Abstract
Recycling of spent lithium (Li)-ion batteries has become a hot research topic due to its surge in the quantity and environmental problems. Herein we demonstrated a new chemical configuration of choline chloride-based deep eutectic solvent (DES) to recover the cobalt from lithium cobalt oxide (LiCoO2), a representative cathode material for Li-ion battery. It was experimentally verified that the leaching efficiency of Co increased rapidly with the increase in the reaction temperature. Nearly 96% of Co can be leached from lithium cobalt oxide after a leaching treatment at 200℃ for 20 h. The leaching mechanism of cobalt in DES was analyzed by using a variety of techniques, including cyclic voltammetric experiments, Fourier transform infrared radiation, and ultraviolet-visible spectra. The results showed that the LiCoO2 dissolve into the DES via reduction of Co(iii) to Co(ii). In particular to this work, the leaching experiments were performed in a higher concentration than those in the previous studies, which significantly promoted the operating efficiency of the leaching process.
1 Introduction
Rechargeable lithium (Li)-ion batteries with a high theoretical efficiency of converting chemical to electrical energy have been widely used in mobile devices, portable electronics, and electric vehicles. Because Li-ion batteries have a limited lifetime of 3–5 years, large numbers of spent Li-ion batteries (SLIB) have been generated, and it is expected that the number of SLIB will reach 900,000 by 2023 [1]. The development of recycling of SLIB is very important. Most of the recoverable value components in SLIB are in the cathode. For example, Cobalt (Co) is a strategic material that typically constitutes up to 15 wt% of LiCoO2 (LCO, one of the main commercial cathode materials) [2,3,4]. The establishment of effective recycling strategies could balance the impact of end-of-life Li-ion batteries and the demands on raw materials in the battery supply chain.
Three routes, namely pyrometallurgy, bio-metallurgy, and wet-chemical methods have been considered as effective ways to recover Co from SLIB-related materials. As a typical method of pyrometallurgy-dominant way, direct reduction of metal oxides in electrodes has been widely studied to recover the Co from LCO-based materials [5,6,7]. Although pyrometallurgy treatment can obtain nearly 100% metal leaching rate, there are some inevitable disadvantages, such as high treatment temperature (>1,000°C), high energy consumption, and harmful gas emission.
Bio-metallurgy has been considered an alternative way to extract metals from LCO-based materials. Biswal et al. [8] used Aspergillus niger strains MM1 and SG1, and Acidithiobacillus thiooxidans 80191 to extract Co and Li from SLIB. After reaction for nearly a week at 30°C, high amount of Co precipitated in the form of cobalt sulfide (100%), cobalt hydroxide (100%), or cobalt oxalate (88%). Huang et al. [9] focused on constructing a bio-electro-hydrometallurgical platform to efficiently recover Co, Li, and Mn. Maximum recoveries of 91.45%, 93.64%, and 87.92% for Co, Li, and Mn, respectively, were achieved. To date, the main challenge of the bio-metallurgy method lies in the difficulty of strain culture and screening which lead to a long recovery period of metals (>1 week). Additionally, only a small number of LCO-based materials could be treated in the bio-metallurgy-dominated routine, which makes it difficult to scale up for the practical purpose.
Wet-chemical method, with its characteristic of high leaching efficiency (often higher than 90%) of Co, is the most popular strategy to recover Co components from LCO-based materials. Barik et al. [10] used hydrochloric acid (concentration of 1.75 M) to recover Co from the cobalt-manganese lithium battery, and 90% of Co was recovered. The cathode material of LiNi1/3Co1/3Mn1/3O2 battery was leached by using 1 M sulfuric acid and 1% hydrogen peroxide. The leaching efficiency of Co was higher than 99.7% [11]. Reduction agents, e.g., H2O2, NaHSO3, and ascorbic acid, were used as additives to reduce the emission of toxic gases [12]. However, inorganic acid, such as sulfuric and hydrochloric acids, has to be added continuously in the recovery process, and environmental issue is often raised in the wet-chemical method. As a consequence, the organic acid is academically considered a suitable candidate for Co leaching. Zeng et al. [13] used oxalic acid to recover Co from LCO-based materials, 97% recovery of Co was achieved. Zhang et al. [14] recovered the LiNi1/3Co1/3Mn1/3O2 from the cathode of a ternary battery by using oxalic acid and the recycled materials were remade to a new cathode. Organic acids possess better characteristics than inorganic acids for Co leaching, e.g., no toxic gas emission and better reducibility [15,16]. Compared with inorganic acid reagents, the organic acid is higher in price, which makes the recovery process not cost-effective.
Recently, deep eutectic solvents (DES) find many applications in electrochemistry, metallurgy, and other fields due to their characteristics of low melting point (typically from room temperature to 180°C) and good solubility of metallic compounds [17,18]. Generally, the behavior of oxides in DES is similar to that in the molten state, and most metal compounds including metal salts have good solubility. Abbott et al. [19] used lactic acid-based DES to extract a large number of metals. Particularly, they mentioned the affinity between DES and Co. The abovementioned characteristic demonstrated that DES could be a good solvent for Co leaching from LCO-based materials.
According to the synthesis mechanism of DES and the principle of green chemistry, low-cost and environment-friendly green materials are preferred. Choline chloride (ChCl) is a commonly available feed additive and is also often used as a hydrogen bond receptor in the synthesis of DESs. There are a few reports on the recovery of waste lithium batteries with ChCl-based DES [20,21,22,23,24,25,26,27,28,29]. Tran et al. [23] are among the first in leaching Co from high concentration Co-DES solution (10 mg LCO with 50 mg DESs, ChCl + ethylene glycol [EG]), and the leaching efficiencies of Co and Li were both higher than 90%. However, the reaction temperature and time were still high to be 220°C and 24 h, respectively, which are not beneficial for commercial operation. Wang et al. [24] dissolved 10 mg of LCO powder in 10 g DES (ChCl + urea), and more than 95% of the Co was leached from LCO-based materials. Schiavi et al. [26] used DES (ChCl + EG) for the selective recovery of Co from mixed LIBs and the leaching yields attained 90% for Co and only 10% for Ni. By using DES (ChCl + formic acid), the leaching efficiency of Co was as high as 99.0% at 70°C [28].
The feasibility of recovering LCO using ChCl-based DES has been proved in the previous studies. However, there are still some problems to be addressed. The ability of ChCl-based DES to treat cathode materials is very limited. Meanwhile, the leaching process of Co still lacks systematic proof. At present, the selection of hydrogen bond donors for ChCl-based DES mainly focuses on urea and EG. The nature and ability of hydrogen bond donors may determine the leaching efficiency of Co [23]. In addition, with the increase in temperature, the leaching is accelerated, especially the short-term leaching effect that needs to be improved. Therefore, there is still room for the selection of hydrogen bond donors to meet larger, faster, and more industrialized processing systems.
In the present work, a new ChCl-based DES system was adopted to achieve better dissolution and higher yield of Co in DES than that in previous studies. Particularly, the leaching experiments were performed at higher concertation than those reported in previous studies, thereby greatly promoting the leaching efficiency of Co.
2 Materials and methods
2.1 Materials
Glycerol (C3H8O3; Gly), absolute ethanol, and sodium carbonate (Na2CO3) are of analytical grade and were purchased from Tianjin Damao Chemical Reagent Factory, China. ChCl (C5H14ClNO) was from American McLean Company. The experimental lithium cobalt(iii) oxide (LiCoO2) was obtained by manual disassembly from a local battery recycling company.
2.2 Preparation of DESs and leaching of Co components
ChCl and Gly were mixed with different molar ratios to prepare the DES. The mixture was melted at 80°C for 1 h with constant stirring until a clear, homogenous DES was obtained. The prepared DES was cooled to room temperature in a desiccator equipped with silica gel to avoid moisture absorption.
Typically, LCO powder (0.1 g) and DES (5 g) were mixed in a glass flask. The glass flask with a condenser tube was placed in an oil bath. The effect of time and temperature on metal leaching was investigated over the time range of 1–10 h and temperature range of 120–200°C. The effect of the molar ratio of ChCl:Gly was also assessed at 200°C for 20 h. The leachates were filtered using a filter membrane with a pore size of 0.45 μm at near to 100°C. The leachates were then precipitated by carbonate and subsequently calcined (500°C, 6 h) to obtain a black powder.
2.3 Characterization of samples
The metal cation concentration of the leachates was determined using inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 5,300 V). The Fourier-transform infrared (FT-IR) and ultraviolet-visible (UV-Vis) spectra of the DES and leachates were obtained using a Nicolet iS50 FT-IR spectrometer in the form of potassium bromide disks, and an EVOLUTION 201 UV-Vis spectrophotometer, with a 1 mm light path quartz cell, respectively.
Electrochemical tests were performed on CHI760E electrochemical workstation. A standard three-electrode system was used with glassy carbon as the working electrode, Ag/AgCl as the reference electrode, and graphite as the counter electrode. The calcined powders were characterized by X-ray diffractometry (XRD, Rigaku Ultima Ⅳ, Japan) using Cu Kα radiation, SEM images of the samples were carried out using Hitachi S-3400N.
2.4 Calculation of leaching efficiency
The leaching efficiency (η) was defined as:
where C is the final concentration of Co in the solution (mg·L−1), V is the volume of the initial resulting solution (in L), and m x is the mass of the initial amount of Co in the original material (in mg).
3 Results and discussion
3.1 Effect of process parameters on leaching efficiency of Co
The effect of the molar ratio of ChCl:Gly on the leaching efficiency of Co is shown in Figure 1a. When the molar ratio of ChCl:Gly was 1:1, 1:1.5, 1:2, and 1:4, the leaching efficiency of Co was 91.7%, 95.41%, 91.18%, and 78.82%, respectively.
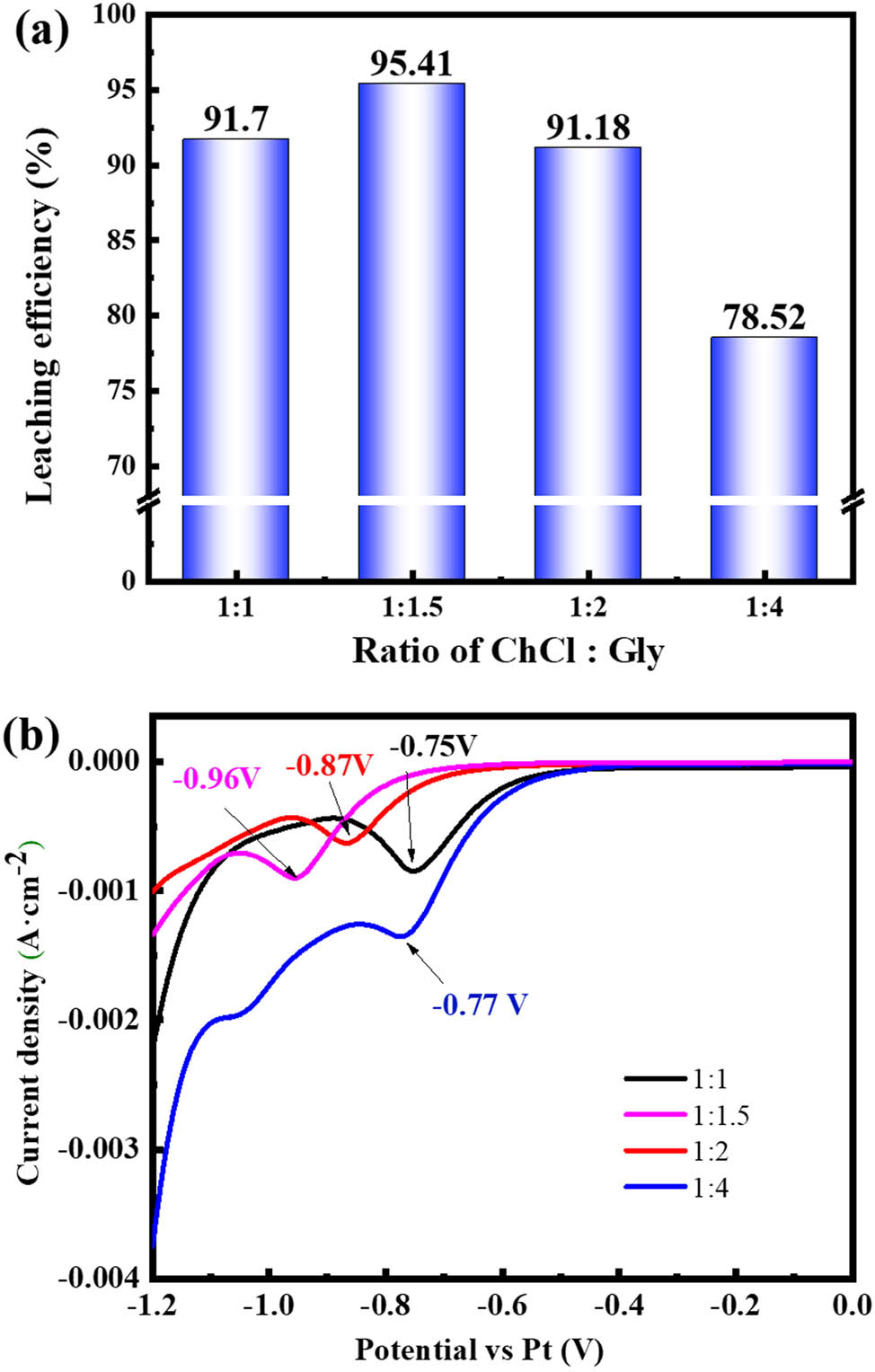
Effect on the leaching of Co from LCO with different molar ratios of ChCl:Gly: (a) the leaching efficiency of cobalt and (b) the CV curves of DES.
The LCO contains a number of polymetallic lithium oxide with a high valence of Co. However, Co(iii) oxides are usually insoluble. Reducing the valence of Co plays a key role in the recycling of valuable elements from spent LCO. In the recycling of valuable elements from spent LCO, the reducibility of DES is one of the factors to be considered. In the recycling of SLIB using ChCl:EG DES, EG could be chemically acting as such an acceptor. This implies the proton acceptor has some reductive ability. The reducibility of DES could be evaluated by its characteristic reduction potential [16]. The results of voltammetric experiments with the different molar ratios of ChCl:Gly DES are shown in Figure 1b. When the molar ratio of ChCl:Gly reaches 1:1.5, DES has the largest reduction potential, −0.96 V (vs Pt wire). In Figure 1a, the highest leaching efficiency of Co attained 95.41% in DES with most negative decomposed onset potential.
There is a good correspondence between the leaching efficiency of Co and the reduction potential of DES. Operating parameters play a key role in the extraction of Co. The effect of the reaction time and temperature on the leaching efficiency of Co is shown in Figure 2. When the temperature was below 160°C, the rate of solid dissolution was very slow, and the leaching efficiency of Co was below 20%, even if the reaction time exceeds 10 h. The leaching efficiency of Co increased with the increase in temperature. When the reaction temperature was 180°C, the reaction speed of the system was accelerated, and the leaching efficiency of Co is rapidly increased to 76.5% in 10 h. When the temperature was further increased to 200°C, the leaching efficiency of Co was increased from 14.0% in 2 h to 90.5% in 6 h. The color of the leaching solution also changed with the change in treatment temperature as shown in the optical image in Figure 2. DES slowly changed from turbid to transparent emerald green and then gradually change to dark green during the dissolution of LCO. The leaching efficiency of Co can be directly reflected in the color of DES. We choose 200°C as the optimal temperature to obtain the highest leaching efficiency. Reaction time is another important experimental factor. Figure 2 also shows that in the first 2 h, the leaching of Co almost did not occur, as the reaction goes on, the leaching efficiency increased rapidly, and reached a plateau at 6 h, indicating the leaching was almost complete. When the reaction temperature is 200°C and the reaction time is 10 h, the leaching efficiency reaches 92.7%.
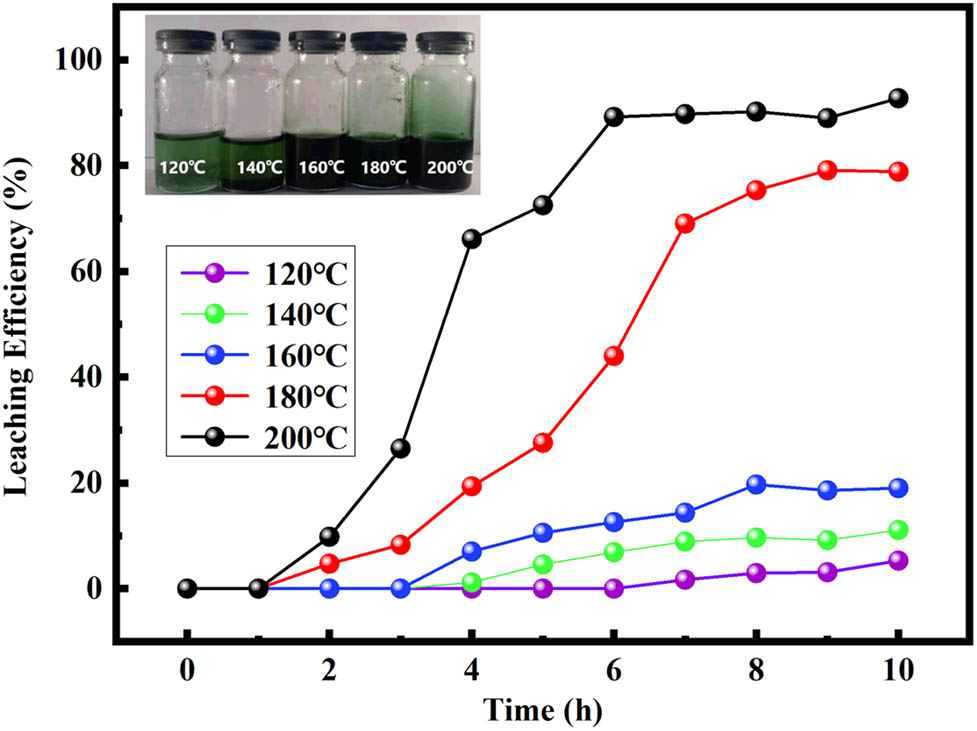
Leaching efficiency of Co at the temperatures from 120°C to 200°C for 6–10 h.
Leaching efficiency data of the previous studies and this work are shown in Table 1. A higher leaching efficiency is seen than the previously reported routes by using other chemical configurations of DES.
Comparison between reported EDS-based approaches for recycling of Co from LIBs
| Entry | Reaction | Reaction conditions | Leaching efficiency (%) | Ref. |
|---|---|---|---|---|
| system | ||||
| 1 | H2SO4 (1 M) | 40℃, 1 h | 40 | [11] |
| 2 | DES (ChCl + EG) | 195℃, 24 h | 69.14 | [23] |
| 3 | DES (ChCl + urea) | 160℃, 2 h | 60 | [24] |
| 4 | DES (ChCl + CA) | 60℃, 4 h | 99 | [30] |
| 5 | DES (ChCl + Gly) | 200℃, 10 h | 92.7 | This work |
3.2 Leaching mechanism of Co in DES
The FT-IR spectra were applied to analyze the functional groups in the eutectic solvents and leachates (Figure 3). The methylene (2,940 cm−1) and hydroxyl absorption peaks (3,400 and 1,150 cm−1) of DES were weakened with the increase in the reaction time, respectively. A new band corresponding to C═O, likely aldehyde or carboxyl group, emerged at 1,740 cm−1, indicating a redox reaction in the leaching process as shown in Figure 3 (purple color line). It is suggested that the polyol structure of Gly is more likely to be oxidized to aldehyde or carboxyl in the presence of two strong oxidants, Co3+ and O2−. Like the traditional wet acid leaching reaction, the emergence of H+ accelerates the destruction of metal oxygen bond in LCO through proton and accelerates the consumption of O2 [23].
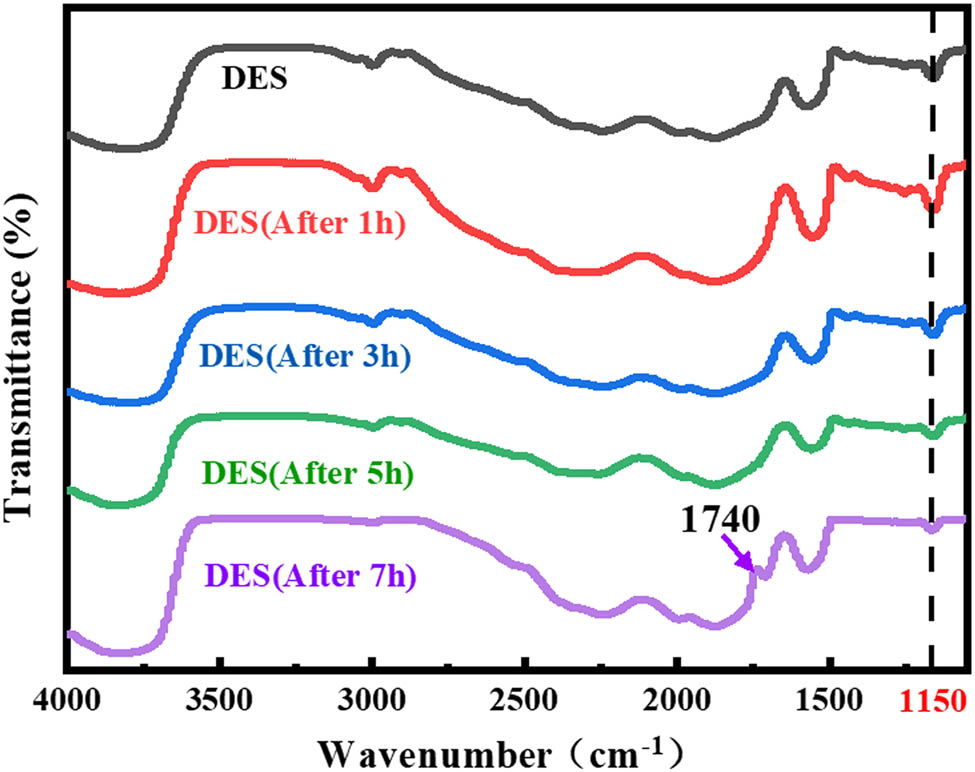
FTIR spectra of neat DES and Co-containing leachates at various reaction times.
The existing forms of cobalt ions in the liquid phase were further analyzed by UV-Vis spectrophotometry (Figure 4). When the temperature was below 180°C, there was no characteristic peak of Co(ii). The adsorption intensity of the broad peak is dependent on the concentration of the Co(ii) in DES. Three absorption peaks at 628, 665, and 690 nm, were observed in the range of 600–700 nm when the temperature reached 180°C. A d–d broadband peak at 628 nm was present due to the formation of Co(ii) complexes. The Co2+ cation can coordinate with Cl− or O2− to be six-coordinated with octahedral geometry. In the chloride-containing system, the absorption peak near 665 nm may be attributed to the presence of [CoCl4]2− anion. The as-observed peaks correspond to the characteristic absorption peaks of [CoCl4]2− anion emerged, which verifies the existence of Co(ii) [28,30] in the Co-containing leachates.
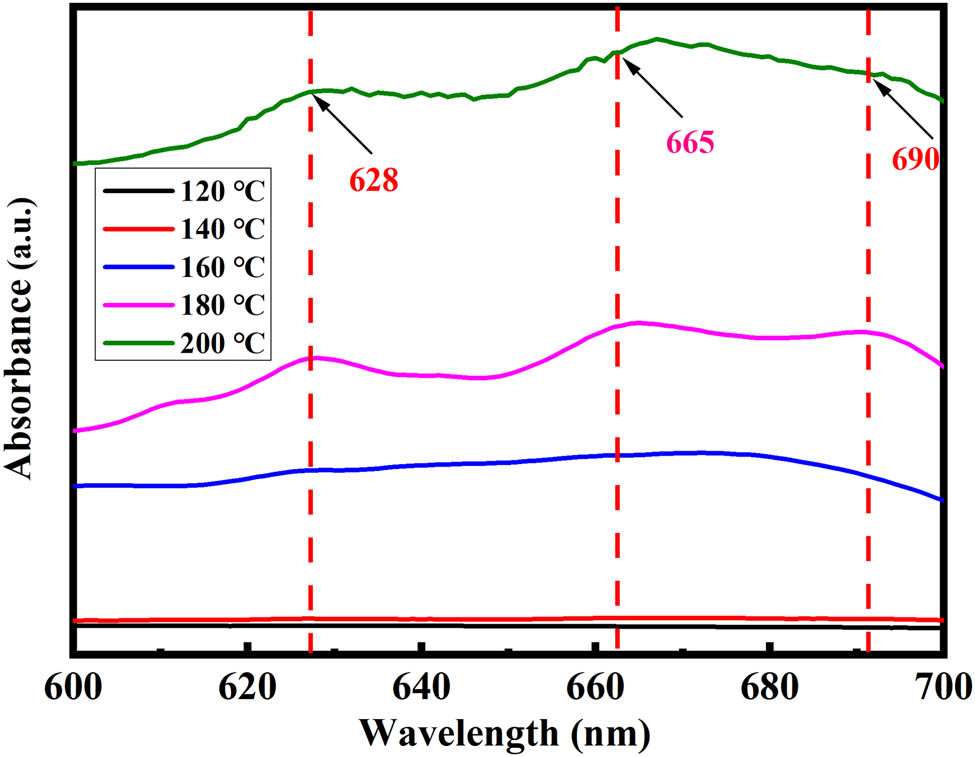
UV-Vis spectra of Co-containing leachates at different temperatures.
The dissolution of LCO in DES resulted in the contact of cobalt(iii) to the part of the system with strong reducibility. The reducibility of the DES promoted the consumption of positive cobalt(iii) in Eq. 3. At the same time, it was oxidized to Aldehyde or carboxyl group, which increased the formation of H, which was rapidly consumed with O2− in Eq. 2, thus increasing the efficiency of the reaction. The coordination of Cl− further stabilized the cobalt(ii) in the solution, thus allowing Eq. 3 to continue.
The leaching process of LCO in ChCl/Gly DES can be expressed as follows:
3.3 Co-containing leachates precipitation and recovery
The cobalt recovery experiment was carried out by precipitation using 20% sodium carbonate solution. The obtained pink precipitate is roasted to obtain a black powder. Figure 5a shows the XRD patterns of the calcined samples, the peak of the crystalline powders can be well matched to cubic cobalt oxide spinel (JCPDS no. 43-1003). SEM image of the acquired Co3O4 powders with particle size ranging from 2 to 10 μm is shown in Figure 5b. The result showed that the most Co in the ChCl:Gly DES was recycled in the process of precipitation–calcination to be cubic cobalt oxide spinel (Co3O4).
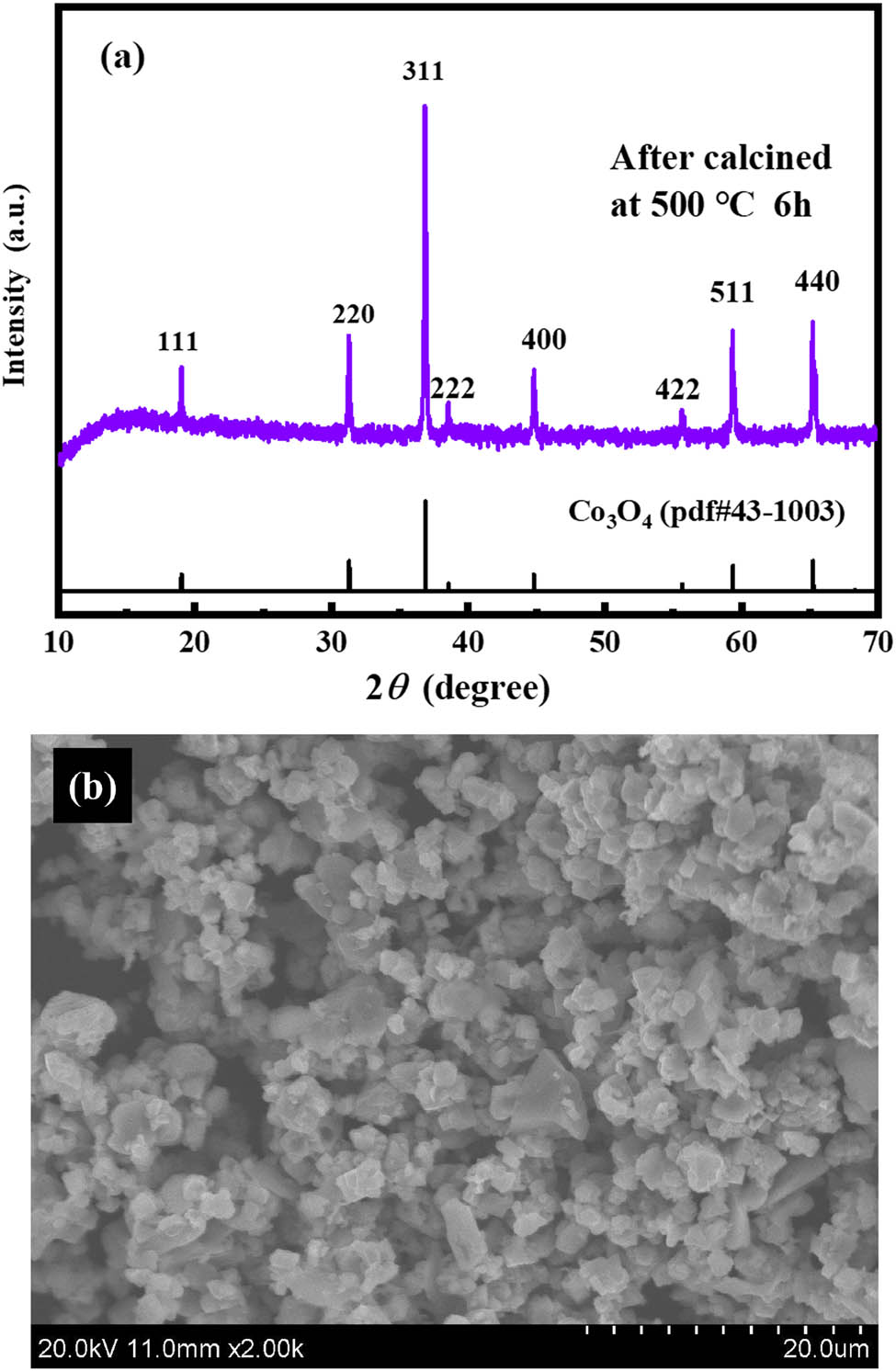
Characterization of calcined precipitate samples from leaching solution: (a) XRD pattern of the sample and (b) SEM image of the sample.
4 Conclusion
In the present work, we demonstrated a new chemical configuration by using the mixture ChCl and Gly to recover the Co from LCO-based materials. The high leaching efficiency of Co 95.7% was achieved at 200°C for 20 h. It was found that the Co component dissolved into the DES by reduction of Co(iii) into Co(ii), the reducibility of DES was evaluated by CV tests, the most negative decomposed onset potential was found to be −0.96 V. The structure of the Co complexes in the choline chloride-based DES was verified from UV spectra. A new band, C═O, emerged at 1,740 cm−1 in DES after leaching, indicating a redox reaction in the leaching process was verified by FTIR as evidenced in CV. Recovery of Co was performed by the carbonate precipitation method, and 94.7% of the Co was achieved in form of Co3O4.
-
Funding information: The National Natural Science Foundation of China (No. U1360204 and No. 51304139) and the Department of Science and Technology of Liaoning Province, China (2019-ZD-0258).
-
Author contributions: Honghao Yu: writing – original draft, methodology, and formal analysis; Shaomian Wang: methodology and project administration; Yin Li: project administration; Qian Qiao: project administration; Kun Wang: project administration; Xin Li: resources and writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Fan ES, Li L, Wang ZP, Lin J, Huang YX, Yao Y, et al. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects. Chem Rev. 2020;120:7020–63. 10.1021/acs.chemrev.9b00535.Search in Google Scholar PubMed
[2] Zeng X, Li J, Singh N. Recycling of spent lithium-ion battery: A critical review. Crit Rev Environ Sci Technol. 2014;44:1129–65. 10.1080/10643389.2013.763578.Search in Google Scholar
[3] Thompson DL, Hartley JM, Lambert SM, Shiref M, Harper GDJ, Kendrick E, et al. The importance of design in lithium ion battery recycling – a critical review. Green Chem. 2020;22:7585–603. 10.1039/D0GC02745F.Search in Google Scholar
[4] Chen X, Li J, Kang D, Zhou T, Ma H. A novel closed-loop process for the simultaneous recovery of valuable metals and iron from a mixed type of spent lithium-ion batteries. Green Chemistry. 2019;21(23):6342–52. 10.1039/C9GC02844G.Search in Google Scholar
[5] Hu X, Mousa E, Tian Y, Ye G. Recovery of Co, Ni, Mn, and Li from Li-ion batteries by smelting reduction - Part I: A laboratory-scale study. J Power Sources. 2021;483:1–8. 10.1016/j.jpowsour.2020.228936.Search in Google Scholar
[6] Stefan WK, Holzer A, Ponak C, Raupenstrauch H. Pyrometallurgical lithium-ion-battery recycling: Approach to limiting lithium slagging with the InduRed reactor concept. Processes. 2021;9:84–99. 10.3390/pr9010084.Search in Google Scholar
[7] Harper G, Sommerville R, Kendrick E, Driscoll L, Slater P, Stolkin R, et al. Recycling lithium-ion batteries from electric vehicles. Nature. 2019;575(7781):75–86. 10.1038/s41586-019-1682-5.Search in Google Scholar PubMed
[8] Biswal BK, Jadhav UU, Madhaiyan M, Ji LH, Yang EH, Cao B. Biological leaching and chemical precipitation methodsfor recovery of Co and Li from spent lithium-ion batteries. ACS Sustainable Chem Eng. 2018;6:12343–52. 10.1021/acssuschemeng.8b02810.Search in Google Scholar
[9] Huang T, Liu LF, Zhang SW. Recovery of cobalt, lithium, and manganese from the cathode active materials of spent lithium-ion batteries in a bio-electro-hydrometallurgical process. Hydrometallurgy. 2019;188:101–11. 10.1016/j.hydromet.2019.06.011.Search in Google Scholar
[10] Barik SP, Prabaharan GL, Kumar J. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. Clean. 2017;147:37–43. 10.1016/j.jclepro.2017.01.095.Search in Google Scholar
[11] He LP, Sun SY, Song XF, Yu JG. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag. 2017;64:171–81. 10.1016/j.wasman.2017.02.011.Search in Google Scholar PubMed
[12] Meshram P, Pandey BD, Mankhand TR. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem Eng J. 2015;281:418–27. 10.1016/j.cej.2015.06.071.Search in Google Scholar
[13] Zeng X, Li J, Shen B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J Hazard Mater. 2015;295:112–8. 10.1016/j.jhazmat.2015.02.064.Search in Google Scholar PubMed
[14] Zhang X, Bian YS, Xu E, Fan Q, Xue Y, Guan F, et al. Innovative application of acid leaching to regenerate Li(Ni1/3Co1/3Mn1/3)O2 cathodes from spent lithium-ion batteries. ACS Sustainable Chem Eng. 2018;6:5959–68. 10.1021/acssuschemeng. 7b04373.Search in Google Scholar
[15] Vieceli N, Nogueira CA, Guimaraes C, Pereira MFC, Durao FO, Margarido F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018;71:350–61. 10.1016/j. wasman.2017.09.032.Search in Google Scholar
[16] Li L, Dunn JB, Zhang XX, Gaines L, Chen RJ, Wu F, et al. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J Power Sources. 2013;233:180–9.10.1016/j.jpowsour.2012.12.089Search in Google Scholar
[17] Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their application. Chem Rev. 2014;114:11060–82. 10.1021/cr300162p.Search in Google Scholar PubMed
[18] Li W, Zhang Z, Han B, Hu S, Song J, Xie Y, et al. Switching the basicity of ionic liquids by CO2. Green Chem. 2008;10:1142–5. 10.1039/b811624e.Search in Google Scholar
[19] Abbott AP, Capper G, Davies DL, McKenzie KJ, Obi SU. Solubility of metal oxides in deep eutectic solvents based on choline chloride. J Chem Eng Data. 2006;51:1280–2. 10.1021/je060038c.Search in Google Scholar
[20] Millia L, Dall'Asta V, Ferrara C, Berbenni V, Quartarone E, Perna FM, et al. Bio-inspired choline chloride-based deep eutectic solvents as electrolytes for lithium-ion batteries. Solid State Ion. 2018;323:44–8. 10.1016/j.ssi.2018.05.016.Search in Google Scholar
[21] Shi Y, Chen G, Chen Z. Effective regeneration of LiCoO2 from spent lithium-ion batteries: a direct approach towards high-performance active particles. Green Chem. 2018;20:851–62. 10.1039/C7GC02831H.Search in Google Scholar
[22] Albler FJ, Bica K, Foreman MR, Holgersson S, Tyumentsev MS. A comparison of two methods of recovering cobalt from a deep eutectic solvent: Implications for battery recycling. J Clean Prod. 2017;167:806–14. 10.1016/j.jclepro.2017.08.135.Search in Google Scholar
[23] Tran MK, Rodrigues MF, Keto K, Babu G, Ajayan PM. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat Energy. 2019;4:339–45. 10.1038/s41560-019-0368-4.Search in Google Scholar
[24] Wang H, Li M, Garg S, Wu Y, Idros MN, Hocking R, et al. Cobalt electrochemical recovery from lithium cobalt oxides in deep eutectic choline chloride + urea solvents. ChemSusChem. 2021;14:2972–83. 10.1002/cssc.202100954.Search in Google Scholar
[25] Wang S, Zhang Z, Lu Z, Xu Z. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chem. 2020;22:4473–82. 10.1039/d0gc00701c.Search in Google Scholar
[26] Schiavi PG, Altimari P, Branchi M, Zanoni R, Simonetti G, Navarra MA, et al. Selective recovery of cobalt from mixed lithium ion battery wastes using deep eutectic solvent. Chem Eng J. 2021;417:129249–58. 10.1016/j.cej.2021.129249.Search in Google Scholar
[27] Chen L, Cha Y, Li X, Zhou G, Lu Q, Hua M, et al. Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries. Green Chem. 2021;23:2177–84. 10.1039/d0gc03820b.Search in Google Scholar
[28] Du K, Ang EH, Wu XL, Liu Y. Progresses in sustainable recycling technology of spent lithium-ion batteries. Energy Environ Mater. 2022;1–25. 10.1002/eem2.12271.Search in Google Scholar
[29] Jena KK, AlFantazi A, Mayyas AT. Comprehensive review on concept and recycling evolution of lithium-ion batteries (LIBs). Energy Fuels. 2021;35:18257–84. 10.1021/acs.energyfuels.1c02489.Search in Google Scholar
[30] Coleman JS. Chloride complexes of cobalt(ii) in anion and cation exchanges. Inorg. 1966;28:2371–8. 10.1016/0022-1902(66)80128-8.Search in Google Scholar
© 2022 Honghao Yu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

