and Engineering: A, Vol. 729, 2018, pp. 439–443.10.1016/j.msea.2018.05.106Search in Google Scholar
[27] Huang, Y., Y. Xie, G. G. Cheng, L. Chen, Y. D. Zhang, and Q. Z. Yan. Effect of rare earth on large size heterogeneous nucleation carbide in H13 steel. Journal of the Chinese Society of Rare Earths, Vol. 35, 2017, pp. 782–789.Search in Google Scholar
[28] Wang, L. Z. Basic research on fine dispersion of non-metallic inclusions in aluminum deoxidized steel. PhD thesis. University of Science and Technology Beijing, Beijing, 2017.Search in Google Scholar
[29] Shi, M. H., P. Y. Zhang, and F. X. Zhu. Toughness and microstructure of coarse grain heat affected zone with high heat input welding in Zr-bearing low carbon steel. Transactions of; the Iron & Steel institute of Japan, Vol. 54, 2014, pp. 188–192.10.2355/isijinternational.54.188Search in Google Scholar
[30] Bramfitt, B. L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metallurgical and Materials Transactions, Vol. 1, 1970, pp. 1987–1995.10.1007/BF02642799Search in Google Scholar
[31] Turnbull, D., and B. Vonnegut. Nucleation catalysis. Industrial and Engineering Chemistry, Vol. 44, 1952, pp. 1292–1294.10.1021/ie50510a031Search in Google Scholar
[32] Zhao, J. L. Calculation and judgment of yield ratio in steel tube tensile test. Petroleum Tubular Goods & Instrument, Vol. 4, 2018, pp. 75–77.Search in Google Scholar
[33] Liu, J., and S. L. Lu. Relationship between microstructure and properties in low carbon steel. Journal of University of Science and Technology Beijing, Vol. 24, 2002, pp. 208−210.Search in Google Scholar
[34] Taheri, M., A. Halvaee, and S. F. Kashani-Bozorg. Effect of Nd:YAG pulsed-laser welding parameters on microstructure and mechanical properties of GTD-111 superalloy joint. Materials Research Express, Vol. 6, 2019, pp. 1–15.10.1088/2053-1591/ab1534Search in Google Scholar
[35] Cui, Y. X., and C. L. Wang. Fracture analysis of metal, Harbin Institute of Technology Press, Harbin, 1998, pp. 27–40.Search in Google Scholar
[36] Liu, L. Q. Rules For Materials And Welding, China Communications Press, Beijing, 2016, pp. 7–8.Search in Google Scholar
[37] Yang, Z. H., and J. M. Fu. Reduction of banded structure in 16Mn plate for special use. Wide and Thick Plate, Vol. 2, 2001, pp. 24–25Search in Google Scholar
[38] Zhang, H. M., L. P. Zhao, X. D. Qin, and X. S. Sun. Effect of cerium on microstructure and hardness of 00 Cr 17 Mo stainless steel. Chinese Rare Earths, Vol. 34, 2013, pp. 27–30.Search in Google Scholar
© 2020 Meng Xianghai et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process
Articles in the same Issue
- Research Article
- Electrochemical reduction mechanism of several oxides of refractory metals in FClNaKmelts
- Study on the Appropriate Production Parameters of a Gas-injection Blast Furnace
- Microstructure, phase composition and oxidation behavior of porous Ti-Si-Mo intermetallic compounds fabricated by reactive synthesis
- Significant Influence of Welding Heat Input on the Microstructural Characteristics and Mechanical Properties of the Simulated CGHAZ in High Nitrogen V-Alloyed Steel
- Preparation of WC-TiC-Ni3Al-CaF2 functionally graded self-lubricating tool material by microwave sintering and its cutting performance
- Research on Electromagnetic Sensitivity Properties of Sodium Chloride during Microwave Heating
- Effect of deformation temperature on mechanical properties and microstructure of TWIP steel for expansion tube
- Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag
- Effects of metallurgical factors on reticular crack formations in Nb-bearing pipeline steel
- Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system
- Energy Conservation and CO2 Abatement Potential of a Gas-injection Blast Furnace
- Experimental validation of the reaction mechanism models of dechlorination and [Zn] reclaiming in the roasting steelmaking zinc-rich dust process
- Effect of substituting fine rutile of the flux with nano TiO2 on the improvement of mass transfer efficiency and the reduction of welding fumes in the stainless steel SMAW electrode
- Microstructure evolution and mechanical properties of Hastelloy X alloy produced by Selective Laser Melting
- Study on the structure activity relationship of the crystal MOF-5 synthesis, thermal stability and N2 adsorption property
- Laser pressure welding of Al-Li alloy 2198: effect of welding parameters on fusion zone characteristics associated with mechanical properties
- Microstructural evolution during high-temperature tensile creep at 1,500°C of a MoSiBTiC alloy
- Effects of different deoxidization methods on high-temperature physical properties of high-strength low-alloy steels
- Solidification pathways and phase equilibria in the Mo–Ti–C ternary system
- Influence of normalizing and tempering temperatures on the creep properties of P92 steel
- Effect of temperature on matrix multicracking evolution of C/SiC fiber-reinforced ceramic-matrix composites
- Improving mechanical properties of ZK60 magnesium alloy by cryogenic treatment before hot extrusion
- Temperature-dependent proportional limit stress of SiC/SiC fiber-reinforced ceramic-matrix composites
- Effect of 2CaO·SiO2 particles addition on dephosphorization behavior
- Influence of processing parameters on slab stickers during continuous casting
- Influence of Al deoxidation on the formation of acicular ferrite in steel containing La
- The effects of β-Si3N4 on the formation and oxidation of β-SiAlON
- Sulphur and vanadium-induced high-temperature corrosion behaviour of different regions of SMAW weldment in ASTM SA 210 GrA1 boiler tube steel
- Structural evidence of complex formation in liquid Pb–Te alloys
- Microstructure evolution of roll core during the preparation of composite roll by electroslag remelting cladding technology
- Improvement of toughness and hardness in BR1500HS steel by ultrafine martensite
- Influence mechanism of pulse frequency on the corrosion resistance of Cu–Zn binary alloy
- An interpretation on the thermodynamic properties of liquid Pb–Te alloys
- Dynamic continuous cooling transformation, microstructure and mechanical properties of medium-carbon carbide-free bainitic steel
- Influence of electrode tip diameter on metallurgical and mechanical aspects of spot welded duplex stainless steel
- Effect of multi-pass deformation on microstructure evolution of spark plasma sintered TC4 titanium alloy
- Corrosion behaviors of 316 stainless steel and Inconel 625 alloy in chloride molten salts for solar energy storage
- Determination of chromium valence state in the CaO–SiO2–FeO–MgO–CrOx system by X-ray photoelectron spectroscopy
- Electric discharge method of synthesis of carbon and metal–carbon nanomaterials
- Effect of high-frequency electromagnetic field on microstructure of mold flux
- Effect of hydrothermal coupling on energy evolution, damage, and microscopic characteristics of sandstone
- Effect of radiative heat loss on thermal diffusivity evaluated using normalized logarithmic method in laser flash technique
- Kinetics of iron removal from quartz under ultrasound-assisted leaching
- Oxidizability characterization of slag system on the thermodynamic model of superalloy desulfurization
- Influence of polyvinyl alcohol–glutaraldehyde on properties of thermal insulation pipe from blast furnace slag fiber
- Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process
- Development and experimental research of a low-thermal asphalt material for grouting leakage blocking
- A downscaling cold model for solid flow behaviour in a top gas recycling-oxygen blast furnace
- Microstructure evolution of TC4 powder by spark plasma sintering after hot deformation
- The effect of M (M = Ce, Zr, Ce–Zr) on rolling microstructure and mechanical properties of FH40
- Phase evolution and oxidation characteristics of the Nd–Fe–B and Ce–Fe–B magnet scrap powder during the roasting process
- Assessment of impact mechanical behaviors of rock-like materials heated at 1,000°C
- Effects of solution and aging treatment parameters on the microstructure evolution of Ti–10V–2Fe–3Al alloy
- Effect of adding yttrium on precipitation behaviors of inclusions in E690 ultra high strength offshore platform steel
- Dephosphorization of hot metal using rare earth oxide-containing slags
- Kinetic analysis of CO2 gasification of biochar and anthracite based on integral isoconversional nonlinear method
- Optimization of heat treatment of glass-ceramics made from blast furnace slag
- Study on microstructure and mechanical properties of P92 steel after high-temperature long-term aging at 650°C
- Effects of rotational speed on the Al0.3CoCrCu0.3FeNi high-entropy alloy by friction stir welding
- The investigation on the middle period dephosphorization in 70t converter
- Effect of cerium on the initiation of pitting corrosion of 444-type heat-resistant ferritic stainless steel
- Effects of quenching and partitioning (Q&P) technology on microstructure and mechanical properties of VC particulate reinforced wear-resistant alloy
- Study on the erosion of Mo/ZrO2 alloys in glass melting process
- Effect of Nb addition on the solidification structure of Fe–Mn–C–Al twin-induced plasticity steel
- Damage accumulation and lifetime prediction of fiber-reinforced ceramic-matrix composites under thermomechanical fatigue loading
- Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3
- Microstructure of metatitanic acid and its transformation to rutile titanium dioxide
- Numerical simulation of nickel-based alloys’ welding transient stress using various cooling techniques
- The local structure around Ge atoms in Ge-doped magnetite thin films
- Friction stir lap welding thin aluminum alloy sheets
- Review Article
- A review of end-point carbon prediction for BOF steelmaking process

