Abstract
Metal–organic frameworks (Sm-MOFs) were prepared using a microwave-assisted ball milling method with a water solution. The structure was characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and SEM, and the thermal stability of the Sm-MOFs was tested by Thermogravimetry (TGA). The results showed that the Sm-MOF material exhibited a favorable effect on removing the organic dye Congo red (CR). When the concentration of CR was 80 ppm, adding 50 mg of Sm-MOF material achieved an adsorption capacity of 396.8 mg·g−1. The experimental data were analyzed theoretically through dynamics, and the experimental results were consistent with the second dynamics model, with correlation coefficients (R 2) all above 0.99. Comprehensive data analysis revealed that the Sm-MOF materials had great potential for future application in wastewater treatment.
1 Introduction
The rapid pace of industrial development and continual increase in urbanization have led to escalating threat of pollution to the water environment. Industrial waste can contain herbicides/pesticides, dyes, hexavalent chromium, and aromatics/organics, and these inorganics and organics should be removed from wastewater. The processes of printing and dyeing produce substantial wastewater with high chromaticity that can cause serious pollution to the environment. Methylene blue, Congo red (CR), and orange II are typical organic dyes that have been widely used in the production of textiles, cosmetics, papers, carpets, and plastics. If these dyes are discharged without treatment, they can cause serious environmental pollution problems, and their toxicity can harm human health. The global textile industry uses more than 10,000 tons of dye each year, and about 5,000 tons of dye and 3,600 tons of different dye-containing waste are discharged into rivers [1,2]. Most dyes in industrial wastewater are toxic, teratogenic, and carcinogenic. Therefore, effective treatment of wastewater from dyeing processes is imperative for the green and sustainable development of the textile industry.
At present, the adsorption method is a simple, direct, cheap, and highly efficient method [3–5] used in the treatment of wastewater, in addition to coagulation [6], photodegradation [7,8], filtration [9,10], among others. As potential effective adsorbents, porous materials have drawn more and more interest in recent years, one of which is the metal–organic frameworks (MOFs).
MOFs are three-dimensional materials formed by linking multifunctional organic ligands and metal ions through coordination bonds. MOFs have structural diversity, high stability, tunable porous properties, a large specific surface area, and renewable metal active sites. MOFs have been widely used in carbon dioxide capture [11,12], catalysis [13,14], energy storage [15,16], chemical sensing [17], adsorption and separation [18–20], antibiotics [21,22,23], drug delivery [24,25], and others [26–30].
Rare earth elements have been widely used in electronics, petrochemical, metallurgy, machinery, energy, light industry, environmental protection, and agriculture fields. In particular, rare-earth metals have been widely applied in fluorescent materials. for example, MOF fluorescence sensing materials formed by lanthanide series as the core has been widely used in detection of inorganic ions [31,32], organic or inorganic small molecules [33,34], explosives [35,36], and temperature [37]. The preparation of MOFs usually includes solvothermal [38,39], ultrasonic [40], and microwave-assisted methods [41–44].
Microwave-assisted ball milling is based on a solid–liquid ball milling approach with a ball milling machine placed in a microwave oven [45]. Microwave heating can speed up the chemical reaction due to the specific microwave effects and high-energy ball milling can effectively suppress the grain growth and refine crystalline particles. Water is used as the solvent. And the use of clean deionized water clear will have the least impact on the environment. Therefore, the method can not only decrease the reaction time from several days to a couple of hours or even minutes, but also avoid the use of a large amount of organic solvent. Thus, the production cost of the adsorbent can obviously decrease.
In this study, Sm(CH3COO)4 was used as the metal ion and terephthalic acid was used as the organic ligand to prepare Sm-MOF materials under the condition of microwave-assisted ball milling. The prepared materials were then used to remove CR.
2 Experimental
The ligands p-phthalic acid and samarium(iii) acetate hydrate were purchased from Aladdin Biological Technology Co., Ltd, Shanghai, China. CR was supplied by Beike New Material Technology Co. Ltd, Beijing, China.
The structure and morphology of the Sm-MOFs were tested by FTIR, XRD, field emission scanning electron microscopy, and TGA.
Briefly, terephthalic acid (0.026 mol), hydrated samarium acetate (0.017 mol), deionized water (700 mL), and stainless-steel balls (480 g) were placed in a Teflon jar. After sealing the gap with insulating tape, the microwave was turned on, and samples were observed at 10 min. The color of the solution gradually turned blue, accompanied by a significant sour taste, until the color no longer changed. The reactant was then filtered, cooled to room temperature, and extracted. Next, the solid part was placed into a beaker containing 100 mL of ethanol, stirred with a magnetic stirrer for 3 h, followed by extraction, filtering, and drying. The light red solids of Sm-MOFs were obtained in 81.57% yield based on hydrated samarium acetate.
To evaluate the removal capacity of the Sm-MOFs, CR was chosen as a model contaminant and dissolved in a 250 mL beaker at indoor temperature (∼15°C). The removal of CR was tested in a beaker containing 30, 40, 50, 80, and 100 ppm of CR solution with 40, 50, 80, and 100 mg Sm-MOFs placed under the action of natural light on a magnetic stirrer. Every 0.5 h, the absorbance at 496 nm [40] from the reaction suspension was monitored by UV-visible spectrophotometer. In this way, the removal rate of CR could be obtained at different time intervals. The quantity of CR removal was calculated according to the following equation:
where C 0 and C e are the initial concentration and equilibrium concentration of the solution (ppm), respectively; V is the volume of the CR solution (L); and m is the amount of the Sm-MOFs (g).
3 Results and discussion
3.1 Structural characterization
As shown in Figure 1a, the antisymmetric and symmetric stretching vibrations of carboxylate were 1,559 and 1,372 cm−1, respectively. This was mainly due to the formation of large bonds after the delocalization of the carboxylic group, resulting in tendency of the two oxygen atoms to be equivalent, while the electron cloud tended to be average. Strong absorption peaks appeared at 1,610–1,550 and 1,420–1,300 cm−1. The stretching vibrations of aromatic ring skeletons are 1,662 and 1,436 cm−1, 1,112 cm−1 is the deformation vibration of C–H bond of aromatic hydrocarbon, and 762 cm−1 is the displacement characteristic peak of aromatic ring. XRD measurement of the Sm-MOFs is shown in Figure 1b, indicating that the material has no wide absorption peak, suggesting that the material has good crystallinity. As it can be seen from the SEM figure shown in Figure 1c and d the Sm-MOF particle morphology had different structures and good dispersion. This was mainly because the intermolecular interactions of organic ligands were weakened or even disappeared, and the deprotonation of organic ligands was enhanced, which promoted the growth of crystal in the aqueous solution.
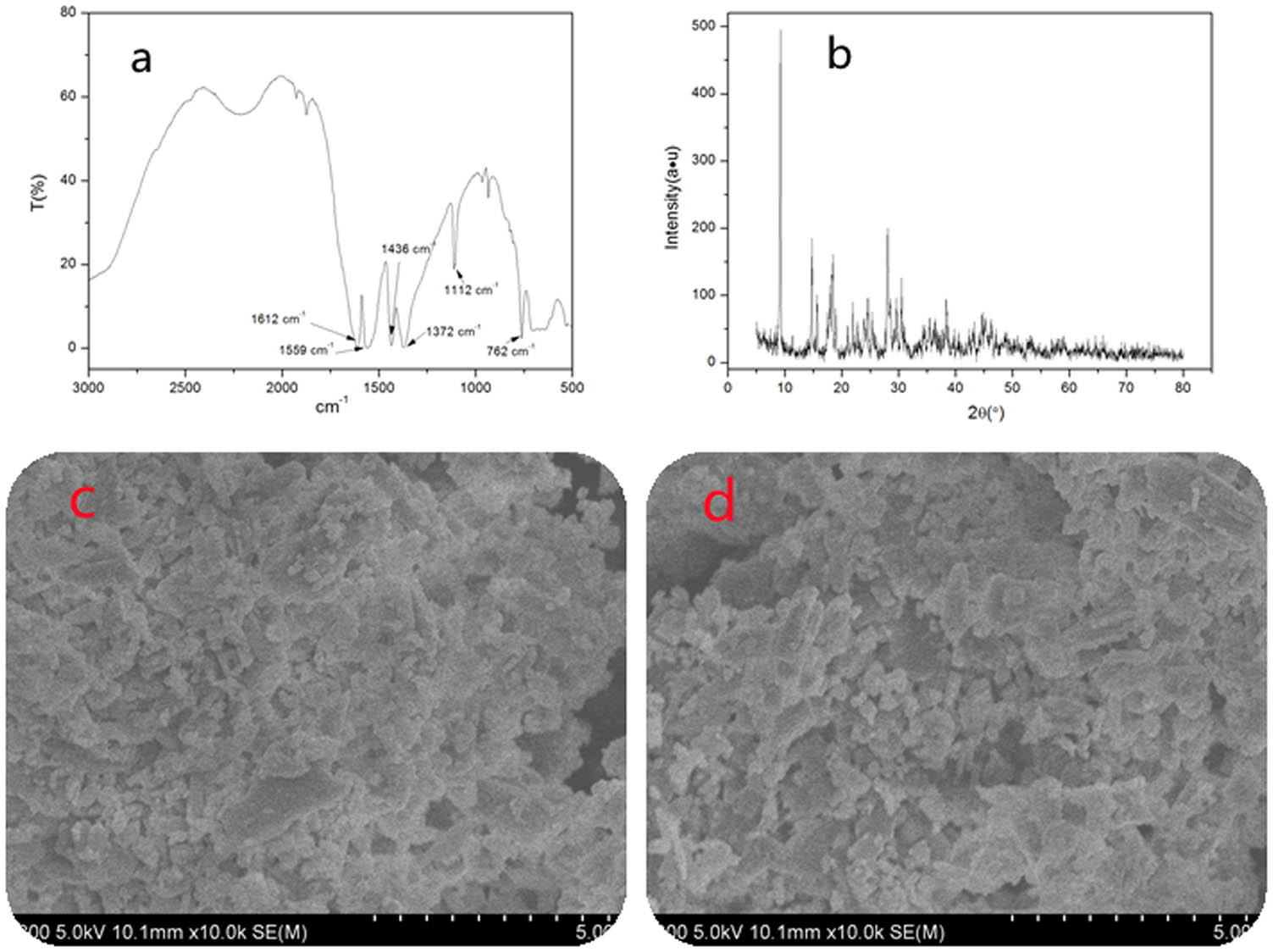
Structural characterization of Sm-MOFs: (a) FTIR, (b) XRD, and (c and d) SEM.
The stability of the material is one of the key factors for its performance. The stability of the Sm-MOFs was tested by the thermogravimetric analyzer and the results are shown in Figure 2, which can be divided into three stages. The MOF material is porous, and the first stage showed that a small amount of solvent (6.3%) was still in the material. The second stage mainly involved the oxidation of unreacted metal ions which accounted for 18.7% of the loss [43,46] between 178°C and 349°C, while the third stage was due to the destruction of the whole frame structure. After reaching 610°C, the whole molecule was completely destroyed. The quality of this loss is stable at 452°C and then completed at 800°C. The residue was 33.9% of the initial mass. Most often the reaction of organic ligands and metal ions via a common synthesis procedure yields MOFs with more stable structures [47].
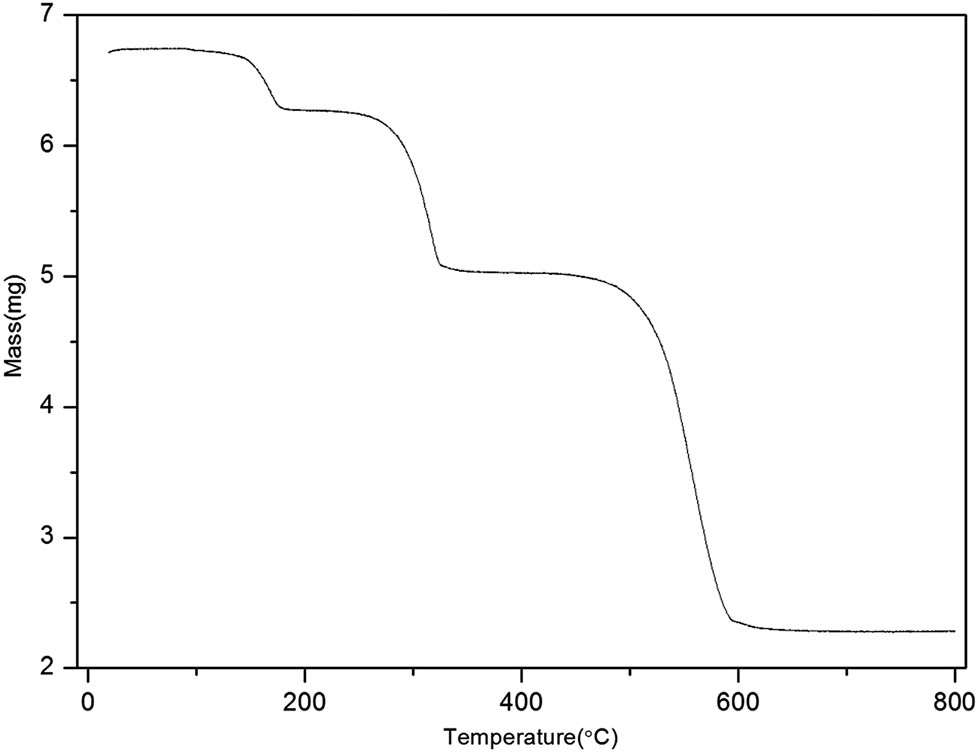
Thermogravimetric analysis of the Sm-MOF.
Figure 3 shows that the Brunauer–Emmett–Teller surface area was 7.89 m²·g−1. The average particle size was 759.6 nm, the adsorption average pore width was 9.6 nm, Barret-Joyner-Halenda (BJH) adsorption average pore width was 16.2 nm, and BJH desorption average pore width was 18.5 nm, indicating the mesoporous nature of the material. As can be seen from Figure 3, the Sm-MOFs have four types of isotherms with H3 hysteresis rings, which represent the mesoporous properties of the sample [48,49].
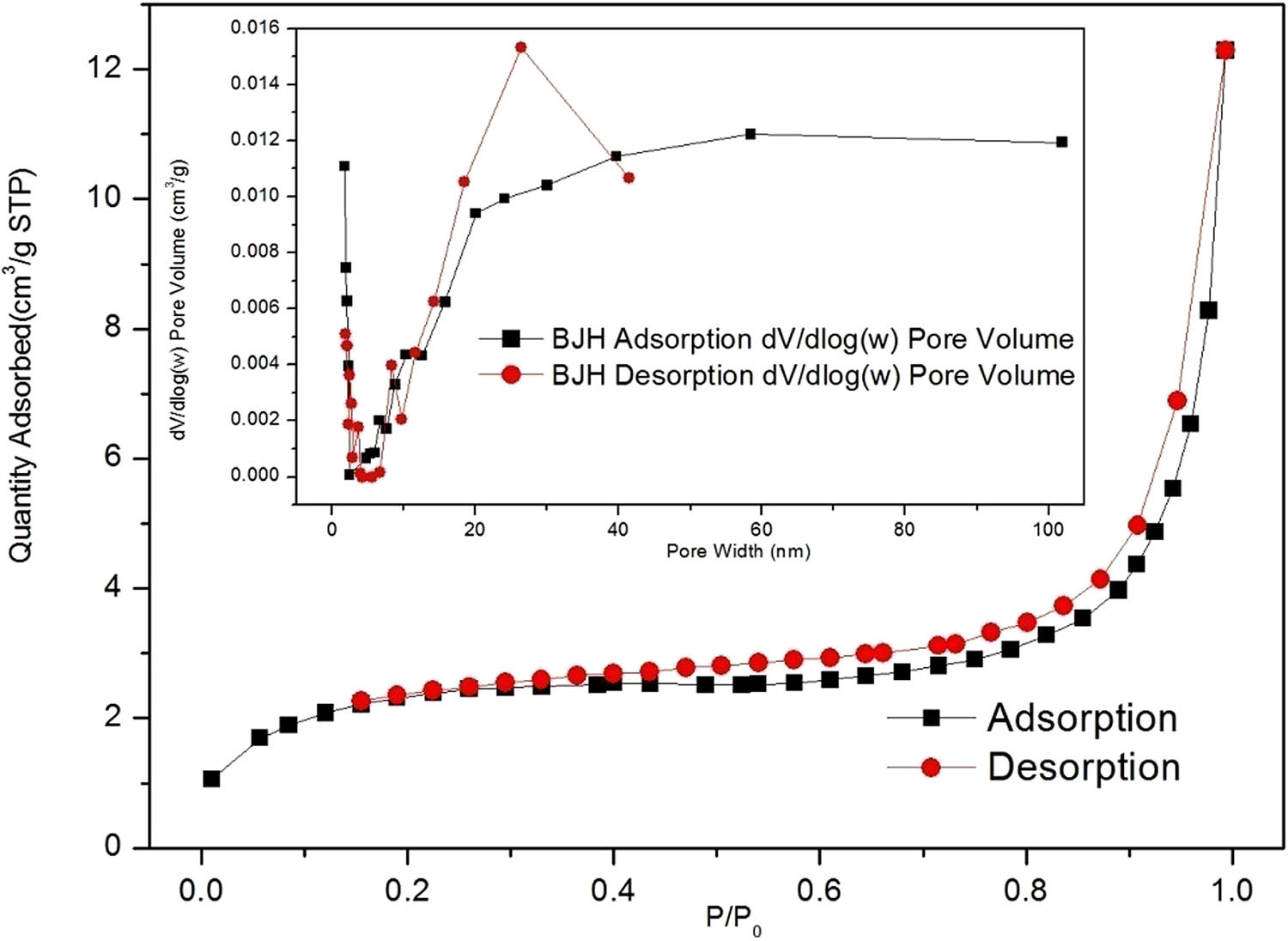
N2 adsorption–desorption isotherms of the Sm-MOF.
3.2 Removal of organic dye
To evaluate the quality of Sm-MOF, CR was used as the target molecule to test Sm-MOF’s ability to remove organic pollutants. Sm-MOFs of different masses were assessed for removal of CR. The results showed that the CR removal rate increased with increasing mass of Sm-MOFs, while decreased with increasing concentrations of CR. The CR removal rate could reach 100% within 30 min for low concentration of CR. When the CR concentration was 80 ppm and the mass of Sm-MOFs was 50 mg, the adsorption capacity was the largest, reaching 396.8 mg·g−1, but when the CR concentration was 100 ppm and the mass of Sm-MOFs was 50 mg, the removal rate was the worst, only 55.7%. The CR adsorption capacity was mainly influenced by the specific area and pore size of Sm-MOFs. First, because the pore size of Sm-MOFs is equivalent to the volume of CR molecules, the CR molecules flow into the pores of Sm-MOFs and adsorb onto the material. Second, Sm-MOFs and CR both have benzene rings and π electrons, which are adsorbed together by π–π bonds. Finally, Sm-MOFs have metal active sites that can adsorb CR; however, the large mass of Sm-MOFs can lead to coverage of active sites, thereby decreasing the adsorption capacity. In addition, there may also be electric charges in the solution. In the system, the presence of π–π interaction, hydrogen bonding, and electrostatic attraction can result in a highly efficient removal of CR by MOFs [50,51]. Therefore, Sm-MOFs have a good CR removal capacity that is due to both physical and chemical adsorption. To test the reusability of Sm-MOFs, the used Sm-MOFs were filtered and washed three times with water and dried. The consequence indicated that the removal rate of Sm-MOFs remained above 80% after four cycles (as shown in Figure 4), indicating a reasonable reusability. The CR adsorptions with other adsorbents are shown in Table 1.
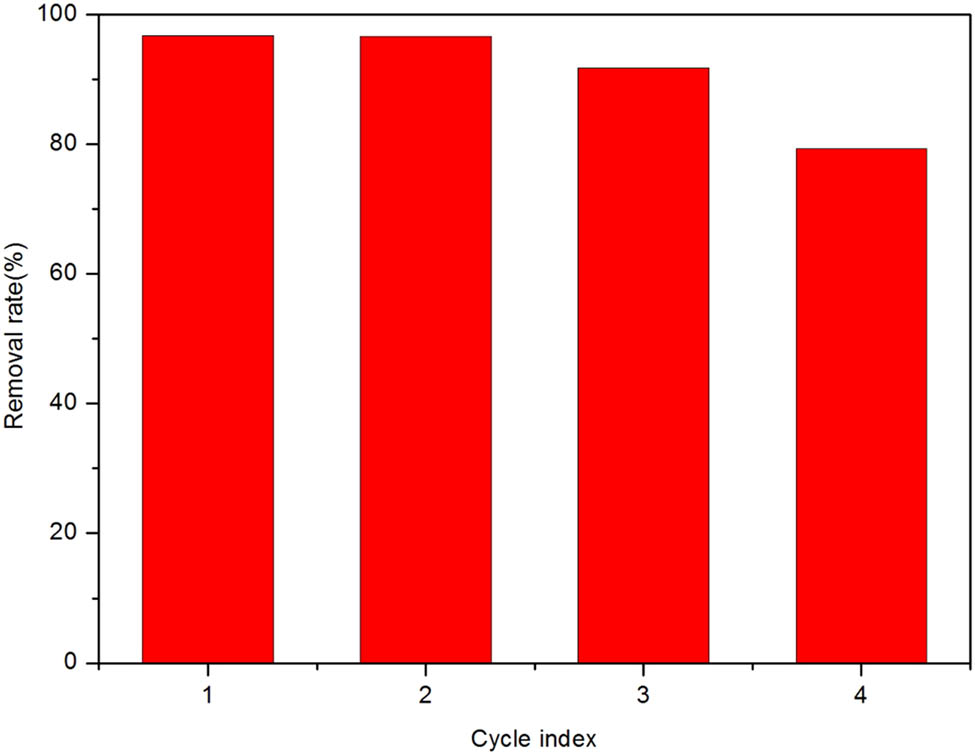
Reuse of Sm-MOF adsorbed CR.
Comparison of maximum adsorption capacities of various adsorbents for CR
| Adsorbent | q max (mg·g−1) | BET (m²·g−1) | Reference |
|---|---|---|---|
| Sm-MOFs | 396.8 | 7.89 | This study |
| In-MOFs-1 | 103.54 | 21 | [45] |
| In-MOFs-2 | 92.29 | 7 | |
| GO/In-MOFs-1 | 108.54 | 14 | |
| GO/In-MOFs-2 | 96.72 | 10 | |
| TUM-7 | 79 | 393 | [52] |
| TUM-39 | 53 | 521 | [53] |
| Uio-66 | 251 | 1,358 | [54] |
| NH2-MIL-68(Al) | 473.93 | 1,393 | [55] |
| HKUST-1 | 58.3 | 1,316 | [56] |
| Fe3O4@SiO2-NH2@HKUST-1 | 49.5 | 1,134 |
A kinetic model was used to verify the consistency between the experiment and theory, and the formula used is presented below.
The pseudo-second-order kinetic model:
where k is the kinetics reaction rate constant (min−1), q t (mg·g−1) is the adsorbing capacity of the Sm-MOFs at time t, and q e is the adsorbing capacity of the MOFs at equilibrium.
It can be seen from Figure 5 and Table 2 that all R 2 of the second kinetic model were greater than 0.99, suggesting the theory that Sm-MOF’s ability to remove CR is consistent with the results obtained in practice.
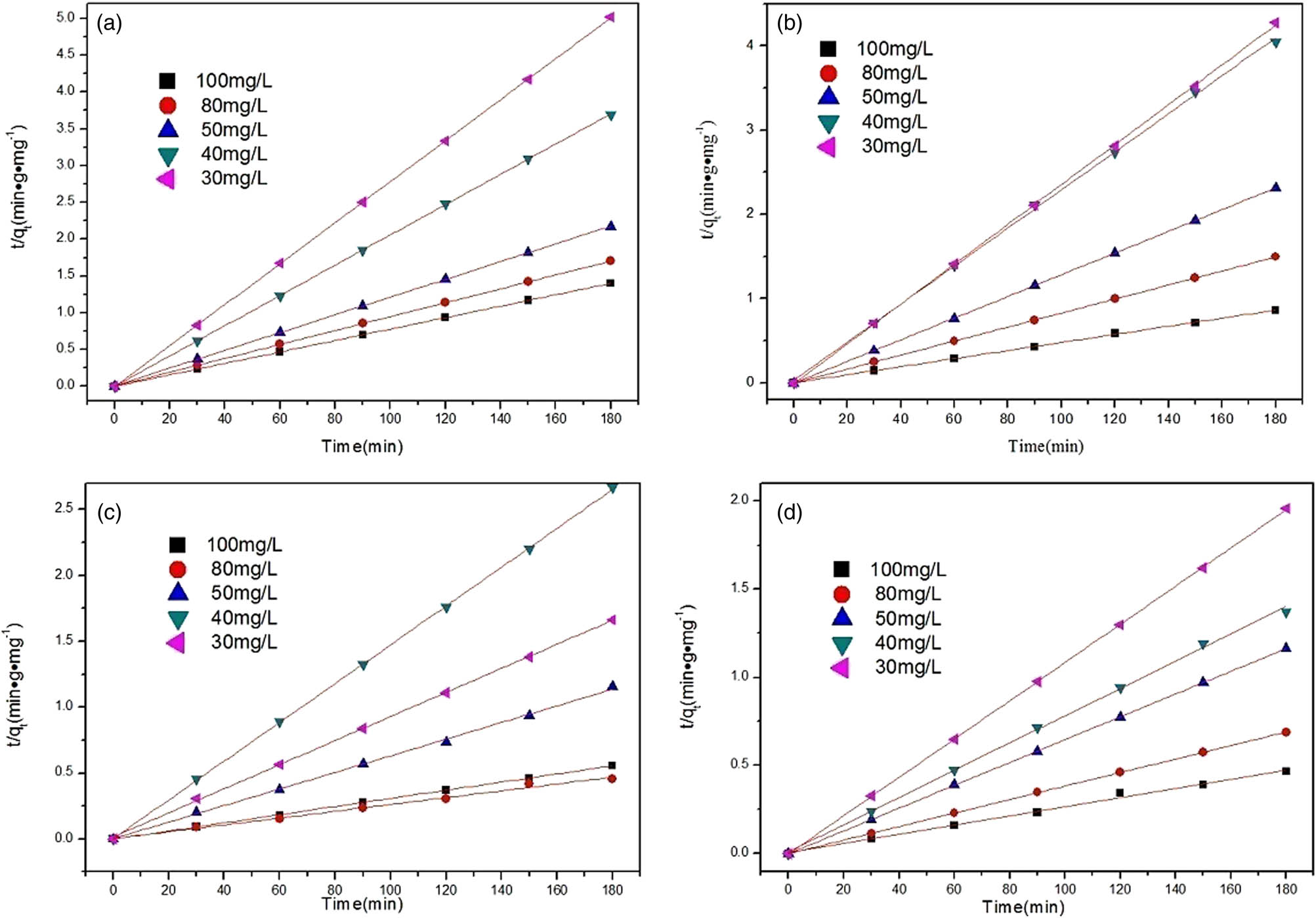
Pseudo-second-order kinetic model for the adsorption over the Sm-MOF: (a) m = 100 mg, (b) m = 80 mg, (c) m = 50 mg, and (d) m = 40 mg.
Kinetic parameters for the adsorption of CR by the Sm-MOFs
| Mass (mg) | Concentration | k | R 2 | q exp (mg·g−1) |
|---|---|---|---|---|
| 100 | 100 | 0.00778 | 0.999 | 128.8 |
| 80 | 0.00946 | 0.999 | 105.8 | |
| 50 | 0.0121 | 0.999 | 166.9 | |
| 40 | 0.0206 | 0.999 | 48.8 | |
| 30 | 0.0279 | 0.999 | 36.1 | |
| 80 | 100 | 0.00479 | 0.999 | 335.1 |
| 80 | 0.00831 | 0.999 | 120.6 | |
| 50 | 0.0129 | 0.999 | 78.1 | |
| 40 | 0.0226 | 0.999 | 72.8 | |
| 30 | 0.0236 | 0.999 | 42.7 | |
| 50 | 100 | 0.00310 | 0.999 | 326.3 |
| 80 | 0.00259 | 0.992 | 396.8 | |
| 50 | 0.00630 | 0.998 | 163.9 | |
| 40 | 0.0147 | 0.999 | 68.8 | |
| 30 | 0.00915 | 0.999 | 108.9 | |
| 40 | 100 | 0.00261 | 0.993 | 308.4 |
| 80 | 0.00383 | 0.999 | 261.5 | |
| 50 | 0.00647 | 0.999 | 154.8 | |
| 40 | 0.00773 | 0.998 | 131.2 | |
| 30 | 0.0108 | 0.999 | 92.5 |
At a certain temperature, the Langmuir isotherm, Freundlich isotherm, and Temkin isotherm models were applied to describe the relationship between the adsorption capacity of the Sm-MOFs and the equilibrium concentration of CR. To determine the efficiency of the adsorbent to remove CR from the environment, the following equations were used:
Figure 6 shows the Temkin isotherm of CR adsorption by MOFs, and Table 2 shows the parameters. The correlation coefficient was 0.982, suggesting that the Temkin isotherm was in good agreement with the experimental data. The q max of MOFs to CR was 304.9 mg·g−1. We also tested the Freundlich isotherm model. As shown in Figure 6 and Table 3, it can be seen that the adsorption of CR by MOFs is a monolayer adsorption. There was no interaction between the adsorbed molecules, and the adsorption equilibrium process was dynamic equilibrium [49].
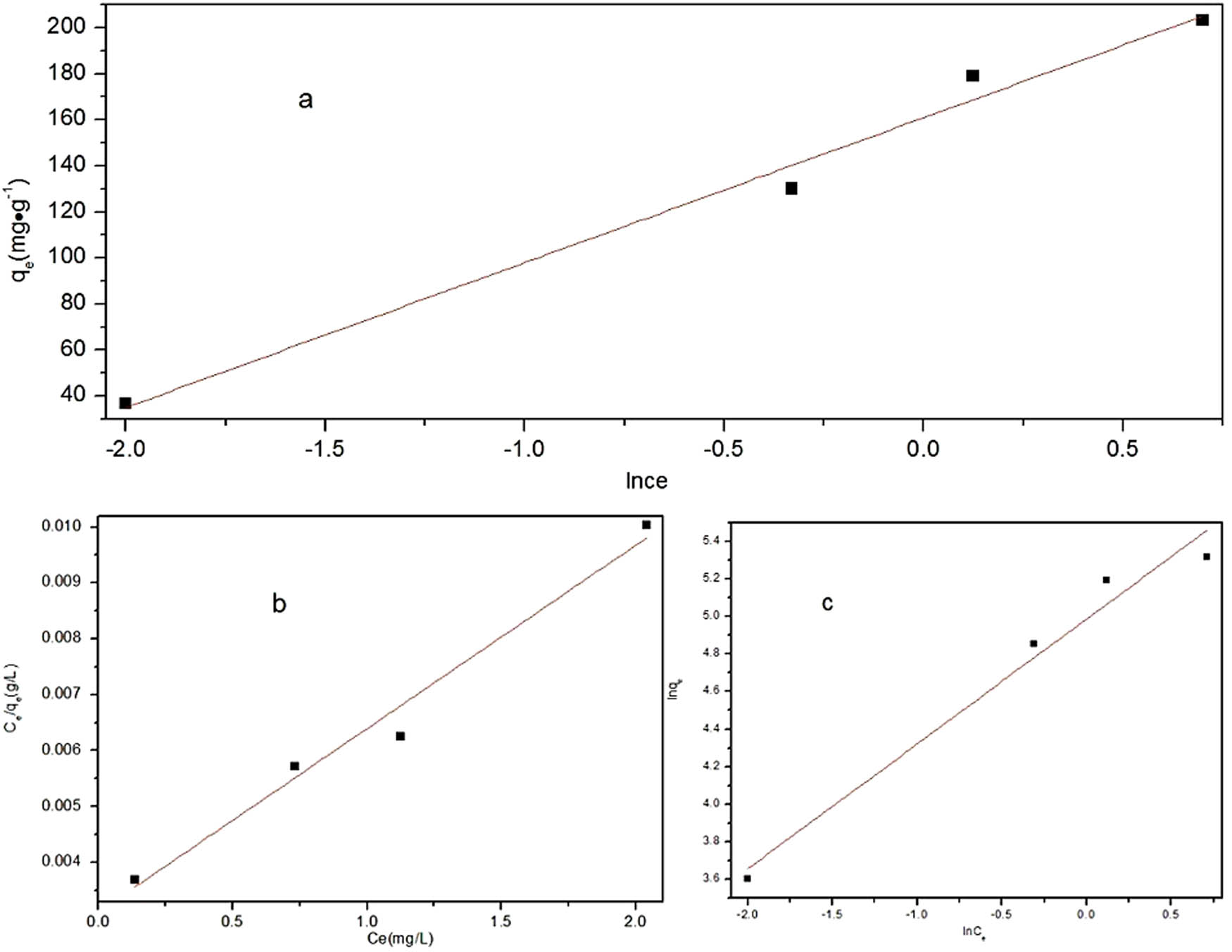
Adsorption isotherms of CR onto Sm-MOFs at room temperature: (a) Temkin isotherm, (b) Langmuir isotherm, and (c) Freundlich isotherm.
Adsorption isotherm parameters of CR onto Sm-MOFs at room temperature
| Adsorbent | Langmuir isotherm | Freundlich isotherm | Temkin isotherm | |||
|---|---|---|---|---|---|---|
| K | R 2 | K | R 2 | K | R 2 | |
| Sm-MOFs | 0.00311 | 0.97099 | 0.66398 | 0.96268 | 1.125 × 102 | 0.98208 |
To further understand the CR adsorption mechanism, the thermodynamic of CR removal was studied. The thermodynamic equilibrium constant and Gibbs free energy change were evaluated by the following formulas:
where K 0 is the Langmuir adsorption constant (L·mol−1) and R is the gas constant. As it can be seen from Figure 7 that ΔG 0, ΔH 0, and ΔS 0 are negative, suggesting that the adsorption of CR by Sm-MOFs is spontaneous, and the adsorption process is exothermic. Therefore, the driving force for CR adsorption by Sm-MOFs is due to both enthalpy effect and entropy effect.
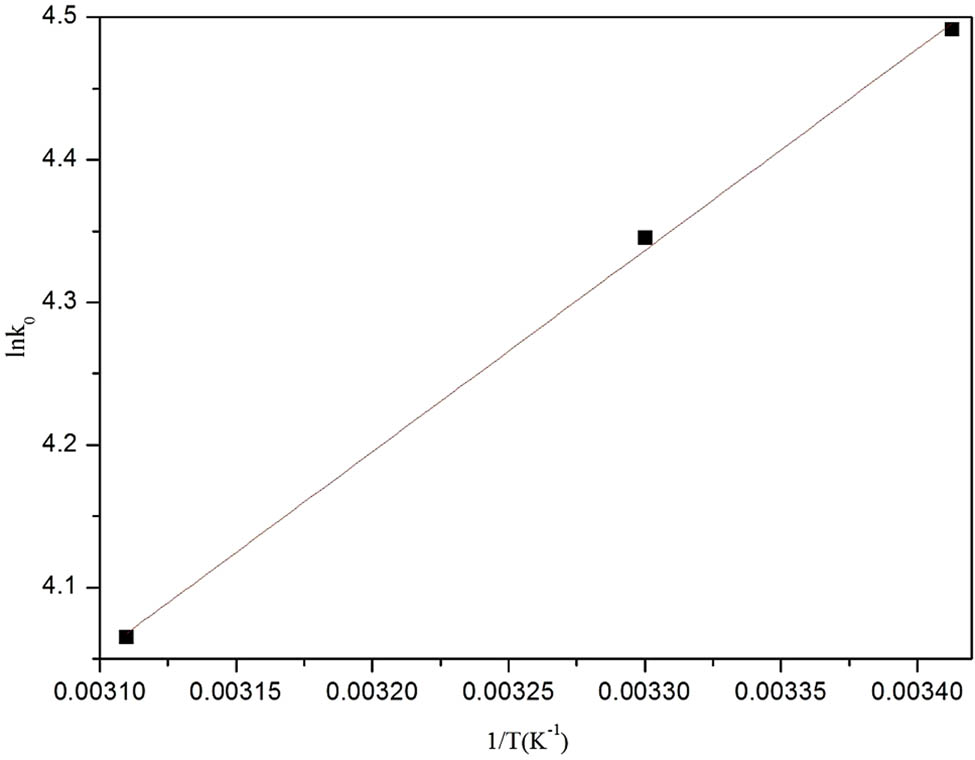
The thermodynamic equilibrium of CR adsorption by Sm-MOFs.
4 Conclusion
In summary, the Sm-MOFs were prepared by microwave-assisted ball milling, and the structure was tested using XRD, SEM, TG, and nitrogen adsorption and desorption. The Sm-MOFs material was tested for removal of CR. The experimental results were in accordance with the second dynamic model, and the correlation coefficients were all above 0.99. Langmuir, Freundlich, and Temkin isotherm analyses were performed to further investigate the adsorption of CR by Sm-MOFs, and the results showed that the correlation coefficients had good coincidence. These results indicate that the theory is consistent with practice. Therefore, Sm-MOFs can be well applied for CR removal in the future.
-
Funding information: This work was supported by Doctoral Fund of Anshun University (asxybsjj202103) and Guizhou Education Department Youth Science and Technology Talents Growth Project (KY[2019]149 and KY[2020]132).
-
Author contributions: Fuhua Wei: writing – original draft, writing – review and editing; Ting Zheng, JunhaoPeng, and Yufu Ma: methodology and investigation; Qinhui Ren and Hongliang Chen: formal analysis; Zhengjun Liu and Zhao Liang: validation and software; Ding Chen: conceptualization.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Murugesan A, Loganathan M, Senthil Kumar P, Vo DVN. Cobalt and nickel oxides supported activated carbon as an effective photocatalysts for the degradation methylene blue dye from aquatic environment. Sustain Chem Pharm. 2021;21:100406.10.1016/j.scp.2021.100406Search in Google Scholar
[2] Renita AA, Vardhan KH, Kumar PS, Ngueagni PT, Abilarasu A, Nath S, et al. Effective removal of malachite green dye from aqueous solution in hybrid system utilizing agricultural waste as particle electrodes. Chemosphere. 2021;273:129634.10.1016/j.chemosphere.2021.129634Search in Google Scholar PubMed
[3] Xiong G, Wang BB, You LX, Ren BY, He YK, Ding F, et al. Hypervalent silicon-based, anionic porous organic polymers with solid microsphere or hollow nanotube morphologies and exceptional capacity for selective adsorption of cationic dyes. Mater Chem. 2019;7(1):393–404.10.1039/C8TA07109HSearch in Google Scholar
[4] Bello K, Sarojini BK, Narayana B, Rao A, Byrappa K. A study on adsorption behavior of newly synthesized banana pseudo-stem derived superabsorbent hydrogels for cationic and anionic dye removal from effluents. Carbohydr Polym. 2018;181:605–15.10.1016/j.carbpol.2017.11.106Search in Google Scholar PubMed
[5] Hayati B, Mahmoodi NM, Maleki A. Dendrimer-titania nanocomposite: synthesis and dye-removal capacity. Res Chem Intermed. 2015;41:3743–57.10.1007/s11164-013-1486-4Search in Google Scholar
[6] Beluci NDL, Mateus GAP, Miyashiro CS, Homem NC, Gomes RG, Fagundes-Klen MR, et al. Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TiO2-modified membranes to improve the removal of reactive black 5 dye. Sci Total Environ. 2019;664:222–9.10.1016/j.scitotenv.2019.01.199Search in Google Scholar PubMed
[7] Lee CG, Javed H, Zhang DN, Kim JH, Westerhoff P, Li QL, et al. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ Sci Technol. 2018;52(7):4285–93.10.1021/acs.est.7b06508Search in Google Scholar PubMed
[8] Mahmoodi NM, Taghizadeh A, Taghizadeh M, Abdi J. In situ deposition of Ag/AgCl on the surface of magnetic metal-organic framework nanocomposite and its application for the visible-light photocatalytic degradation of Rhodamine dye. Hazard Mater. 2019;378:120741.10.1016/j.jhazmat.2019.06.018Search in Google Scholar PubMed
[9] Li CY, Lou T, Yan X, Long YZ, Cui GP, Wang XJ. Fabrication of pure chitosan nanofibrous membranes as effective absorbent for dye removal. Int J Biol Macromol. 2018;106:768–74.10.1016/j.ijbiomac.2017.08.072Search in Google Scholar PubMed
[10] Mahmoodi NM, Bashiri M, Moeen SJ. Synthesis of nickel–zinc ferrite magnetic nanoparticle and dye degradation using photocatalytic ozonation. Mater Res Bull. 2012;47:4403–8.10.1016/j.materresbull.2012.09.036Search in Google Scholar
[11] Mahmoodi NM, Saffar-Dastgerdi MH. Clean Laccase immobilized nanobiocatalysts (graphene oxide–zeolite nanocomposites): from production to detailed biocatalytic degradation of organic pollutant. Appl Catal B Environ. 2020;268:118443.10.1016/j.apcatb.2019.118443Search in Google Scholar
[12] Sumida K, Rogow DL, Mason JA. Carbon dioxide capture in metal-organic frameworks. Chem Rev. 2012;112:724–81.10.1021/cr2003272Search in Google Scholar PubMed
[13] Wang CC, Li JR, Lv XL. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ Sci. 2014;7:2831–67.10.1039/C4EE01299BSearch in Google Scholar
[14] Yin RT, Zhang SS, Xu YY, Xue JM, Bi JQ, Liu RJ. Adsorption mechanism and electrochemical properties of methyl blue onto magnetic CoxCu(1-x)Fe2O4 nanoparticles prepared via an alcohol solution of nitrate combustion and calcination process. J Inorg Organomet Polym Mater. 2021;31(8):3584–94.10.1007/s10904-021-01986-3Search in Google Scholar
[15] Wang H, Yuan XZ, Wu Y, Zeng GM, Dong HR, Chen XH, et al. In situ synthesis of In2S3@MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl Catal B-Environ. 2016;186:19–29.10.1016/j.apcatb.2015.12.041Search in Google Scholar
[16] Mahmoodi NM, Najafi F, Neshat A. Poly (amidoamine-co-acrylic acid) copolymer: synthesis, characterization and dye removal ability. Ind Crop Prod. 2013;42:119–25.10.1016/j.indcrop.2012.05.025Search in Google Scholar
[17] Kreno LE, Leong K, Farha OK. Metal-organic framework materials as chemical sensors. Chem Rev. 2012;112:1105–25.10.1021/cr200324tSearch in Google Scholar PubMed
[18] Nasrollahi N, Aber S, Vatanpour V, Mahmoodi NM. The effect of amine functionalization of CuO and ZnO nanoparticles used as additives on the morphology and the permeation properties of polyethersulfone ultrafiltration nanocomposite membranes. Compos Part B Eng. 2018;154:388–409.10.1016/j.compositesb.2018.09.027Search in Google Scholar
[19] Chen D, Feng PF, Wei FH. Preparation of Fe(III)-MOFs by microwave-assisted ball for efficiently removing organic dyes in aqueous solutions under natural light. Chem Eng Process Process Intensif. 2019;135:63–7.10.1016/j.cep.2018.11.013Search in Google Scholar
[20] Bajpai VK, Shukla S, Khan I, Kang SM, Haldorai Y, Tripathi KM, et al. A sustainable graphene aerogel capable of the adsorptive elimination of biogenic amines and bacteria from soy sauce and highly efficient cell proliferation. ACS Appl Mater Interfaces. 2019;11:43949–63.10.1021/acsami.9b16989Search in Google Scholar PubMed
[21] Pan S, Yu QM, Yu LL, Xu YY, Liu RJ. Preparation and anti-microbial performance of Ni0.5Zn0.5Fe2O4@Ag nanocomposites. J Inorg Organomet Polym Mater. 2021;31(2):875–85.10.1007/s10904-020-01768-3Search in Google Scholar
[22] Mahmoodi NM, Hayati B, Bahrami H, Arami M. Dye adsorption and desorption properties of Mentha pulegium in single and binary systems. J Appl Polym Sci. 2011;122:1489–99.10.1002/app.34235Search in Google Scholar
[23] Kumari S, Kumari P, Panda PK, Patel P, Jha E, Mallick MA, et al. Biocompatible biogenic silver nanoparticles interact with caspases on an atomic level to elicit apoptosis. Nanomedicine. 2020;15(22):2119–32.10.2217/nnm-2020-0138Search in Google Scholar
[24] Mahmoodi NM. Synthesis of magnetic carbon nanotube and photocatalytic dye degradation ability. Environ Monit Assess. 2014;186:5595–604.10.1007/s10661-014-3805-7Search in Google Scholar PubMed
[25] Li Y, Pan S, Yu QM, Ding X, Liu RJ. Adsorption mechanism and electrochemical performance of methyl blue onto magnetic Ni(1-x-y)CoyZnxFe2O4 nanoparticles prepared via the rapid-combustion process. Ceram Int. 2020;46(3):3614–22.10.1016/j.ceramint.2019.10.080Search in Google Scholar
[26] Mohan A, Dipallini S, Lata S, Mohanty S, Pradhan PK, Patel P, et al. Oxidative stress induced antimicrobial efficacy of chitosan and silver nanoparticles coated Gutta-percha for endodontic applications. Mater Today Chem. 2020;17:100299.10.1016/j.mtchem.2020.100299Search in Google Scholar
[27] Hayati B, Mahmoodi NM, Mazaheri F. Dye removal from colored textile wastewater by poly(propylene imine) dendrimer: operational parameters and isotherm studies. Clean-Soil Air Water. 2011;39:673–9.10.1002/clen.201000182Search in Google Scholar
[28] Mahmoodi NM, Oveisi M, Bakhtiari M, Hayati B, Shekarchi AA, Bagheri A, et al. Environmentally friendly ultrasound-assisted synthesis of magnetic zeolitic imidazolate framework – graphene oxide nanocomposites and pollutant removal from water. J Mol Liq. 2019;282:115–30.10.1016/j.molliq.2019.02.139Search in Google Scholar
[29] Oveisi M, Asli MA, Mahmoodi NM. Carbon nanotube based metal-organic framework nanocomposites: synthesis and their photocatalytic activity for decolorization of colored wastewater. Inorg Chim Acta. 2019;487:169–76.10.1016/j.ica.2018.12.021Search in Google Scholar
[30] Chen F, Yang Q, Li X, Zeng G, Wang D, Niu C, et al. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: an efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl Catal B. 2017;200:330–42.10.1016/j.apcatb.2016.07.021Search in Google Scholar
[31] Yang GH, Zhang DQ, Zhu G, Zhou TR, Song MT, Qu LL, et al. A Sm-MOF/GO nanocomposite membrane for efficient organic dye removal from wastewater. RSC Adv. 2020;10:8540–7.10.1039/D0RA01110JSearch in Google Scholar PubMed PubMed Central
[32] Mahmoodi NM, Taghizadeh A, Taghizadeh M, Azimi M. Surface modified montmorillonite with cationic surfactants: preparation, characterization, and dye adsorption from aqueous solution. J Environ Chem Eng. 2019;7:103243.10.1016/j.jece.2019.103243Search in Google Scholar
[33] Han LJ, Kong YJ, Hou GZ, Chen HC, Zhang XM, Zheng HGA. Europium-based MOF fluorescent probe for efficiently detecting malachite green and uric acid. Inorg Chem. 2020;59:7181–7.10.1021/acs.inorgchem.0c00620Search in Google Scholar PubMed
[34] Yu LL, Li Y, Pan S, Huang W, Liu RJ. Adsorption mechanisms and electrochemical properties of methyl blue onto magnetic Ni(x)Mg(y)Zn((1-x-y))Fe(2)O(4) nanoparticles fabricated via the ethanol-assisted combustion process. Water Air Soil Pollut. 2020;231(6):316.10.1007/s11270-020-04686-9Search in Google Scholar
[35] Almasian A, Olya ME, Mahmoodi NM. Preparation and adsorption behavior of diethylenetriamine/polyacrylonitrile composite nanofibers for a direct dye removal. Fibers Polym. 2015;16:1925–34.10.1007/s12221-015-4624-3Search in Google Scholar
[36] Wu N, Guo H, Xue R, Wang MY, Cao YJ, Wang XQ, et al. A free nitrogen-containing Sm-MOF for selective detection and facile removal of mercury(II). Colloids Surf A-Physicochem Eng Asp. 2021;618:126484.10.1016/j.colsurfa.2021.126484Search in Google Scholar
[37] Liu JQ, Pei L, Xia ZG, Xu Y. Hierarchical accordion-like lanthanide-based metal–organic frameworks: solvent-free syntheses and ratiometric luminescence temperature-sensing properties. Cryst Growth Des. 2019;19:6586–91.10.1021/acs.cgd.9b01014Search in Google Scholar
[38] Wei FH, Zhang H, Ren QH, Chen HL, Yang LL, Ding B, et al. Removal of organic contaminants from wastewater with GO/MOFs composites. PLoS One. 2021;16(7):e0253500.10.1371/journal.pone.0253500Search in Google Scholar PubMed PubMed Central
[39] Wei FH, Ren QH, Zhang H, Yang LL, Chen HL, Liang Z, et al. Removal of tetracycline hydrochloride from wastewater by Zr/Fe-MOFs/GO composites. RSC Adv. 2021;11(17):9977–84.10.1039/D1RA01027ASearch in Google Scholar PubMed PubMed Central
[40] Zhao SQ, Chen D, Wei FH, Chen NN, Liang Z, Luo Y. Removal of CR dye from aqueous solution with nickel-based metal-organic framework/graphene oxide composites prepared by ultrasonic wave-assisted ball milling. Ultrason Sonochem. 2017;38:845–52.10.1016/j.ultsonch.2017.06.013Search in Google Scholar PubMed
[41] Wei FH, Chen D, Liang Z, Zhao SQ. Comparison study on the adsorption capacity of rhodamine B, CR, and orange II on Fe-MOFs. Nanomaterials. 2018;8(4):248.10.3390/nano8040248Search in Google Scholar PubMed PubMed Central
[42] Wei FH, Chen D, Liang Z, Zhao SQ, Luo Y. Preparation of Fe-MOFs by microwave-assisted ball milling for reducing CR(VI) in wastewater. Dalton Trans. 2017;46(47):16525–31.10.1039/C7DT03776GSearch in Google Scholar PubMed
[43] Wei FH, Chen D, Liang Z, Zhao SQ, Luo Y. Synthesis and characterization of metal-organic frameworks fabricated by microwave-assisted ball milling for adsorptive removal of CR from aqueous solutions. RSC Adv. 2017;7(73):46520–28.10.1039/C7RA09243ASearch in Google Scholar
[44] Ren QH, Wei FH, Chen HL, Chen D, Ding B. Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and CR from wastewater. Green Process Synth. 2021;10(1):125–33.10.1515/gps-2021-0020Search in Google Scholar
[45] Wei FH, Ren QH, Liang Z, Chen D. Synthesis of graphene oxide/metal-organic frameworks composite materials for removal of CR from wastewater. ChemSelect. 2019;4(19):5755–62.10.1002/slct.201900363Search in Google Scholar
[46] Ren QH, Nie M, Yang LL, Wei FH, Ding B, Chen HL, et al. Synthesis of MOFs for RhB adsorption from wastewater. Inorganics. 2022;10(3):27.10.3390/inorganics10030027Search in Google Scholar
[47] Farha OK, Hupp JT. Rational design, synthesis, purification, and activation of metal−organic framework materials. Acc Chem Res. 2010;43:1166–75.10.1021/ar1000617Search in Google Scholar PubMed
[48] Yang XC, Wang Z, Jing MX, Liu RJ, Jin LN, Shen XQ. Efficient removal of dyes from aqueous solution by mesoporous nanocomposite Al2O3/Ni0.5Zn0.5Fe2O4 microfibers. Water Air Soil Pollut. 2014;225:1819.10.1007/s11270-013-1819-3Search in Google Scholar
[49] Li Y, Wang TC, Zhang SS, Zhang YL, Yu LL, Liu RJ. Adsorption and electrochemical behavior investigation of methyl blue onto magnetic nickel-magnesium ferrites prepared via the rapid combustion process. J Alloy Compd. 2021;885(34):160969.10.1016/j.jallcom.2021.160969Search in Google Scholar
[50] Khajeh M, Oveisi AR, Barkhordar A, Sorinezami Z. Co-Fe-layered double hydroxide decorated amino-functionalized zirconium terephthalate metal-organic framework for removal of organic dyes from water samples. Spectrochim Acta Part A Mol Biomol Spectrosc. 2020;234:118270.10.1016/j.saa.2020.118270Search in Google Scholar PubMed
[51] Oveisi M, Asli MA, Mahmoodi NM. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J Hazard Mater. 2018;347:123–40.10.1016/j.jhazmat.2017.12.057Search in Google Scholar PubMed
[52] Masoomi MY, Bagheri M, Morsali A. Porosity and dye adsorption enhancement by ultrasonic synthesized Cd(II) based metal-organic framework. Ultrason Sonochem. 2017;37:244–50.10.1016/j.ultsonch.2017.01.018Search in Google Scholar PubMed
[53] Abdollahi N, Masoomi MY, Morsali A, Junk PC, Wang J. Sonochemical synthesis and structural characterization of a new Zn(II) nanoplate metal-organic framework with removal efficiency of Sudan red and Congo red. Ultrason Sonochem. 2018;45:50–6.10.1016/j.ultsonch.2018.03.001Search in Google Scholar PubMed
[54] Han Y, Liu M, Li K, Sun Q, Zhang W, Song C, et al. In situ synthesis of titanium doped hybrid metal-organic framework UiO-66 with enhanced adsorption capacity for organic dyes. Inorg Chem Front. 2017;4:1870–80.10.1039/C7QI00437KSearch in Google Scholar
[55] Wu Z, Yuan X, Zhong H, Wang H, Jiang L, Zeng G, et al. Highly efficient adsorption of Congo red in single and binary water with cationic dyes by reduced graphene oxide decorated NH2-MIL-68(Al). J Mol Liq. 2017;247:215–29.10.1016/j.molliq.2017.09.112Search in Google Scholar
[56] Xu Y, Jin J, Li X, Han Y, Meng H, Wang T, et al. Fabrication of hybrid magnetic HKUST-1 and its highly efficient adsorption performance for Congo red dye. RSC Adv. 2015;5:19199–202.10.1039/C5RA00384ASearch in Google Scholar
© 2022 Fuhua Wei et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

