Abstract
The hydrolysis of phosphinic esters is an important transformation that may be performed under acidic or basic conditions on conventional heating. A series of cyclic phosphinates, 1-alkoxy-3-methyl or 3,4-dimethylphospholane oxides, has now been hydrolyzed under microwave (MW) conditions in the presence of 0.1 or 0.5 equivalents of p-toluenesulfonic acid that served not only as the catalyst but also as a MW absorber. The later phenomenon was proved separately. The pseudo-first-order rate constants for the hydrolyses performed by the new approach were determined and a reactivity order was setup. The model reactions investigated were transplanted into flow MW accomplishment.
Graphical abstract

1 Introduction
Change of the P-function, such as esterification of P-acids, is an often-emerging task during organic syntheses [1]. Hydrolysis of P-esters, as the opposite direction, is also a typical transformation [2,3] performed, in most cases, under acidic conditions [4,5,6,7,8,9] and in other cases applying base catalysts [10,11,12,13]. Among the acid catalyst, hydrochloric acid is the most often applied agent [4,5,6,7]. However, in most cases, the hydrolyses remained unoptimized, applying the acid or base in excessive quantities and allowing too long reaction times. We rationalized and optimized the HCl-catalyzed hydrolysis of cyclic and acyclic phosphinates [14,15], as well as phosphonates [16,17] in term of catalyst quantity and reaction time. The two-step transformation of phosphonates deserved special attention.
There are only a few cases when microwave (MW) irradiation was utilized in hydrolyses. Czech authors elaborated the MW-promoted HCl-catalyzed hydrolysis of acyclic nucleoside phosphonate diesters at 130–140°C [18]. During our studies on acidic hydrolyses, we described the MW-assisted hydrolysis of 1-methoxy and 1-etoxy-3-methyl-3-phospholene oxide [14] and that of alkyl diphenylphosphinates [15]. In both cases, p-toluenesulfonic acid (PTSA) was the catalyst to avoid corrosion problems of the MW reactor caused by HCl. Now we wished to revisit this problem for two reasons: (1) to study the MW absorbing effect of the PTSA additive as a dipolar agent (in the sense that it is an acid that can be dissociated [deprotonated] or protonated) and (2) to elaborate a MW-assisted flow chemical variation. Our earlier experience showed that dipolar additives, such as quaternary ammonium and phosphonium salts, may promote MW reactions [19]. Alloying the two aims means a novel kind of accomplishment for MW-enhanced hydrolysis. To realize our proposes, we wished to study the acidic hydrolysis of a series of 1-alkoxyphospholane oxides as a new model.
2 Materials and methods
2.1 General
The 31P NMR spectra were taken on a Bruker DRX-500 spectrometer (Bruker Corporation, Billerica, Massachusetts, USA) operating at 202.4, 125.7, and 500 MHz, respectively. LC–MS measurements were performed with an Agilent 1200 liquid chromatography system coupled with a 6130 quadrupole mass spectrometer equipped with an ESI ion source (Agilent Technologies, Palo Alto, CA, USA).
The starting phosphinates (1a, 1b, and 3) were prepared as described earlier [20]. PTSA (≥99%) was purchased from Sigma Aldrich.
2.2 General procedure for the acidic hydrolysis of 1-alkoxy-3-methylphospholane oxide (1a/b) and 1-methoxy-3,4-dimethylphospholane oxide (3) under MW conditions
A mixture of 0.68 mmol of phosphinates (1a: 0.10 g, 1b: 0.11 g, and 3: 0.11 g), 0.06 g or 0.01 g (0.35 or 0.07 mmol) of PTSA, and 1.0 mL of water was irradiated in a sealed tube placed in CEM MW reactor at 160–180°C (100 W) for 0.5–5 h. After evaporating the water, the residue so obtained was taken up in 10 mL of dichloromethane, and then washed 3× with 3 mL of water, and dried (Na2SO4). The product was analyzed by 31P NMR spectroscopy.
2.3 General procedure for the continuous flow hydrolysis of 1-alkoxy-3-methylphospholane oxide (1a/1b) and 1-methoxy-3,4-dimethylphospholane oxide (3)
A mixture of 21.0 mmol of the starting phosphinate (1a: 3.0 g, 1b: 3.3 g, and 3: 3.3 g), 1.80 g of PTSA (10.5 mmol), and 30 mL of water was homogenized by stirring for 5 min at 25°C. The reactor was flushed with 20 mL of the mixture with a flow rate of 10 mL·min−1 at 25°C and 17 bar. Then, the flow rate was set to 0.15 mL·min−1, and the system was irradiated at 160°C for 2 h after an instationary stage of 1 h. Excess of the water of the collected fraction was removed under reduced pressure, and the residue so obtained was taken up in 30 mL of dichloromethane and then washed with 2 × 5 mL of water. The product obtained after drying (Na2SO4) and evaporating the solvent was analyzed by 31P NMR spectroscopy.
31P NMR and MS data of the starting materials (1a, 1b, and 3), and products (2 and 4) can be found in Table 1.
Characterization of cyclic phosphinates (1a, 1b, and 3) and phosphinic acids (2, 4)
| Compounds | δ 31P NMR | [M + H]+* | |
|---|---|---|---|
| Found | Literature | ||
| 1a | 81.3 (broad) | 81.1 [20] | 149 |
| 1b | 79.0 (broad) | 79.4 [20] | 163 |
| 3 | Isomers: 74.9 | Isomers: 74.7 | 163 |
| 80.9 | 80.6 | ||
| 81.5 | 81.3 [20] | ||
| 2 | 80.1 (broad) | 80.2 [21] | 135 |
| 4 | Isomers: 73.3, | Isomers: 72.9, | 149 |
| 80.0 | 79.5 [21] | ||
*Obtained by LC-MS.
2.4 Use of the 31P NMR spectra in quantitative analysis
Composition of the reaction mixture was determined by the integration of the areas under the corresponding peaks of the starting material and product in the 31P NMR spectra.
2.5 Curve fitting on the time – relative quantity data pairs
The acidic hydrolyses were modeled assuming pseudo-first-order kinetics. The concentration of water and PTSA was constant during the reaction. The calculated time – composition curves were fitted on the experimental data using nonlinear least-squares method. The pseudo-first-order rate constants were optimized that the sum of squares of the residuals (i.e., the difference of the experimental and the calculated composition) to be the minimal. The approximate values of the rate constants were found iteratively, using the nonlinear generalized reduced gradient method of Microsoft Excel Solver.
3 Results and discussion
Before the synthetic work, we studied how water absorbs heat in the absence and presence of PTSA. Figure 1 shows how different amounts absorb MW: (a) 0 mg of PTSA in 3 mL of water, (b) 18 mg of PTSA in 3 mL of water, (c) 36 mg of PTSA in 3 mL of water, (d) 180 mg of PTSA in 3 mL of water, and (e) 360 mg of PTSA in 3 mL of water. Applying an irradiation of 20 W, depending on the quantity of PTSA, the temperature increased from 122°C to 152–180°C. The additive had a significant effect on the warming of the solution via its MW absorbing ability. However, it was surprising that the increase in the temperature was the highest in the presence of the less amount (18 mg per 3 mL) of PTSA. With the increasing amount of the PTSA additive [b) → c) → d) → e)] the warming somewhat decreased. It means that the concentration has an optimum regarding the maximum heat absorbing ability that is in our case is 6 mg·mL−1 or 0.034 mmol·mL−1. The small, but significant difference between cases “b” and “c” was confirmed by parallel measurements.

The effect of PTSA additive on MW heating in water solutions.
In our case, PTSA/H2O mixtures of c = 0.07 and 0.35 mmol·mL−1 corresponding to 0.1 and 0.5 equivalents, respectively, were applied in the MW-assisted hydrolyses in a volume of 1 mL for 0.68 mmol of the phosphinate.
The first model reaction was the hydrolysis of 1-methoxy-3-methylphospholane oxide (1a). The MW-assisted hydrolysis was carried out at 160°C in the presence of 0.5 equivalents of PTSA. Monitoring the transformation by 31P NMR spectroscopy showed that the conversion was 93% after 3 h (Table 2, entries 1–5). Completion takes 3 h 15 min. Concentration profile of the components may be seen in Figure 2. The pseudo-first-order rate constant was 1.57 h–1. In the comparative thermal experiment carried out at 160°C per 3 h, the conversion was only 28% (Table 2, entry 6). The hydrolysis was much faster at 180°C applying 0.5 equivalents of PTSA: complete conversion was attained after 20 min (Table 2, entry 7). In the comparative thermal experiment, the conversion was only 25% (Table 2, entry 8). Comparing the results of the MW-assisted and conventionally heated hydrolyses, one may conclude the significant difference. To be more eco-friendly, the hydrolysis of phosphinate 1a was also performed using only 0.1 equivalents of the catalyst and MW absorber at 180°C. In this case, again ca 3 h 10 min was the reaction time (Table 2, entries 9–13 and Figure 3) confirmed by a k value of 1.42 h–1 similar to the previous one.
Acidic hydrolysis of 1-methoxy-3-methylphospholane oxide (1a) under MW conditions

|
|||||
|---|---|---|---|---|---|
| Entry | Temperature (°C) | Heating | Time (h) | PTSA (equiv.) | Conversion (%) |
| 1 | 160 | MW | 0.25 | 0.5 | 28 |
| 2 | 160 | MW | 0.5 | 0.5 | 55 |
| 3 | 160 | MW | 1 | 0.5 | 85 |
| 4 | 160 | MW | 2 | 0.5 | 90 |
| 5 | 160 | MW | 3 | 0.5 | 93 |
| 6 | 160 | Δ | 3 | 0.5 | 28 |
| 7 | 180 | MW | 0.33 | 0.5 | 100* |
| 8 | 180 | Δ | 0.33 | 0.5 | 25 |
| 9 | 180 | MW | 0.25 | 0.1 | 20 |
| 10 | 180 | MW | 0.5 | 0.1 | 59 |
| 11 | 180 | MW | 1 | 0.1 | 78 |
| 12 | 180 | MW | 2 | 0.1 | 88 |
| 13 | 180 | MW | 3 | 0.1 | 95 |
*Isolated yield: 94%.

Concentration profile for the components during the hydrolysis of methoxy-3-methylphospholane (1a) at 160°C using 0.5 equivalents of PTSA. The R 2 measure of goodness of fit is 0.9607.

Concentration profile for the components during the hydrolysis of methoxy-3-methylphospholane (1a) at 180°C using 0.1 equivalents of PTSA. The R 2 measure of goodness of fit is 0.9408.
Then, we studied the PTSA-catalyzed hydrolysis of 1-ethoxy-3-methylphospholane oxide (1b) under similar conditions (Table 3). Using 0.5 equivalents of the catalyst at 160°C, the hydrolysis was complete after 5 h (Table 3, entry 7). The similar reaction at 180°C took place in a reaction time of 1.5 h (Table 3, entry 10). A decrease in the quantity of the additive to 0.1 equivalents had to be compensated by a reaction time of 5 h (Table 3, entry 11). One can see that the hydrolysis of the ethoxyphospholane oxide (1b) was significantly slower than that of the methoxy derivative (1a); otherwise, the tendencies were exactly the same.
Acidic hydrolysis of 1-ethoxy-3-methylphospholane oxide (1b)
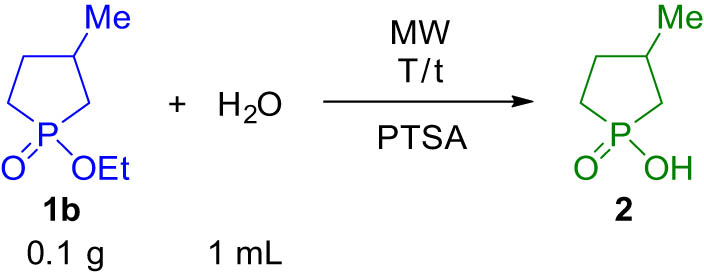
|
|||||
|---|---|---|---|---|---|
| Entry | Temperature (°C) | Heating | Time (h) | PTSA (equiv.) | Conversion (%) |
| 1 | 160 | MW | 0.25 | 0.5 | 14 |
| 2 | 160 | MW | 0.5 | 0.5 | 31 |
| 3 | 160 | MW | 1 | 0.5 | 37 |
| 4 | 160 | MW | 2 | 0.5 | 65 |
| 5 | 160 | MW | 3 | 0.5 | 83 |
| 6 | 160 | MW | 4 | 0.5 | 88 |
| 7 | 160 | MW | 5 | 0.5 | 100* |
| 8 | 160 | Δ | 5 | 0.5 | 30 |
| 9 | 180 | MW | 1 | 0.5 | 93 |
| 10 | 180 | MW | 1.5 | 0.5 | 100 |
| 11 | 180 | MW | 5 | 0.1 | 100 |
*Isolated yield: 96%.
Data of the kinetic study (Table 3, entries 1–7 and Figure 4) suggested a pseudo-first-order rate constant of 0.56 h–1. The low conversion of 30% for the comparative thermal experiment (Table 3, entry 8) is again noteworthy. This may suggest a relatively high enthalpy of activation for the hydrolyses investigated, similarly to the direct esterifications [22].

Concentration profile for the components during the hydrolysis of ethoxy-3-methylphospholane (1b) at 160°C using 0.5 equivalents of PTSA. The R 2 measure of goodness of fit is 0.9655.
The next model for the MW-assisted PTSA-catalyzed hydrolysis was 1-methoxy-3,4-dimethylphospholane oxide (3) comprising three diastereomers. The hydrolyzed product (4) consisted of two diastereomers. The experimental results are listed in Table 4. Completion of the hydrolysis at 160°C in the presence of 0.5 equivalents of catalyst required a somewhat longer reaction time than 4.5 h (Table 4, entry 7). At 180°C, the hydrolysis took place in 1.5 h (Table 4, entry 9). Decreasing the quantity of PTSA to 0.1 equivalents, complete conversion could be attained after 5.5 h (Table 4, entry 11). All results, including the pseudo-first-order rate constant of 0.58 h–1 obtained at 160°C using 0.5 equivalents of the additive (Table 4, entry 1–7 and Figure 5) were rather similar to the data collected with the 1-ethoxy-3-methylphospholane oxide 1b that is not surprising if the substitution patterns are compared.
MW-assisted hydrolysis of the 1-methoxy-3,4-dimethylphospholane oxide

|
|||||
|---|---|---|---|---|---|
| Entry | Temperature (°C) | Time (h) | PTSA (equiv.) | Conversion (%) | Ratio of the diastereomers (%) |
| 1 | 160 | 0.25 | 0.5 | 17 | 59:41 |
| 2 | 160 | 0.5 | 0.5 | 21 | 86:14 |
| 3 | 160 | 1 | 0.5 | 41 | 68:32 |
| 4 | 160 | 2 | 0.5 | 71 | 70:30 |
| 5 | 160 | 3 | 0.5 | 79 | 70:30 |
| 6 | 160 | 4 | 0.5 | 93 | 70:30 |
| 7 | 160 | 4.5 | 0.5 | 96 | 70:30 |
| 8 | 180 | 1 | 0.5 | 88 | 70:30 |
| 9 | 180 | 1.5 | 0.5 | 100* | 67:33 |
| 10 | 180 | 4 | 0.1 | 88 | 70:30 |
| 11 | 180 | 5 | 0.1 | 93 | 70:30 |
*Isolated yield: 91%.

Concentration profile for the components during the hydrolysis of methoxy-3,4-dimethylphospholane (3) at 160°C using 0.5 equivalents of PTSA. The R 2 measure of goodness of fit is 0.9758.
The reactivity order for the hydrolysis of the cyclic phosphinates was the following: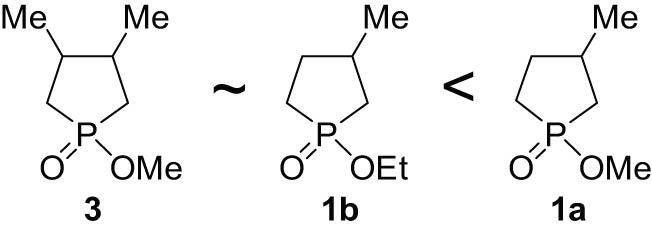
Then, we wished to realize the PTSA-catalyzed hydrolysis of an unsaturated P-cycle, 1-methoxy-3-methyl-3-phospholene oxide (5) under the above-applied conditions. However, it was found that under MW irradiation at 160°C for 1 h, or at 180°C for 1 h, in the presence of 0.5 equivalents of PTSA, decomposition took place, especially at 180°C (Scheme 1), and the expected phosphinic acid 6 formed after isomerization was only a minor by-product, along with 1,3-dihydroxy-4-methylphospholane oxide (7) formed by the addition of one molecule of water on the double bond (Scheme 1). Species 6 and 7 were identified by 31P NMR, LC-MS, and HRMS (6: δP (DMSO) 69.7, δP [14] (CDCl3) 79.7, [M + H]+ = 133, [M + H]+ found = 133.0422, C5H10O2P requires 133.0418; 7: δP (DMSO) 43.3, [M + H]+ = 151, [M + H]+ found = 151.0526, C5H12O3P requires 151.0524). It is noted that according to our earlier examination, the 5 → 6 conversion took place neatly on irradiation at 140°C for 1 h in the presence of three equivalents of PTSA. In this case, phosphinic acid 6 was formed in 88%, along with 12% of the 1-hydroxy-3-methyl-3-phospholene oxide [14].

The inefficient hydrolysis of 1-methoxy-3-methyl-3-phospholene 1-oxide (5).
Only two other examples can be found in the literature for MW-assisted hydrolysis of P-esters. Earlier, we described the MW-promoted hydrolysis of a few phosphinates in the presence of three equivalents of PTSA at a lower temperature of 120–140°C [14]; however, this was not “green” due to the excess amount of the acid applied. However, the HCl-catalyzed hydrolysis of acyclic nucleoside phosphonate diesters was disclosed [18]; however, no data were provided on the possible corrosion problems. The method described in this study may be the superior protocol.
In the final stage of our study, we wished to utilize the results of the batch experiments in developing the MW flow variation. The equipment is shown in Figure 6, whereas the results were summarized in Table 5. In all experiments, a mixture containing 0.5 equivalents of PTSA was fed in the MW reactor at a flow rate of 0.15 mL·min−1 at a temperature of 160°C. Regarding the hydrolysis of the methoxyphospholane oxide 1a, the conversion was 63%. Recycling the mixture collected from the first run, the conversion was complete (Table 5, entry 1). Based on our earlier results, it was obvious that the hydrolysis of the monomethyl P-ethoxy and the dimethyl P-methoxy models (1b and 3, respectively) led to similar results. However, there was a need for three runs due to the somewhat lower reactivity of these phosphinates (1b and 3). In the first round, the conversion was 59/61%, in the second run it was 77/82%, whereas in the third cycle 90/92% was reached (Table 5, entries 2 and 3). The phosphinic acids 2 and 4 were obtained in yields of 82–89%.

The flow MW equipment used for the hydrolysis of cyclic phosphinates (1a, 1b, and 3).
Continuous flow hydrolysis of saturated cyclic phosphinates (1a, 1b, and 3)

|
||||
|---|---|---|---|---|
| Entry | R 1 | R 2 | Conversion (%) | Yield (%) |
| 1 | Me (1a) | H | Round 1: 63 | — |
| Round 2: 100 | 89 (2) | |||
| 2 | Et (1b) | H | Round 1: 59 | — |
| Round 2: 77 | — | |||
| Round 3: 90 | 82 (2) | |||
| 3 | Me (3) | Me | Round 1: 61 | — |
| Round 2: 82 | — | |||
| Round 3: 92 | 86 (4) | |||
4 Conclusion
After proving the MW absorbing effect of PTSA in separate experiments, batch MW-assisted hydrolysis of a series of 1-alkoxyphospholane oxides was elaborated. Hence, PTSA had a double role serving also as the acid catalyst. The hydrolyses of the 1-methoxy- and ethoxy derivatives, as well as the 3-methyl- and 3,4-dimethyl model compounds were characterized by pseudo-first-order rate constants, and a reactivity order was set up. Finally, the hydrolyses were transplanted into a MW-assisted flow system allowing a more productive hydrolysis.
-
Funding information: This project was supported by the National Research, Development and Innovation Office (K134318).
-
Author contributions: György Keglevich: finding out the project, getting funds, supervision, managing the research work, drawing the conclusions, and writing up the manuscript; Nikoletta Harsági: literature survey, carrying out the experimental work, data processing, and writing up the experimental.
-
Conflict of interest: The corresponding author (György Keglevich) is a member of the Editorial Board of Green Processing and Synthesis.
-
Supplementary material: 31P NMR spectra of the best experiments including those of the pure phosphinic acids. Representative LC-MS spectra were also included.
References
[1] Quin LD. A guide to organophosphorus chemistry. New York: Wiley; 2000.Search in Google Scholar
[2] Virieux D, Volle J-N, Bakalara N, Pirat J-L. Synthesis and biological applications of phosphinates and derivatives. In: Montchamp JL, editor. Phosphorus chemistry I. Topics in current chemistry. Vol 360. Cham: Springer; 2014. p. 39–114. 10.1007/128_2014_566.Search in Google Scholar PubMed
[3] Harsági N, Keglevich G. The hydrolysis of phosphinates and phosphonates – a review. Molecules. 2021;26(10):2840. 10.3390/molecules26102840.Search in Google Scholar PubMed PubMed Central
[4] Gavande N, Yamamoto I, Salam NK, Ai TH, Burden PM, Johnston GAR, et al. Novel cyclic phosphinic acids as GABAC receptor antagonists: design, synthesis, and pharmacology. ACS Med Chem Lett. 2011;2(1):11–6. 10.1021/ml1001344.Search in Google Scholar PubMed PubMed Central
[5] Dennis EA, Westheimer FH. The rates of hydrolysis of esters of cyclic phosphinic acids. J Am Chem Soc. 1966;88(14):3431–2. 10.1021/ja00966a045.Search in Google Scholar PubMed
[6] Froestl W, Mickel SJ, von Sprecher G, Diel PJ, Hall RG, Maier L, et al. Phosphinic acid analogues of GABA. 2. Selective, orally active GABAB antagonists. J Med Chem. 1995;38(17):3313–31. 10.1021/jm00017a016.Search in Google Scholar PubMed
[7] Wang Y, Wang Y, Yu J, Miao Z, Chen R. Stereoselective synthesis of α-amino(phenyl)methyl(phenyl)phosphinic acids with O-pivaloylated D-galactoslamine as chiral auxiliary. Chem Eur J. 2009;15(37):9290–3. 10.1002/chem.200901419.Search in Google Scholar PubMed
[8] Reiter LA, Jones BP. Amide-assisted hydrolysis of β-carboxamido-substituted phosphinic acid esters metal ions, and appropriately substituted phosphinic responsible for promoting the cleavage of the phosphinic acid esters. J Org Chem. 1997;62(9):2808–12. 10.1021/jo962275w.Search in Google Scholar PubMed
[9] Bunnett JF, Edwards JO, Wells DV, Brass HJ, Curci R. The hydrolysis of methyl methylarylphosphinates in perchloric acid solution. J Org Chem. 1973;38(15):2703–7. 10.1021/jo00955a028.Search in Google Scholar
[10] Cook RD, Diebert CE, Schwarz W, Turley PC, Haake P. Mechanism of nucleophilic displacement at phosphorus in the alkaline hydrolysis of phosphinate esters. J Am Chem Soc. 1973;95(24):8088–96. 10.1021/ja00805a023.Search in Google Scholar
[11] Clarke FB, Westheimer FH. Substituted 1-oxyphosphole. J Am Chem Soc. 1971;93(18):4541–5. 10.1021/ja00747a034.Search in Google Scholar
[12] Hong HJ, Lee J, Bae AR, Um IH. Kinetics and reaction mechanism for alkaline hydrolysis of Y-substituted-phenyl diphenylphosphinates. Bull Korean Chem Soc. 2013;34(7):2001–5. 10.5012/bkcs.2013.34.7.2001.Search in Google Scholar
[13] Cevasco G, Thea S. The Quest for carbanion-promoted dissociative pathways in the hydrolysis of aryl phosphinates. J Chem Soc Perkin Trans. 1993;2(6):1103–6. 10.1039/P29930001103.Search in Google Scholar
[14] Keglevich G, Rádai Z, Harsági N, Szigetvári Á, Kiss NZ. A study on the acidic hydrolysis of cyclic phosphinates: 1-Alkoxy-3-phospholene 1-oxides, 1-ethoxy-3-methylphospholane 1-oxide, and 1-ethoxy-3-methyl-1,2,3,4,5,6-hexahydrophosphinine 1-oxide. Heteroat Chem. 2017;28(5):e21394. 10.1002/hc.21394.Search in Google Scholar
[15] Harsági N, Szőllősi B, Kiss NZ, Keglevich G. MW irradiation and ionic liquids as green tools in hydrolyses and alcoholyses. Green Process Synth. 2021;10(1):1–10. 10.1515/gps-2021-0001.Search in Google Scholar
[16] Harsági N, Rádai Z, Kiss NZ, Szigetvári Á, Keglevich G. Two step acidic hydrolysis of dialkyl arylphosphonates. Mendeleev Commun. 2020;30(1):38–9. 10.1016/j.mencom.2020.01.012.Search in Google Scholar
[17] Harsági N, Rádai Z, Szigetvári Á, Kóti J, Keglevich G. Optimization and a kinetic study on the acidic hydrolysis of dialkyl α-hydroxybenzylphosphonates. Molecules. 2020;25(17):3793. 10.3390/molecules25173793.Search in Google Scholar PubMed PubMed Central
[18] Jansa P, Baszczyňski O, Procházková E, Dračínský M, Janeba Z. Microwave-assisted hydrolysis of phosphonate diesters: an efficient protocol for the preparation of phosphonic acids. Green Chem. 2012;14(8):2282–8. 10.1039/c2gc35547g.Search in Google Scholar
[19] Hohmann E, Keglevich G, Greiner I. The effect of onium salt additives on the Diels-Alder reactions of a 1-phenyl-1,2-dihydrophosphinine oxide under microwave conditions. Phosphorus Sulfur Silicon. 2007;182(10):2351–7. 10.1080/10426500701441473.Search in Google Scholar
[20] Jablonkai E, Henyecz R, Milen M, Kóti J, Keglevich G. T3P®-assisted esterification and amidation of phosphinic acids. Tetrahedron. 2014;70(44):8280–5. 10.1016/j.tet.2014.09.021.Search in Google Scholar
[21] Keglevich G, Bálint E, Kiss NZ, Jablonkai E, Hegedűs L, Grün A, et al. Microwave-assisted esterification of phosphinic acids. Curr Org Chem. 2011;15(11):1802–10. 10.2174/138527211795656570.Search in Google Scholar
[22] Keglevich G, Kiss NZ, Mucsi Z, Körtvélyesi T. Insights into a surprising reaction: The microwave-assisted direct esterification of phosphinic acids. Org Biomol Chem. 2012;10(10):2011–8. 10.1039/C2OB06972E.Search in Google Scholar
© 2022 Nikoletta Harsági and György Keglevich, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal
Articles in the same Issue
- Research Articles
- Kinetic study on the reaction between Incoloy 825 alloy and low-fluoride slag for electroslag remelting
- Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications – A green approach
- Protective role of foliar application of green-synthesized silver nanoparticles against wheat stripe rust disease caused by Puccinia striiformis
- Effects of nitrogen and phosphorus on Microcystis aeruginosa growth and microcystin production
- Efficient degradation of methyl orange and methylene blue in aqueous solution using a novel Fenton-like catalyst of CuCo-ZIFs
- Synthesis of biological base oils by a green process
- Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature
- Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing
- Regioselectivity in the reaction of 5-amino-3-anilino-1H-pyrazole-4-carbonitrile with cinnamonitriles and enaminones: Synthesis of functionally substituted pyrazolo[1,5-a]pyrimidine derivatives
- A numerical study on the in-nozzle cavitating flow and near-field atomization of cylindrical, V-type, and Y-type intersecting hole nozzles using the LES-VOF method
- Synthesis and characterization of Ce-doped TiO2 nanoparticles and their enhanced anticancer activity in Y79 retinoblastoma cancer cells
- Aspects of the physiochemical properties of SARS-CoV-2 to prevent S-protein receptor binding using Arabic gum
- Sonochemical synthesis of protein microcapsules loaded with traditional Chinese herb extracts
- MW-assisted hydrolysis of phosphinates in the presence of PTSA as the catalyst, and as a MW absorber
- Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification
- Superior photocatalytic degradation performance for gaseous toluene by 3D g-C3N4-reduced graphene oxide gels
- Catalytic performance of Na/Ca-based fluxes for coal char gasification
- Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil
- Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers
- Influence of biosynthesized silver nanoparticles using red alga Corallina elongata on broiler chicks’ performance
- Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract
- Vitamin supplements enhance Spirulina platensis biomass and phytochemical contents
- Malachite green dye removal using ceramsite-supported nanoscale zero-valent iron in a fixed-bed reactor
- Green synthesis of manganese-doped superparamagnetic iron oxide nanoparticles for the effective removal of Pb(ii) from aqueous solutions
- Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis
- Biological fabrication of zinc oxide nanoparticles from Nepeta cataria potentially produces apoptosis through inhibition of proliferative markers in ovarian cancer
- Effect of stabilizers on Mn ZnSe quantum dots synthesized by using green method
- Calcium oxide addition and ultrasonic pretreatment-assisted hydrothermal carbonization of granatum for adsorption of lead
- Fe3O4@SiO2 nanoflakes synthesized using biogenic silica from Salacca zalacca leaf ash and the mechanistic insight into adsorption and photocatalytic wet peroxidation of dye
- Facile route of synthesis of silver nanoparticles templated bacterial cellulose, characterization, and its antibacterial application
- Synergistic in vitro anticancer actions of decorated selenium nanoparticles with fucoidan/Reishi extract against colorectal adenocarcinoma cells
- Preparation of the micro-size flake silver powders by using a micro-jet reactor
- Effect of direct coal liquefaction residue on the properties of fine blue-coke-based activated coke
- Integration of microwave co-torrefaction with helical lift for pellet fuel production
- Cytotoxicity of green-synthesized silver nanoparticles by Adansonia digitata fruit extract against HTC116 and SW480 human colon cancer cell lines
- Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches
- Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells
- Fabrication and characterization of lysine hydrochloride Cu(ii) complexes and their potential for bombing bacterial resistance
- First report of biocellulose production by an indigenous yeast, Pichia kudriavzevii USM-YBP2
- Biosynthesis and characterization of silver nanoparticles prepared using seeds of Sisymbrium irio and evaluation of their antifungal and cytotoxic activities
- Synthesis, characterization, and photocatalysis of a rare-earth cerium/silver/zinc oxide inorganic nanocomposite
- Developing a plastic cycle toward circular economy practice
- Fabrication of CsPb1−xMnxBr3−2xCl2x (x = 0–0.5) quantum dots for near UV photodetector application
- Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells
- Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment
- A low-cost and eco-friendly fabrication of an MCDI-utilized PVA/SSA/GA cation exchange membrane
- Synthesis, microstructure, and phase transition characteristics of Gd/Nd-doped nano VO2 powders
- Biomediated synthesis of ZnO quantum dots decorated attapulgite nanocomposites for improved antibacterial properties
- Preparation of metal–organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater
- A green approach in the biological base oil process
- A cost-effective and eco-friendly biosorption technology for complete removal of nickel ions from an aqueous solution: Optimization of process variables
- Protective role of Spirulina platensis liquid extract against salinity stress effects on Triticum aestivum L.
- Comprehensive physical and chemical characterization highlights the uniqueness of enzymatic gelatin in terms of surface properties
- Effectiveness of different accelerated green synthesis methods in zinc oxide nanoparticles using red pepper extract: Synthesis and characterization
- Blueprinting morpho-anatomical episodes via green silver nanoparticles foliation
- A numerical study on the effects of bowl and nozzle geometry on performances of an engine fueled with diesel or bio-diesel fuels
- Liquid-phase hydrogenation of carbon tetrachloride catalyzed by three-dimensional graphene-supported palladium catalyst
- The catalytic performance of acid-modified Hβ molecular sieves for environmentally friendly acylation of 2-methylnaphthalene
- A study of the precipitation of cerium oxide synthesized from rare earth sources used as the catalyst for biodiesel production
- Larvicidal potential of Cipadessa baccifera leaf extract-synthesized zinc nanoparticles against three major mosquito vectors
- Fabrication of green nanoinsecticides from agri-waste of corn silk and its larvicidal and antibiofilm properties
- Palladium-mediated base-free and solvent-free synthesis of aromatic azo compounds from anilines catalyzed by copper acetate
- Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application
- Co-chlorination of low-density polyethylene in paraffin: An intensified green process alternative to conventional solvent-based chlorination
- Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta
- Recovery of cobalt from spent lithium-ion battery cathode materials by using choline chloride-based deep eutectic solvent
- Synthesis of insoluble sulfur and development of green technology based on Aspen Plus simulation
- Photodegradation of methyl orange under solar irradiation on Fe-doped ZnO nanoparticles synthesized using wild olive leaf extract
- A facile and universal method to purify silica from natural sand
- Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors
- Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity
- Off-gas detection and treatment for green air-plasma process
- Ultrasonic-assisted food grade nanoemulsion preparation from clove bud essential oil and evaluation of its antioxidant and antibacterial activity
- Construction of mercury ion fluorescence system in water samples and art materials and fluorescence detection method for rhodamine B derivatives
- Hydroxyapatite/TPU/PLA nanocomposites: Morphological, dynamic-mechanical, and thermal study
- Potential of anaerobic co-digestion of acidic fruit processing waste and waste-activated sludge for biogas production
- Synthesis and characterization of ZnO–TiO2–chitosan–escin metallic nanocomposites: Evaluation of their antimicrobial and anticancer activities
- Nitrogen removal characteristics of wet–dry alternative constructed wetlands
- Structural properties and reactivity variations of wheat straw char catalysts in volatile reforming
- Microfluidic plasma: Novel process intensification strategy
- Antibacterial and photocatalytic activity of visible-light-induced synthesized gold nanoparticles by using Lantana camara flower extract
- Antimicrobial edible materials via nano-modifications for food safety applications
- Biosynthesis of nano-curcumin/nano-selenium composite and their potentialities as bactericides against fish-borne pathogens
- Exploring the effect of silver nanoparticles on gene expression in colon cancer cell line HCT116
- Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report
- Double [3 + 2] cycloadditions for diastereoselective synthesis of spirooxindole pyrrolizidines
- Green synthesis of silver nanoparticles and their antibacterial activities
- Review Articles
- A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications
- Applications of polyaniline-impregnated silica gel-based nanocomposites in wastewater treatment as an efficient adsorbent of some important organic dyes
- Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review
- Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis
- Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications
- Special Issue: Use of magnetic resonance in profiling bioactive metabolites and its applications (Guest Editors: Plalanoivel Velmurugan et al.)
- Stomach-affecting intestinal parasites as a precursor model of Pheretima posthuma treated with anthelmintic drug from Dodonaea viscosa Linn.
- Anti-asthmatic activity of Saudi herbal composites from plants Bacopa monnieri and Euphorbia hirta on Guinea pigs
- Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach
- Synthetic pathway of 2-fluoro-N,N-diphenylbenzamide with opto-electrical properties: NMR, FT-IR, UV-Vis spectroscopic, and DFT computational studies of the first-order nonlinear optical organic single crystal

