Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
-
Habibi Hidayat
Abstract
A facile synthesis method of gold nanoparticles (AuNPs) utilizing Lantana camara flower extract (LFE) using visible light illumination towards the bio-reduction system has been conducted. The systematic characterizations of AuNPs were employed using transmission electron microscopy, X-ray powder diffraction, scanning electron microscopy, and X-ray photoelectron spectroscopy. The nanoparticles having a particle size of ranging 4.8–25 nm were obtained with dependence on the LFE concentration of the extract and time of light irradiation. The antibacterial activity of AuNPs was evaluated against Escherichia coli, Staphylococcus aureus, Propionibacterium acnes, and Pseudomonas aeruginosa, and the photocatalytic activity was examined in methylene blue photooxidation. The overall results point to a promising photochemical synthesis of AuNPs utilizing plant extract and the potential activities of synthesized nanoparticles as antibacterial agents and photocatalysts.
1 Introduction
With the rapid development of nanotechnology, research and innovative applications of nanomaterials consisting of nanoparticles are also interesting concerns. Nanoparticles’ excellent characteristics such as optonic, sensor, antibacterial, and antioxidative properties were employed for miscellaneous innovative applications in medical and environmental fields [1,2,3]. In particular, the adoption of nanotechnology and nanomaterials in pharmaceutical and biomedical sectors is in very high demand. Some of these are the efficacy of nanomedicine including the use of green-synthesized metal nanoparticles and nanocomposite in drug delivery systems for various disease conditions, especially cancer, biodetection, and antibacterial coating. Within this scheme, gold nanoparticles (Au NPs) are one of the exciting nanoparticles, and the increasing applicability of Au NPs forced the innovation for its sustainable and green synthesis [3].
One of many possible approaches within the eco-friendly process for preparation is the use of plant extract as a facile agent in the bio-reduction step in the synthesis. The high content of secondary metabolites having potency to reduce Au NPs precursor, especially from diverse medicinal plants, is an interesting issue to explore the Au NPs green synthesis utilizing plant extract [4,5]. Besides cheap, abundant, and convenient features of the plant extracts, capability of the extract to be a capping agent for synthesized Au NPs brings many advantages related to bioactivity and stability. In addition, different shapes that can be controlled by different methods in the synthesis are also interesting synthesis variables [6,7].
Various medicinal plant extracts such as Moringa oleifera, Aloe vera, Magnifea indica, Mimosa tenuiflora, and Stevia rebaudiana have been reported for Au NPs synthesis; however, some other techniques and specific conditions for optimum synthesis have attracted the attention in the field [8,9,10]. A well-known garden plant of Lantana camara Linn. (L. camara) was previously reported as bio-reductor in the Au NPs synthesis. An intensive study of chemical constituents of leaves and flowers revealed the high content of polyphenols [11]. The leaves of the plant are used in the traditional medical treatment of malaria, and rheumatic, anti-urolithiatic, antiseptic, and carminative properties have also been reported [12,13]. It is also confirmed by the high antioxidant and enzymatic activity of the extract. In addition, it was profound that the higher activity was found in flowers, which is related to the quantitative composition of the antioxidant [14]. The rich content of secondary metabolite in L. camara leaf and flower extracts has also been employed in synthesizing metal nanoparticles. In this regard, L. camara has been utilized as a bio-reductor in the synthesis of silver, gold, and CuO nanoparticles [15,16,17].
Such intensification methods of microwave-assisted, ultrasound irradiation, and photochemical-assisted methods were studied. Photochemical process of metal nanoparticles is an interesting method gaining attention in the fabrication of metallic NPs due to controllability and simplicity in operation. A typical experiment exposes the metal precursor and reductor solutions to visible or ultraviolet (UV) light at ambient conditions. The photochemical process begins with photon absorption by a reducing agent that accelerates the reduction of the metal precursor, from n+ valence (Mn+) to its zero-valence state (M0). Furthermore, controlling metal formation to prevent agglomeration as well as directing the certain particle size are other important steps. Some studies utilized reducing agents from polymers as the capping agents and stabilizers. Such polymers of poly(vinyl pyrrolidone), polymethacrylate, poly-, mono-saccharides, and proteins (chitosan, glucose, dextrose, and gelatin) were reported in the synthesis of AuNPs and Ag NPs. The controlling factors such as specific interactions and composition of reactant trigger significant variations in the size and shape of the obtained metallic NPs [18,19].
Based on this mechanism, secondary metabolites from plants consisting pigments are potential agents for conducting this action. The presence of secondary metabolites in plant extracts, including from the flower extract of L. camara, could act as the photon catcher, stabilizers, and capping agents during the photochemical process. To our knowledge, photochemical synthesis mechanism utilizing plant extract has not been studied yet; therefore, studies on the use of L. camara flower extract for the photochemical synthesis of Au NPs become interesting to be explored. The study will be an initial step for developing photochemical synthesis with other potential plant extracts. Particularly, the composition of extract and precursor solution as well as the time of irradiation were considered crucial factors for the AuNPs formation. Instrumental analyses utilizing X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope, and X-ray photoelectron spectroscopy analyses were performed, and toward the potential applicability of the synthesized nanoparticles, photocatalytic and antibacterial studies were also conducted. Photocatalytic oxidation of methylene blue (MB) was chosen as a simple reaction to evaluate the optical photocatalytic property of AuNPs; meanwhile, corresponding to the possible future perspective of Au NPs in medical applications, the antibacterial activity of AuNPs was evaluated against Escherichia coli, Staphylococcus aureus, Propionibacterium acnes, and Pseudomonas aeruginosa.
2 Materials and methods
2.1 Materials
Fresh L. camara flower was collected from Universitas Islam Indonesia Park. The identification and authentication of plant taxonomy were performed at Biology Department, Universitas Gadjah Mada, Yogyakarta, Indonesia. About 10 g of the flowers was washed using distilled water followed by crushing and soaking in water for 4 h. The filtration was furthermore performed to obtain the filtrate which was denoted as L. camara flower extract (called LFE). Other chemicals consisting of HAuCl4·3H2O, MB, and H2O2 were purchased from Merck (Darmstadt, Germany). All the chemicals were of analytical grade and used without any purification.
2.2 Preparation of Au NPs by photochemical method
Photochemical procedure of AuNPs synthesis was conducted by mixing 1 mL of LFE and 1 mL of HAuCl4·3H2O (0.5 mM) followed by dilution with water until the total volume of 25 mL. The mixture was exposed to xenon light (λ = 550 nm) at various times: 5, 10, 20, and 30 min. The Au NPs from these variations were encoded as Au-5, Au-10, Au-20, and Au-30, respectively. The experiments were also studied on varied concentrations of LFE by 0.5, 1, 2, and 3 mL over irradiation for 10 min, and so the samples were encoded as Au-0.5, Au-1.0, Au-2.0, and Au-3.0, respectively. The formation of Au NPs was monitored by UV-Vis spectrophotometry analysis using HITACHI U–2010 spectrophotometer operated at a resolution of 1 nm. Physicochemical characterization of Au NPs was also carried out on the solid Au NPs by using XRD, SEM, transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). For XRD analysis, a Bruker AXS D8 diffractometer operated using Cu Kα radiation at λ = 0.154 nm was employed; meanwhile, the surface morphology analysis using SEM was determined using a Phenom X microscope. TEM identification was performed on a JEOL-JEM-2100 (Tokyo, Japan) operated at 20 kV was employed, and a ULVAC instrument (Quantera SXM, Japan) was used for XPS analysis. For all solid analyses, samples were dried at a temperature of 60°C, and for TEM analysis, the samples were dried on carbon-coated copper grid.
2.3 Antibacterial activity test
Antibacterial activity of Au NPs was evaluated on Escherichia coli (ATCC 11303), Staphylococcus aureus (ATCC 25923), Propionibacterium acnes (ATCC 11828), and Pseudomonas aeruginosa (ATCC 87110). The nutrient medium was utilized for bacterial growth and prepared by suspending nutrient agar in distilled water and autoclaved it before use. About 5 μL of Au NPs solution was loaded onto a 6 mm filter disc, and bacterial culture was evenly spread throughout the Petri plate, followed by 24 h incubation. The evaluation of antibacterial activity was performed based on the inhibition zone of each sample.
2.4 Photocatalytic activity of AuNPs
The photocatalytic activity of the Au NPs samples was studied on the MB photooxidation using visible light illumination. The Xenon (40 W, Philips) was utilized as a photon source. For each experiment, about 100 mL of 5 mg·L−1 of MB solution was mixed with 0.2 g of Au NPs sample and 0.5 mL of 30% H2O2 solution. The sampling of treated solutions was performed consecutively. The samples were analyzed using UV-Vis spectrophotometry. Degradation efficiency (DE) was determined based on the change in absorbance of the solution referred to the following equation:
where Abs0 and Abs t are the absorbance of the initial solution and the absorbance of the treated solution, measured at the wavelength of 635 nm.
3 Results and discussion
3.1 Synthesis and physicochemical characterization of AuNPs
The formation of AuNPs over photochemical synthesis was first evaluated using UV-vis spectroscopy. The formation of Au NPs can be confirmed as identified absorbance at the range of 500–700 nm [20], centered at ∼520 nm originating from the localized surface plasmon resonances of AuNPs. Additional peaks below 400 nm (216 and 320 nm) were assigned to ligand-to-metal charge transfer (LMCT) (π → σ*) Cl pπ → 5dx 2–y 2 bands of the various chloro hydroxo aurate. As can be seen from Figure 1a, LFE shows the spectrum as a representation of secondary metabolite content consisting of aromatic functional groups as identified by the peak at 200–350 nm, which is characteristic of flavonoid content, including the aromatic rings from some pigment from chlorophyll [21]. Previous investigation reported secondary metabolites content such as triterpenoids, flavonoids, as well as b-sitosteryl-3-O-b-d-glucoside and a mixture of campesterol, stigmasterol, and b-sitosterols in the extract of L. camara. In addition, the presence of phenolic compounds was obtained as it gave positive response to the ferric chloride test. This implies and was proven that the extract has the capability to reduce chloroauric acid into atomic gold.
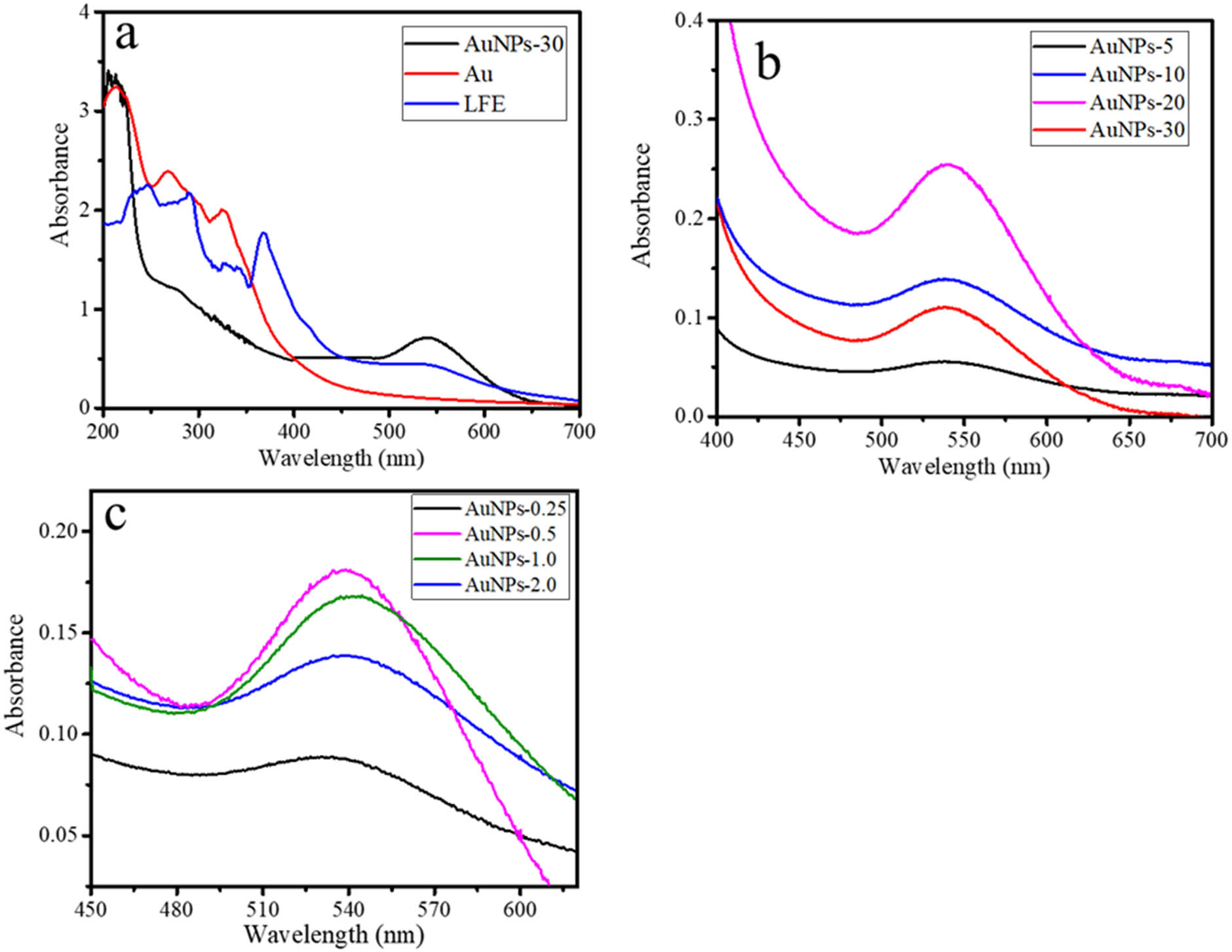
UV-Vis spectra of (a) AuNPs in comparison with Au3+ and LFE. (b) AuNPs at varied time of light irradiation, (c) AuNPs at varied LFE concentrations.
The first step of the study was to evaluate the effect of time of visible light irradiation on the bio-reduction of the gold atoms will lead to the formation of nano-sized particles and get stabilized by the polyphenols, quinones, and other coordinating phytochemicals. To test this, we treated aqueous HAuCl4 solutions with increasing concentrations of the leaf extract of L. camara. From the compared spectra presented in Figure 1, it is seen that Au-0.25 does not express any SPR peak, as identification of the absence of AuNPs formation, and interestingly, Au-0.5 gives the highest absorbance at the smallest wavelength of the SPR at 519 nm.
AuNPs formation was also identified using FTIR analysis, and the comparison of LFE and AuNPs spectra is presented in Figure 2. The LFE spectrum consists of some observed peaks at 3,430, 2,940, 2,900, 1,629, 1,415, 1,361, 1,155, and 1,105 cm−1 that are associated with the aromatic, C–C, C═C, amide, N–H, C═O, O–H, and aliphatic amine functional groups, and corresponding to the phenolic, flavonoids, and some secondary metabolite in the LFE. In AuNPs, some peaks are shifted to the higher wavenumber, and some peaks are disappeared as the result of the reduction reaction. The shifted peaks are those associated with aromatic functional group (3,420 cm−1), amide (1,629 cm−1), N–H (1,415 cm−1), and O–H (1,105 cm−1) [22,23]. The shifting peak is the identification of the attached Au metal into the functional groups which is related to the function of secondary metabolite as a capping agent of AuNPs. This is also in line with some disappeared peaks such as the peak at 2,940 and 2,900 cm−1.
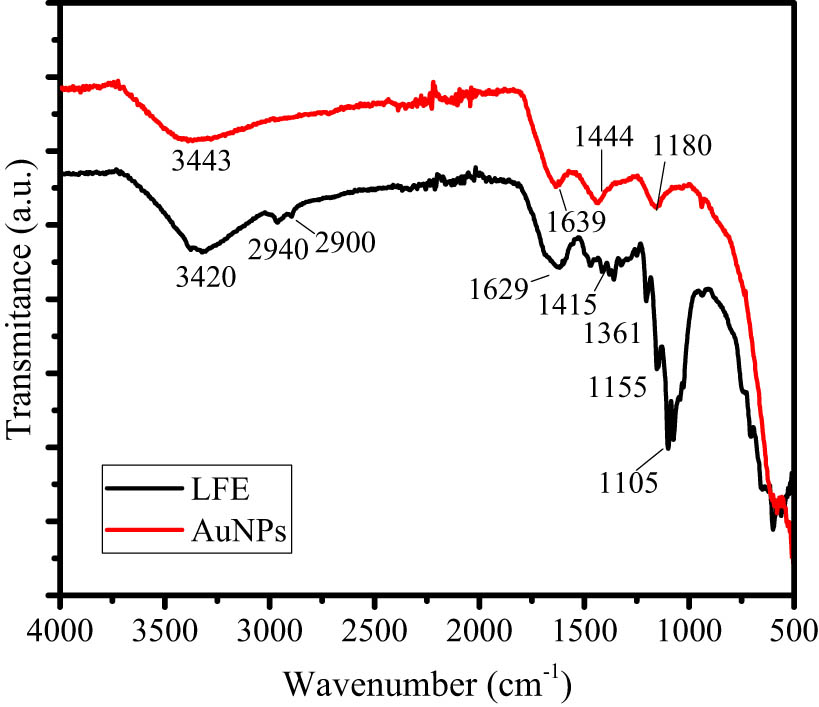
Comparison of FTIR spectra of LFE and AuNPs.
In addition, the higher LFE concentration in other samples tends to produce a lower absorbance and larger wavelength. It is conclusively found that the optimum concentration of LFE in the composition of synthesis is 0.5 mL. The highest absorbance of the SPR represents the intensity of the nanoparticle’s formation, and generally, the lower wavelength is attributed to the smaller particle size [24]. The particle size data of the AuNPs obtained from TEM analysis selected TEM images and HRTEM images are presented in Figure 3. All sample represent the dominantly spherical shape of AuNPs. The distinctive profile found for Au-0.5 that the particles are uniformly sized compared to other samples, and it is also seen that Au-2.0 shows a bulky aggregation of the particles, along with the presence of LFE as a capping agent. HRTEM images (Figure 3e and f) show lattice spacing of 0.24 nm, assigned to the (111) plane of Au referring to JCPDS standard No. 04-0784 [25,26]. In addition, the particle size distribution of Au NPs as a function of time of light illumination and LFE concentration are presented in Figure 3. The pattern of the images of Au-HA samples suggests that the higher LFE concentration tends to obey the particle aggregation. In general, the particle size distributions (Figure 4a and b) are in accordance with the trend of UV-Vis spectra that highlight the optimum time of synthesis for 10 min and the LFE volume of 0.5 mL.
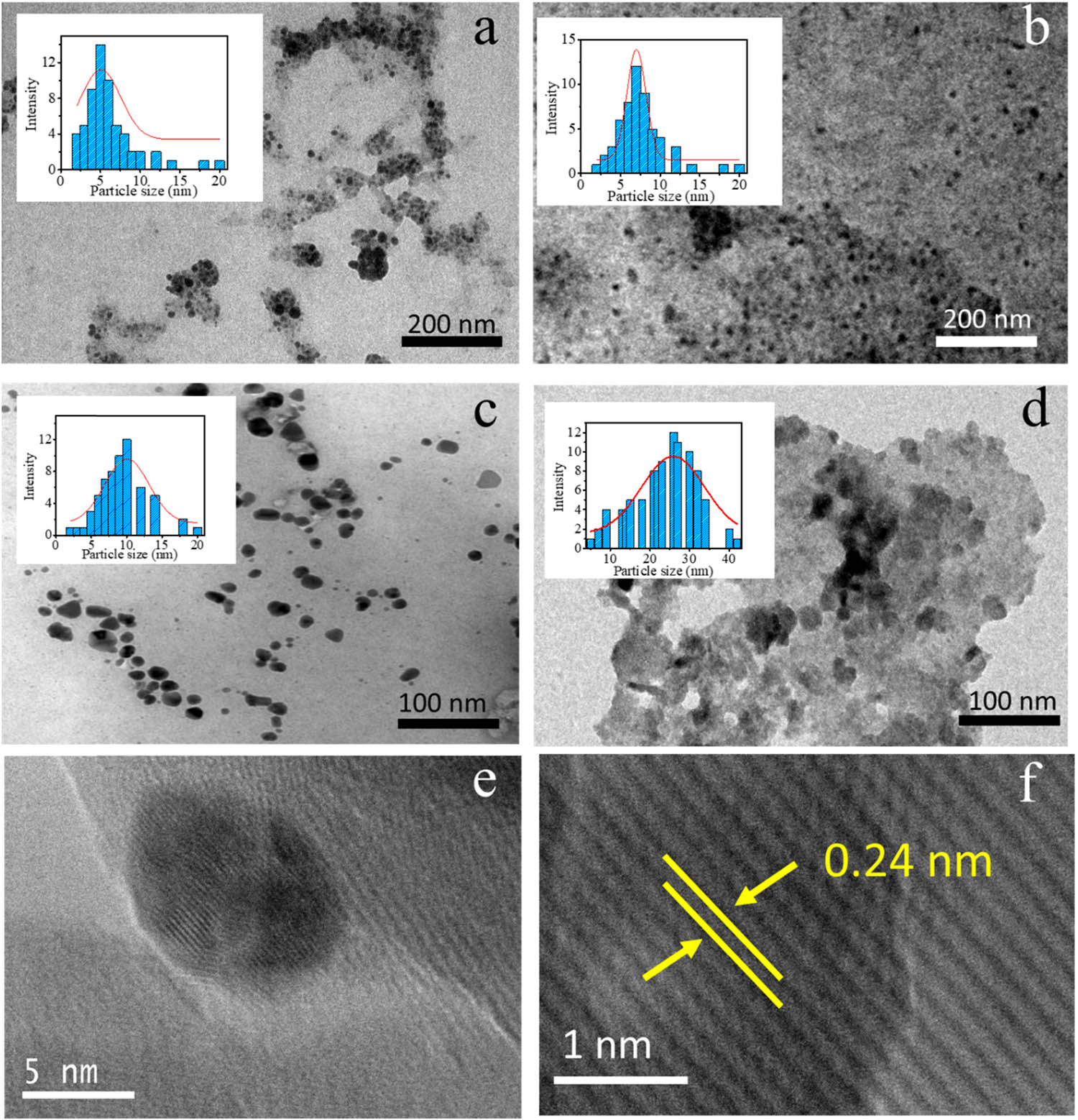
TEM image and particles distribution of (a) AuNPs-0.25, (b) AuNPs-0.5, (c) AuNPs-1.0, (d) AuNPs-2.0, and (e,f) HRTEM images of AuNPs.
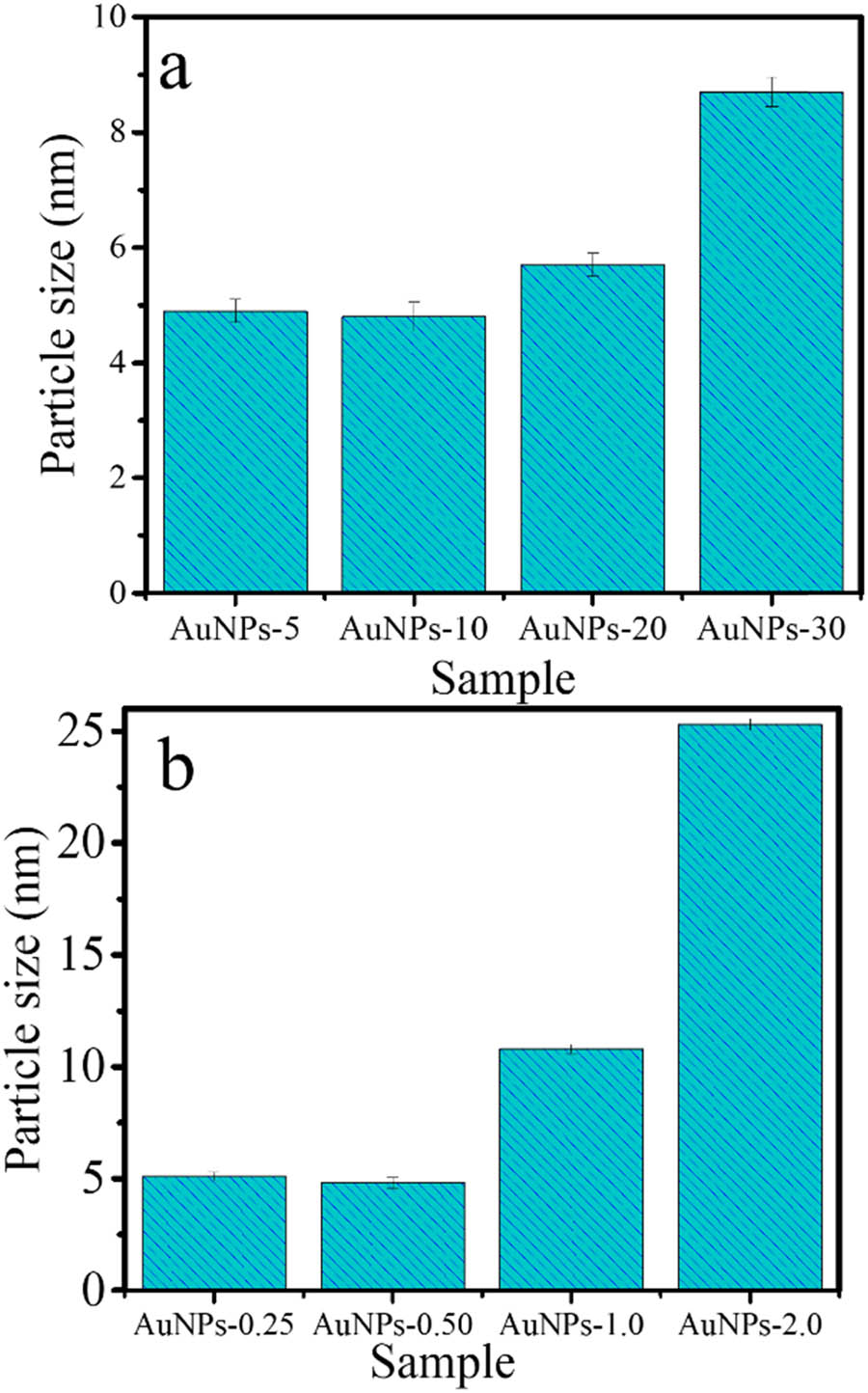
Particle size of AuNPs as a function of (a) light illumination and (b) LFE concentration.
An important thing to be pointed out from the spectra and TEM images is that the photochemical reaction occurred, and it is related to the capability of LFE to absorb light radiation. Referring to the previous study [27], a radiative energy transfer by the emission of a photon of a higher wavelength can be conducted by a chromophore. Pigments and other chromophores from double-bond structures in the secondary metabolite of LFE could absorb and utilize the photon to furthermore produce radicals from water by the following equation: 2H2O → 4H+ + 4e− + O2˙.
The breaking O–H bond requires ∼464 kJ·mol−1 of energy, which is directly possible only by a photon of ∼257 nm (UV range) and at a visible wavelength spectrum with sufficient photon transfer. The effect of time of light irradiation on the AuNPs formation as shown by UV-Vis spectra and TEM image in Figure 3 confirmed the presence of a photochemical reaction. In more detail, the change in absorbance along with increasing time of irradiation confirmed by the particle size distribution reflects the kinetics of nanoparticles formation with the influence of photon source. Refer to several references, the proposed mechanism can be described as follow:
Considered that polyphenol is one of the components of the LFE, the hydroxyl functional group from secondary metabolites can form the radicals coming from the interaction with the hydroxyl radicals formed by the interaction of water and light. The reduction of [AuCl2 −] species to Au0 atoms is a slower process than that of [AuCl4]− to [AuCl2], and it is associated with particles size formation.
The dried AuNPs was identified using XRD, SEM, and XPS analyses with the results presented in Figure 5. The XRD pattern demonstrates the peaks associated with (111), (200), and (220) reflections are in accordance with JCPDS standard No. 04-0784 [28,29]. It suggests a single phase of Au formed in the synthesis, which is also confirmed by XPS spectra (Figure 3c and d). The survey scan and the deconvolution to the Au4f spectrum revealed the Au0 species as the doublet peaks are found at 83.7 and 87.4 eV that are ascribed to Au4f7/2 and Au4f5/2, respectively. A regular morphology of AuNPs with homogeneous spherical forms is observed in clear morphology using SEM analysis.
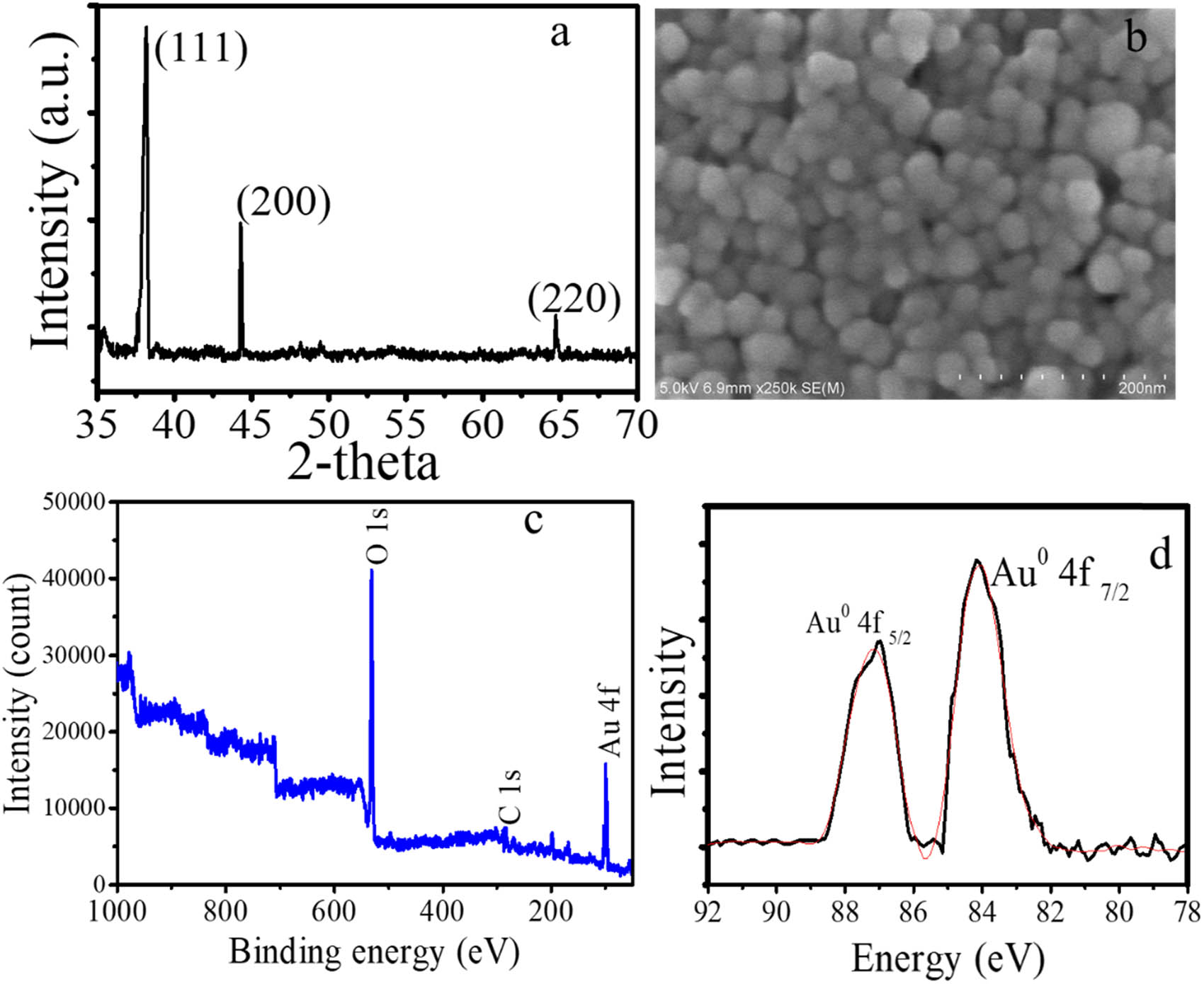
(a) XRD pattern of AuNPs, (b) SEM profile of AuNPs, (c) survey scan spectrum of AuNPs, and (d) spectrum of Au 4f.
From these physicochemical characterization data, it can be concluded AuNPs have been successfully prepared under the photochemical method. The method is simpler and timeless compared to other procedures such as Reflux, microwave-irradiation, or ultrasound-irradiation method which suggest the potency for scaling up on industrial scale.
3.2 Antibacterial activity of AuNPs
Antimicrobial effects of AuNPs gram-positive bacteria and gram-negative bacteria are found to be effective, as shown by some tests presented in Figure 6, and tabulated the inhibition zones (in mm) in Table 1. From various AuNPs samples, it is obtained that AuNPs 0.5 exhibited the highest activity as the largest inhibition zone appeared. The antibiotic ampicillin and double distilled water were used as positive and negative controls, respectively. Each positive and negative control produced a 23 mm and no zone of inhibition zones.
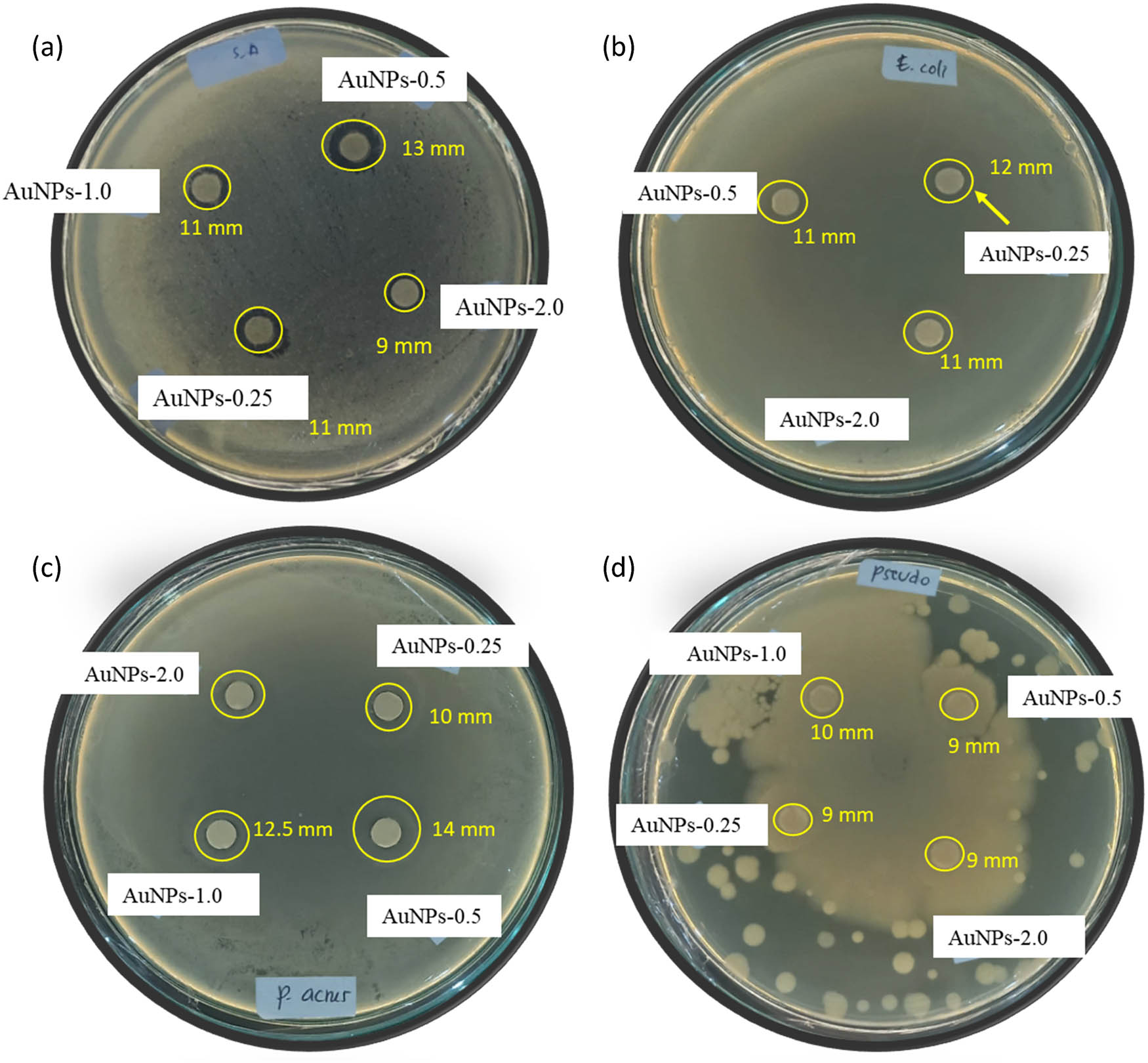
Some images from antibacterial activity test of AuNPs against (a) S. aureus, (b) E. coli, (c) P. acnes, (d) P. aeruginosa.
Zone of inhibition for antibacterial effect of AuNPs (tested concentration, 5 μg·mL−1)
| Tested bacteria | Inhibition zone by tested sample (mm) | |||
|---|---|---|---|---|
| AuNPs-0.25 | AuNPs-0.5 | AuNPs-1.0 | AuNPs-2.0 | |
| E. coli | 11 ± 0.2 | 12 ± 0.2 | 11 ± 0.1 | 11 ± 0.1 |
| S. aureus | 11 ± 0.2 | 13 ± 0.2 | 11 ± 0.1 | 9 ± 0.1 |
| P. acne | 10 ± 0.2 | 14 ± 0.3 | 12.5 ± 0.2 | 10 ± 0.1 |
| P. aeruginosa | 9 ± 0.1 | 9 ± 0.2 | 10 ± 0.2 | 9 ± 0.1 |
The inhibition of bacterial growth occurs by the penetration of ionic Au from the Au NPs surface into the cell wall which prevents the formation of ATP and DNA replication of bacteria. As more ions enter the cytoplasm, the cell membrane will be penetrated by the reaction of ions with the −SH (thiol) group of cell proteins. This reaction leads to the release of ATP synthesis during cellular respiration and then losing protons, and the cell metabolism system is disrupted [33].
From the inhibition zone data, the larger inhibition zones on S. aureus and P. acne, which are gram-positive bacteria, were greater than those of E. coli and P. aeruginosa, indicating the stronger antibacterial effects against gram-positive bacteria rather than gram-negative bacteria. This phenomenon reflects the stronger surface interaction of AuNPs which has a negative surface, as the zeta potential measurement is −21 mV. On the contrary, less interaction occurs with the gram-negative bacteria, caused by the repulsion by the electrostatic force equilibrium. Based on the larger inhibition zones, activities of these samples are higher compared to those of other AuNPs from previous works, for example, AuNPs synthesized using chitosan and Lignosus rhinocerotis [30], Uncaria gambir [31], and macadamia nut shells [32] that showed the inhibition at a range of 8–10 mm at higher concentration. Comparison with other plant-mediated AuNPs is presented in Table 2. The activity toward E. coli obtained in this research is higher compared to those synthesized using Platycodon grandiflorum [33], Nigella arvensis [34], and Pimenta dioica [35]. The superiority in antibacterial activity is related to the smaller concentration to give the same range of inhibition zone, especially for S. aureus and E. coli. In addition, the short time required for the synthesis is an advantage from the time effectivity perspective. Unfortunately, the comparison in antibacterial against P. aeruginosa and P. acne was not much presented for Au NPs. In summary, the antibacterial and other biological activities of the green synthesized nanoparticles are influenced by many factors such as morphology, particle size, capping agent, and surface chemistry [36,37].
Comparison of antibacterial activity of AuNPs using various plant extract
| Plant extract | Tested bacteria | Remark | Reference |
|---|---|---|---|
| Pimenta dioica | S. aureus and E. coli | The inhibition zone of 30 μg·mL−1 nanoparticle gave were 3 and 9 mm for S. aureus and E. coli, respectively | [35] |
| Nigella arvensis | S. aureus and E. coli | The inhibition zone of 125 μg·mL−1 nanoparticles showed an inhibition zone of 11 mm against S. aureus and μg·mL−1 nanoparticles showed an inhibition zone of 9 mm against E. coli, respectively | [34] |
| Platycodon grandiflorum | E. coli | Au NPs were synthesized by mixing the plant extract with AuCl3 under stirring at 50°C. The inhibition zone of 11 mm was exhibited by 10 μg·mL−1 of Au NPs | [33] |
| Jatropha integerrima Jacq. flower extract | S. aureus and E. coli | Au NPs were synthesized by mixing the plant extract with AuCl3 under stirring at 75°C. The inhibition zone of 10 μg·mL−1 nanoparticles gave was 16 mm for both S. aureus and E. coli | [38] |
| Digera muricata | S. aureus and E. coli | The inhibition zone of 50 μg·mL−1 nanoparticles gave was 7 mm for both S. aureus and 13 mm for E. coli, respectively | [39] |
3.3 Photocatalytic activity of AuNPs
The photocatalytic activity of AuNPs is expressed by the capability of the material to degrade MB on photocatalytic oxidation under visible light. It is revealed by the spectral change of MB solution presented in Figure 7a and the kinetics plot of MB degradation in Figure 7b. The degradation efficiency of 88% for 1 h of treatment was observed, which is higher compared to other similar works [40,41]. The kinetic evaluation indicates the fitness of the reaction to pseudo-first-order kinetics as reflected by plots in Figure 6c, refer to the following equation (Eq. 2):
where C o and C t are initial concentrations of MB, k is the kinetic constant, and t is the time of reaction. It confirmed for all varied AuNPs dose in the photocatalytic system the correlation coefficient (R 2) are high (∼1).
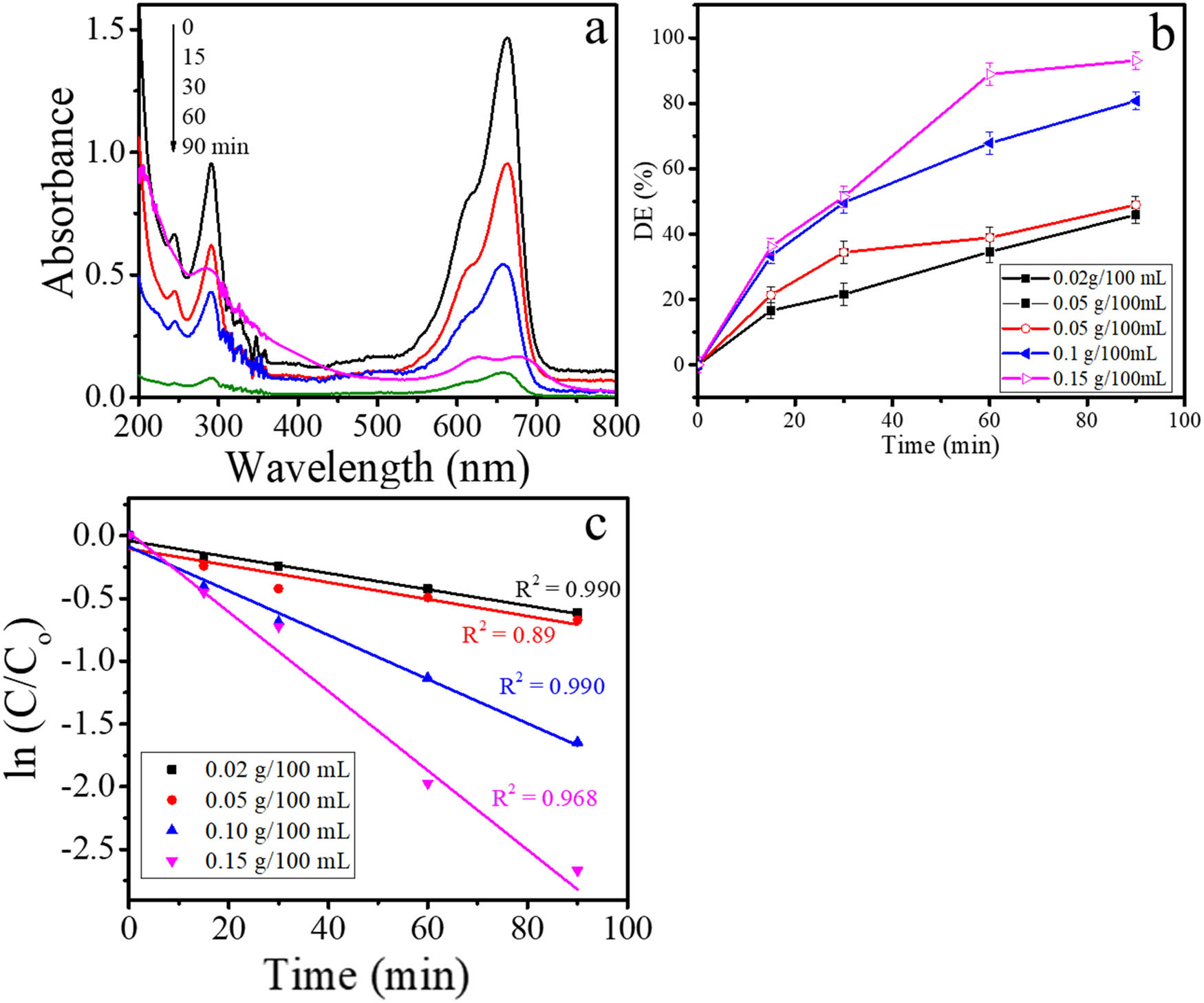
(a) Spectral change of MB by photocatalytic degradation, (b) kinetics plot of MB removal and (c) pseudo-first order plot of MB degradation.
From varied AuNPs content, it is seen that within the range from 0.02 g/100 mL to 0.15 g/100 mL of AuNPs as a photocatalyst, the reaction rate and DE increase as the increasing photocatalyst dose, implies the role of nanoparticles in the mechanism. The optimum degradation was shown by the DE of 88% reached over 0.15 g/100 mL of MB solution for 90 min of treatment. The value is similar to what was reported using a higher AuNPs dose under UV light [42] and also higher compared to Au–TiO2 [43]. In summary, photochemical synthesized AuNPs demonstrated a facile synthesis by photochemical method with the nanoparticles having active as antibacterial and photocatalyst. To our knowledge, the present work is a photochemical method utilizing plant extract first reported for the synthesis of nanoparticles.
4 Conclusion
The photochemical method of AuNPs synthesis using L. camara flower extract has been successfully conducted. The synthesized nanoparticles are spherical within the particle size range of 4.8–25 nm, depending on the concentration of the extract and time of light irradiation in the synthesis. The AuNPs show the potential activity as to degrade MB via photooxidation under visible light illumination with an efficiency of about 88% for 1 h. The material also demonstrated antibacterial activity against E. coli, S.aureus, P. aeruginosa, and P. acne. The results represented that the photochemical process utilizing visible light and secondary metabolite in plant extract is one of the considerable methods for an eco-friendly AuNPs synthesis.
Acknowledgments
Authors acknowledge the DRPM Ministry of Education, Culture, Research and Technology, Republic of Indonesia for research funding.
-
Funding information: This research is funded by DRPM Ministry of Education, Culture, Research and Technology, Republic of Indonesia, contract no: 071/E5/PG.02.00.PT/2022.
-
Author contributions: Habibi Hidayat: formal analysis, writing – original draft; Gani Purwiandono: writing – review and editing, visualization; Tohari Tohari: formal analysis; Bambang Hernawan Nugroho: resource, writing – review and editing; Muhammad Husnu Jauhari: formal analysis; Satria Bagus Widyaputra: formal analysis; Is Fatimah: writing – original draft, project administration.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data used to support the findings of this study are included in the article. Should further data or information be required, these are available from the corresponding authors upon request.
References
[1] Huang W, Tao F, Li F, Mortimer M, Guo LH. Antibacterial nanomaterials for environmental and consumer product applications. NanoImpact. 2020;20:100268. 10.1016/j.impact.2020.100268.Search in Google Scholar
[2] Kumar R, Aadil KR, Ranjan S, Kumar VB. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J Drug Deliv Sci Technol. 2020;57:101617. 10.1016/j.jddst.2020.101617.Search in Google Scholar
[3] Bradford SA, Shen C, Kim H, Letcher RJ, Rinklebe J, Ok YS, et al. , Environmental applications and risks of nanomaterials: An introduction to CREST publications during 2018–2021. Crit Rev Env Sci Technol. 2021;52:1–11.10.1080/10643389.2021.2020425Search in Google Scholar
[4] Khalafi T, Buazar F, Ghanemi K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci Rep. 2019;9:1–10. 10.1038/s41598-019-43368-3.Search in Google Scholar PubMed PubMed Central
[5] Koopi H, Buazar F. A novel one-pot biosynthesis of pure alpha aluminum oxide nanoparticles using the macroalgae Sargassum ilicifolium: A green marine approach. Ceram Int. 2018;44:8940–5. 10.1016/j.ceramint.2018.02.091.Search in Google Scholar
[6] Amina SJ, Guo B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int J Nanomed. 2020;15:9823–57. 10.2147/IJN.S279094.Search in Google Scholar PubMed PubMed Central
[7] Nadeem M, Abbasi BH, Younas M, Ahmad W, Khan T. A review of the green syntheses and anti-microbial applications of gold nanoparticles. Green Chem Lett Rev. 2017;10:216–27. ://doi.org/10.1080/17518253.2017.1349192.Search in Google Scholar
[8] Boruah JS, Devi C, Hazarikad U, Reddy PVB, Chowdhury D, Barthakur M, et al. Green synthesis of gold nanoparticles using an antiepileptic plant extract: in vitro biological and photo-catalytic activities. RSC Adv. 2021;11:28029–41.10.1039/D1RA02669KSearch in Google Scholar PubMed PubMed Central
[9] Sadeghi B, Mohammadzadeh M, Babakhani B. Green synthesis of gold nanoparticles using Stevia rebaudiana leaf extracts: Characterization and their stability. J Photochem Photobiol B Biol. 2015;148:101–6. 10.1016/j.jphotobiol.2015.03.025.Search in Google Scholar PubMed
[10] Sierra JA, Vanoni CR, Tumelero MA, Plá Cid CC, Faccio R, Franceschini DF, et al. Biogenic approaches using citrus extracts for the synthesis of metal nanoparticles: The role of flavonoids in gold reduction and stabilization. N J Chem. 2016;40:1420–9. 10.1039/c5nj02128f.Search in Google Scholar
[11] Dash SS, Bag BG, Hota P. Lantana camara Linn leaf extract mediated green synthesis of gold nanoparticles and study of its catalytic activity. Appl Nanosci. 2015;5:343–50. 10.1007/s13204-014-0323-4.Search in Google Scholar
[12] Ezzat MI, El Gendy SN, Saad AS, Abdo WS, EL Sayed AM, Elmotayam AK. Secondary metabolites from Lantana camara L. flowers extract exhibit in vivo anti-urolithiatic activity in adult Wistar albino rats. Nat Prod Res. 2020;36(4):1–4. 10.1080/14786419.2020.1853726.Search in Google Scholar PubMed
[13] Hardur Ven A, Amrutanand T, Majumdar SP, Harish M. Application of Lantana camara flower extract as a natural coloring agent with preservative action. Asian J Biol Sci. 2020;13:361–9. 10.3923/ajbs.2020.361.369.Search in Google Scholar
[14] Mansoori A, Singh N, Dubey SK, Thakur TK, Alkan N, Das SN, et al. Phytochemical characterization and assessment of crude extracts From Lantana camara L. for antioxidant and antimicrobial activity. Front Agron. 2020;2:582268. 10.3389/fagro.2020.582268.Search in Google Scholar
[15] Chowdhury R, Khan A, Rashid MH. Green synthesis of CuO nanoparticles using: Lantana camara flower extract and their potential catalytic activity towards the aza-Michael reaction. RSC Adv. 2020;10:14374–85. 10.1039/d0ra01479f.Search in Google Scholar PubMed PubMed Central
[16] Ajitha B, Reddy AK, Reddy PS. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater Sci Eng C. 2015;49:373–81. 10.1016/j.msec.2015.01.035.Search in Google Scholar PubMed
[17] Ali D, Dawood S. Biosynthesis, characterization and antiproliferative activity of green synthesized gold nanoparticles using Lantadene A extracted from Lantana camara KIJOM. 2021;7(4):7. 10.33640/2405-609X.3158.Search in Google Scholar
[18] Marin ML, McGilvray KL, Scaiano JC. Photochemical strategies for the synthesis of gold nanoparticles from Au(III) and Au(I) using photoinduced free radical generation. J Am Chem Soc. 2008;130:16572–84. 10.1021/ja803490n.Search in Google Scholar PubMed
[19] Jara N, Milán NS, Rahman A, Mouheb L, Boffito DC, Jeffryes C, et al. Photochemical synthesis of gold and silver nanoparticles–A review. Molecules. 2021;26:4585.10.3390/molecules26154585Search in Google Scholar PubMed PubMed Central
[20] Amendola V, Pilot R, Frasconi M, Maragò OM, Iatì MA. Surface plasmon resonance in gold nanoparticles a review. J Phys Condens Matter. 2017;29(20):203002.10.1088/1361-648X/aa60f3Search in Google Scholar PubMed
[21] Varliklioz Er S, Eksi-Kocak H, Yetim H, Boyaci IH. Novel spectroscopic method for determination and quantification of saffron adulteration. Food Anal Methods. 2017;10:1547–55. 10.1007/s12161-016-0710-4.Search in Google Scholar
[22] Shikha S, Chaudhuri SR, Bhattacharyya MS. Facile one pot greener synthesis of sophorolipid capped gold nanoparticles and its antimicrobial activity having special efficacy against gram negative vibrio cholerae. Sci Rep. 2020;10:1–13. 10.1038/s41598-019-57399-3.Search in Google Scholar PubMed PubMed Central
[23] Kumar H, Bhardwaj K, Kuča K, Kalia A, Nepovimova E, Verma R, et al. Flower-based green synthesis of metallic nanoparticles: Applications beyond fragrance. Nanomaterials. 2020;10:766. 10.3390/nano10040766.Search in Google Scholar PubMed PubMed Central
[24] Yulizar Y, Ariyanta HA, Abdurrachman L. Green synthesis of gold nanoparticles using aqueous garlic (Allium sativum L.) extract and its interaction study with melamine. Bull Chem React Eng Catal. 2017;12:212–8. 10.9767/bcrec.12.2.770.212-218.Search in Google Scholar
[25] Susan Punnoose M, Bijimol D, Mathew B. Microwave assisted green synthesis of gold nanoparticles for catalytic degradation of environmental pollutants. Env Nanotechnol Monit Manag. 2021;16:100525. 10.1016/j.enmm.2021.100525.Search in Google Scholar
[26] Prema P, Boobalan T, Arun A, Rameshkumar K, Suresh Babu R, Veeramanikandan V, et al. Green tea extract mediated biogenic synthesis of gold nanoparticles with potent anti-proliferative effect against PC-3 human prostate cancer cells. Mater Lett. 2022;306:130882. 10.1016/j.matlet.2021.130882.Search in Google Scholar
[27] Manoj KM, Manekkathodi A. Light’s interaction with pigments in chloroplasts: The murburn perspective. J Photochem Photobiol. 2021;5:100015. 10.1016/j.jpap.2020.100015.Search in Google Scholar
[28] Das RK, Borthakur BB, Bora U. Green synthesis of gold nanoparticles using ethanolic leaf extract of Centella asiatica. Mater Lett. 2010;64(13):1445–7. 10.1016/j.matlet.2010.03.051.Search in Google Scholar
[29] Dwivedi AD, Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A Physicochem Eng Asp. 2010;369:27–33.10.1016/j.colsurfa.2010.07.020Search in Google Scholar
[30] Katas H, Lim CS, Nor Azlan AYH, Buang F, Busra MF. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm J. 2019;27:283–92. 10.1016/j.jsps.2018.11.010.Search in Google Scholar PubMed PubMed Central
[31] Arief S, Nasution FW, Labanni A. High antibacterial properties of green synthesized gold nanoparticles using Uncaria gambir Roxb. leaf extract and triethanolamine. J Appl Pharm Sci. 2020;10:124–30. 10.7324/JAPS.2020.10814.Search in Google Scholar
[32] Dang H, Fawcett D, Poinern GEJ. Green synthesis of gold nanoparticles from waste macadamia nut shells and their antimicrobial activity against Escherichia coli and Staphylococcus epidermis. Int J Res Med Sci. 2019;7:1171. 10.18203/2320-6012.ijrms20191320.Search in Google Scholar
[33] Anbu P, Gopinath SCB, Jayanthi S. Synthesis of gold nanoparticles using Platycodon grandiflorum extract and its antipathogenic activity under optimal conditions. Nanomater Nanotechnol. 2020;10:1–9. 10.1177/1847980420961697.Search in Google Scholar
[34] Chahardoli A, Karimi N, Sadeghi F, Fatani A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial activity, antioxidant, cyotoxicity and catalytic activities. Artif Cells Nanomed Biotechnol. 2017;43:579–88.10.1080/21691401.2017.1332634Search in Google Scholar
[35] Fadaka A, Aluko O, Awawu S, Theledi K. Green synthesis of gold nanoparticles using pimenta dioica leaves aqueous extract and their application as photocatalyst, antioxidant, and antibacterial agents. J Multidiscip Appl Nat Sci. 2021;1:78–88. 10.47352/jmans.v1i2.81.Search in Google Scholar
[36] Barabadi H, Vahidi H, Damavandi Kamali K, Rashedi M, Saravanan M. Antineoplastic biogenic silver nanomaterials to combat cervical cancer: A novel approach in cancer therapeutics. J Clust Sci. 2020;31:659–72. 10.1007/s10876-019-01697-3.Search in Google Scholar
[37] Saravanana M, Barabadi H, Vahidi H. Green nanotechnology: isolation of bioactive molecules and modified approach of biosynthesis, in “Biogenic Nanoparticles for Cancer Theranostics: A volume in Micro and Nano Technologies”. Amsterdam: Elsevier B.V.; 2021. pp. 101–122. 10.1016/C2019-0-02790-2.Search in Google Scholar
[38] Suriyakala G, Sathiyaraj S, Babujanarthanam R, Alarjani KM, Hussein DS, Rasheed RA, et al. Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J King Saud Univ - Sci. 2022;34:101830. 10.1016/j.jksus.2022.101830.Search in Google Scholar
[39] Ayaz Ahmed KB, Subramanian S, Sivasubramanian A, Veerappan G, Veerappan A. Preparation of gold nanoparticles using Salicornia brachiata plant extract and evaluation of catalytic and antibacterial activity, Spectrochim. Acta - Part A Mol Biomol Spectrosc. 2014;130:54–8. 10.1016/j.saa.2014.03.070.Search in Google Scholar PubMed
[40] Kumar B, Smita K, Cumbal L, Debut A. One pot synthesis and characterization of gold nanocatalyst using Sacha inchi (Plukenetia volubilis) oil: Green approach. J Photochem Photobiol B Biol. 2016;158:55–60. 10.1016/j.jphotobiol.2016.02.023.Search in Google Scholar PubMed
[41] Rodriguez-Leon E, Rodriguez-Vazquez BE, Martinez-Higuera A, Rodriguez-Beas C, Larios-Rodriguez E, Navaro RE, et al. Iniguez-Palomares, synthesis of gold nanoparticles using Mimosa tenilora, assesments of cytotoxicity, cellular uptake, and catalysis. Nanoscale Res Lett. 2019;14:1–16.10.1186/s11671-019-3158-9Search in Google Scholar PubMed PubMed Central
[42] Singh RK, Behera SS, Singh KR, Mishra S, Panigrahi B, Sahoo TR, et al. Biosynthesized gold nanoparticles as photocatalysts for selective degradation of cationic dye and their antimicrobial activity. J Photochem Photobiol A Chem. 2020;400:112704. 10.1016/j.jphotochem.2020.112704.Search in Google Scholar
[43] Arias M-C, Aguilar C, Piza M, Zarazua E, Anguebes F, Anguebes F, et al. Removal of the methylene blue dye (MB) with catalysts of Au-TiO2: Kinetic and degradation pathway. Mod Res Catal. 2021;10:1–14. 10.4236/mrc.2021.101001.Search in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

