Abstract
Bioactive compounds are highly susceptible to oxidation and degradation, limiting their stability, bioavailability, and effectiveness, particularly in food applications where preservation is critical. Microencapsulation presents a promising strategy to protect these compounds and enhance their functional performance. This review explores key factors influencing microencapsulation efficiency, including extraction methods, encapsulation techniques – such as fluidized-bed spray coating, emulsification, emulsion solidification, liposomal entrapment, coacervation, and ionic gelation – and their effects on capsule structure, controlled release, and bioaccessibility. Findings from in vitro and in vivo studies are synthesized to evaluate the outcomes of microencapsulated compounds. The results show that optimizing the entire microencapsulation process – from extraction and formulation to production techniques – can enhance stability and bioavailability, ultimately supporting the development of functional foods with protective and health-promoting properties. The review highlights microencapsulation as a valuable tool for the food industry, offering broad potential for innovation and application.
1 Introduction
Bioactive constituents, such as vitamins, bioactive lipids (e.g., ω −3 and ω −6 fatty acids), bioactive peptides, essential oils, and probiotics, are widely acknowledged for their potential health benefits. However, these bioactive compounds in their free form are prone to autooxidation and other forms of degradation [1]. The observed susceptibility results in constraints on bioavailability, physical characteristics (such as color), applications in industry, and contributes to instability during the storage of products that incorporate these compounds. Microencapsulation technology demonstrates efficacy in regulating the release characteristics of active compounds, thus improving the bioavailability of administered active components [2]. In addition to microencapsulation, several other innovative systems have been developed for delivering bioactive compounds in food applications. These include gelled systems (such as oleogels, bigels, emulgels, and hydrogels), intelligent carriers, and stimuli-responsive delivery systems. While these alternatives offer promising benefits – such as improved stability, controlled release, and targeted delivery – they also present notable limitations. For instance, the release behavior of gelled systems depends heavily on the type and concentration of gelators, making formulation more complex [3]. Intelligent carriers and stimuli-responsive systems can enhance bioavailability and enable site-specific release by responding to physiological conditions, but they often require sophisticated design and face challenges in maintaining consistency and stability under variable conditions [4,5]. Due to these challenges, microencapsulation remains a more practical and widely adopted strategy in the food and nutraceutical industries, offering a balance of effectiveness, scalability, and formulation flexibility.
In the food industry, microencapsulation involves encapsulating various food materials within microscopic shells or coatings for protection and potential later release. This technology is employed to safeguard ingredients from degradation caused by environmental factors like water, oxygen, heat, and light, ultimately extending the shelf life of active materials [6]. Additionally, this technique enables controlled delivery and release of active food components. Driven by rising health consciousness, aging populations, and advances in food technology, the functional food sector continues to experience robust global growth. Consumers, particularly in developed regions with higher disposable incomes, are increasingly seeking products that offer health benefits beyond basic nutrition [7]. Older adults, in particular, are fueling demand for functional foods aimed at preventing age-related diseases and supporting long-term wellness [8]. Alongside these demographic drivers, economic and technological advancements – such as agricultural diversification and the development of bioactive-rich formulations – are accelerating the innovation and availability of functional products [7,9]. However, to ensure widespread adoption, industry stakeholders must navigate regulatory frameworks and safeguard consumer trust through responsible health claims and transparent labeling [10]. Microencapsulation offers a strategic platform for addressing these demands, positioning it as a key enabler in the evolution of next-generation functional foods.
Microencapsulation is a process that involves enclosing a substance (core or sensitive material) within another substance, known as the encapsulant, coating agent, or wall material. The resulting product is referred to as the encapsulate or microcapsule. The encapsulation efficiency represents the ratio of the core material that is successfully contained within the encapsulate to the initial amount introduced into the encapsulation system. Various factors during the initial stage of microencapsulation can affect the yield of microcapsules. Microcapsules may exhibit different internal or external microstructures, depending on the extraction and microencapsulation methodologies employed. The internal microstructure refers to the composition and organization of components inside the microcapsule, such as the core composition (solid or liquid), core morphology, which may be mononuclear (single core), polynuclear (multiple cores), or even in matrix form where the active ingredient spreads throughout the shell material, as well as the pore structure [11–13]. The external microstructure pertains to the shell or coating that encases the core, such as shell composition, wall thickness, and surface morphology, which includes texture and porosity [12,13]. Both internal and external microstructures play a significant role in determining the encapsulation efficiency, retention of core material, and controlled release behavior. Microencapsulation systems characterized by lower core loads generally demonstrate greater encapsulation efficiency. This is attributed to enhanced wall coverage, improved process yields, stable morphology, optimized diffusion dynamics, and advantageous stability properties [14–16]. Therefore, the microencapsulation structure affects the encapsulation efficiency and the control release of the core material, ultimately impacting its bioavailability.
Recently, there has been an increasing focus on investigating and comprehending the processes through which bioactive substances are metabolized and made available within the human body. The bioavailability, bioaccessibility, and bioactivity of bioactive substances are currently significant topics of investigation owing to the existing uncertainties in this field [17]. Despite the common misconceptions, these phrases convey distinct meanings. The concept of “bioavailability” pertains to the extent to which a substance is accessible and utilized by the organism, encompassing processes such as digestion, absorption, metabolism, distribution within tissues, potential biotransformation, and physiological responses [18]. The challenges associated with impracticality and ethical considerations in research limit the precise evaluation of the bioavailability of particular active compounds in food products. The study of bioaccessibility, which refers to the quantity of active compounds that are liberated from food into the digestive system and may be available for absorption or bioactivity, is currently a significant area of research in contemporary science.
Despite the noted observations, comprehensive information on the factors influencing encapsulation structure and efficiency, and how this structure impacts the bioavailability of the encapsulated compound, is currently scattered, lacking a comprehensive review. The purpose of this review is to offer a thorough summary of the influence of various extraction and encapsulation methods and conditions on the controlled release and bioavailability of encapsulated compounds. Key factors influencing microstructures are highlighted, and potential solutions to achieve desirable microstructures for higher encapsulation efficiency, improved retention, and better-controlled release behavior of encapsulated materials are also discussed.
2 Factors influencing encapsulation structure and efficiency
In the realm of encapsulation technology, there are several key elements that play a crucial role in shaping the structure and efficiency of the encapsulation process. Factors influencing encapsulation structure and efficiency include the choice of encapsulation method or system, the microstructure of the encapsulates, the properties of the encapsulants, the load of core materials, and the solidification conditions. It is essential to recognize that the factors influencing encapsulation efficiency are not limited solely to the details of the encapsulation process itself. Even the specific steps of the initial stages, such as how bioactive compounds are extracted, can significantly impact the overall efficiency of microencapsulation [19]. For instance, residual solvents from extraction processes may interfere with matrix formation by altering interactions between core and wall materials [20]. Additionally, a mismatch in polarity between the extracted bioactive compounds and the encapsulant can hinder the formation of table encapsulation systems, ultimately lowering encapsulation efficiency and stability [20,21]. Core material or bioactive compound characteristics, such as the molecular structure, interaction between natural components and coating material, molecular weight, polarity, and solubility, impact encapsulation efficiency, stability, and release kinetics [16]. Several parameters in forming a microencapsulation system, as illustrated in Figure 1, significantly affect the microencapsulation efficiency.
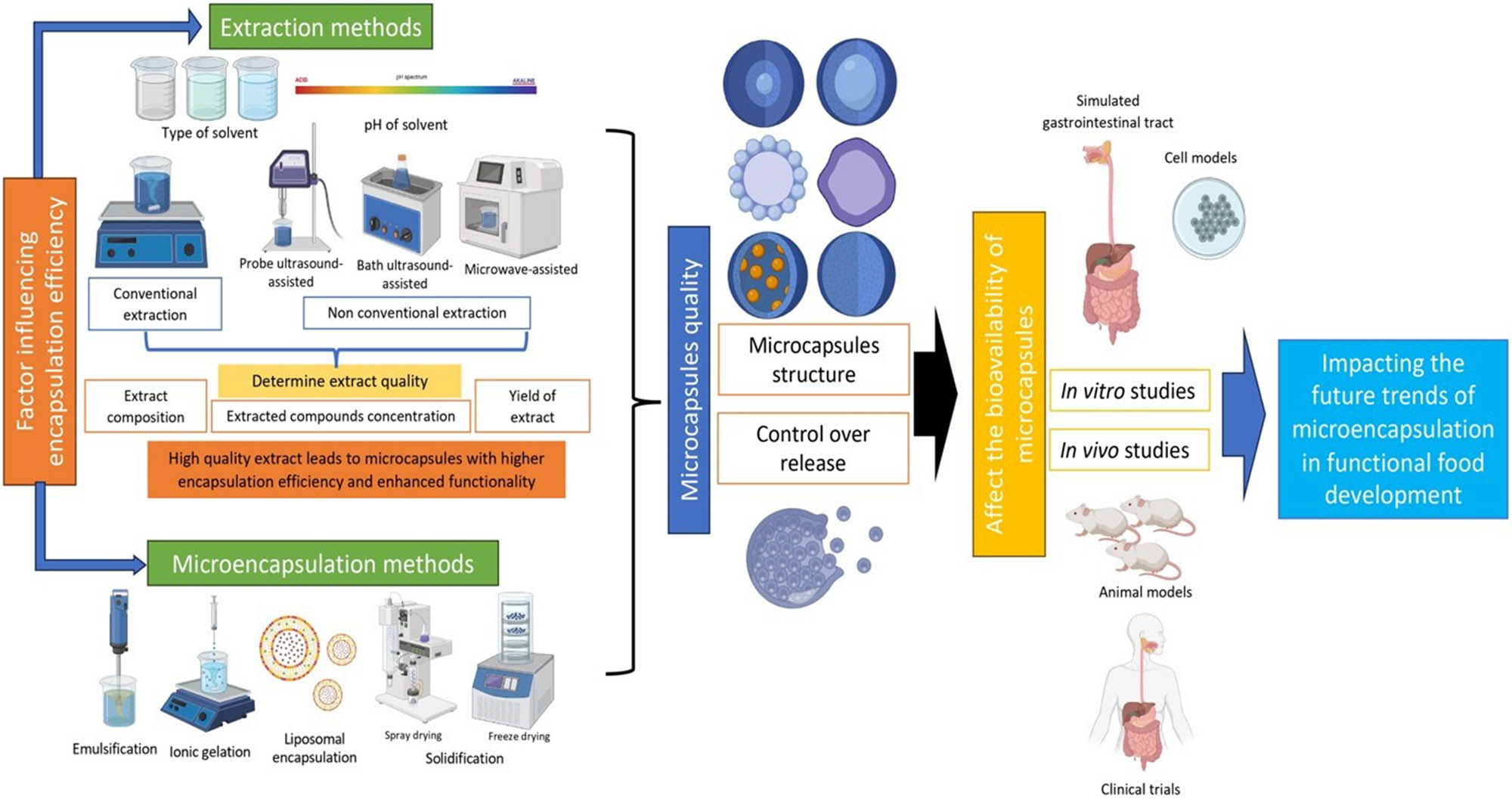
Factors affecting the structure and bioavailability of microcapsules.
2.1 Extraction methods and their impact on microencapsulation
Several parameters within the chosen extraction methodology can significantly influence the microencapsulation efficiency. These parameters encompass the classification of the extraction method, whether it is conventional or unconventional, the selection of the solvent, as well as the precise application of time and temperature conditions. The precise application of time and temperature conditions during extraction is critical to optimizing yield and preserving the bioactivity of compounds. Optimal temperature ranges vary depending on the source material: for example, garlic husk (Allium sativum) extraction is most effective between 60 and 70°C [22], and carrot (Daucus carota) compounds are efficiently extracted within 30–70°C [23]. Chamaenerion angustifolium extracts best at 58°C [24], while Levisticum officinale shows maximum extraction efficiency between 75 and 95°C [25]. Fucus vesiculosus significant yields are observed at temperatures of 75°C and above, with peak yields around 120°C [26].
Extraction time also plays a vital role, with optimal durations differing across materials. Chamaenerion angustifolium extraction times range from 20 to 60 min, peaking at around 35 min [24]. Levisticum officinale requires 20–40 min, optimally near 35.7 min [25], whereas garlic husk extractions are best sustained for 60–90 min [22]. Carrot extraction time appears less critical but has been tested between 17 and 57 min [23]. For Fucus vesiculosus, longer durations of 1–4 h, especially at higher temperatures, yield the best results [26].
Different extraction methods, such as microwave extraction (ME), ultrasound extraction (UE), and solvent ethanol–water extraction (SEWE), have been shown to have varying extraction efficiencies and yield different bioactive compounds [27]. The quality of bioactive extracts obtained through various extraction methods plays a crucial role in determining the efficiency and stability of the subsequent microencapsulation process. Parameters such as the solvent type, extraction temperature, and duration influence not only the yield but also the composition of the extract, particularly the concentration and types of bioactive compounds present. Extracts with higher purity and concentration of desired compounds tend to produce microcapsules with improved encapsulation efficiency, better retention, and enhanced functional performance [27]. For instance, overly high extraction temperatures may degrade sensitive compounds, reducing the effectiveness of encapsulation. Conversely, optimized extraction conditions can preserve compound integrity and improve interaction with encapsulating materials, resulting in microcapsules with favorable morphology, release characteristics, and bioavailability. Previous studies reported how the extraction methods, as one of the stages of microencapsulation preparation, influence the final microcapsules' properties physicochemically and biologically, as presented in Table 1.
A combination of the extraction methods and extract encapsulation
| Sample | Extraction | Optimal conditions of microencapsulation | Encapsulation efficiency (%) | Particle size (μm/nm) | Findings | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Optimal method | Extracted compounds | Coating material (s) | Method | |||||
| Prickly pear | Sample was extracted by aqueous glycerol (23.15%). Sample to solvent ratio 1:10 at 31.15°C for 10.43 min | Betalain (betaxanthin and betacyanin) | Glycerol 23.15%, core material concentration was 10% | UAE combined with glycerol as the encapsulating agent | 93.76% | Not available | UAE followed by encapsulation using glycerol as a green agent was highlighted as an effective method. The optimization achieved high encapsulation efficiency and high betalain content | [28] |
| Grape pomace | Two steps extraction with gliadin-rich hydroalcoholic solution | Phenolics | Wheat gliadin as the core material and Arabic gum ratio 1:1 | Solvent evaporation | 62.9% in terms of epicatechin | 506.6 nm | Using gliadin-rich hydroalcoholic solution to extract polyphenol from gape pomace significantly enhanced the yield and composition of extract as well as improved the encapsulating ability | [29] |
| Pomegranate peel | SEWE, pomegranate peel was combined with acidified ethanol (0.01% citric acid) and a mixture of acidified ethanol and water (1:1) for 5 min with electric mixer soaked at a maximum speed | Phenolics and anthocyanins | Mixture of 0.5 g of indigeneous gum and 4.5 g of 15% maltodextrin in water. Anthocyanin extract and coating materials ratio was 1:2 (w/w) | Freeze drying | 86.57% | 112 nm | The average size distribution of nanoencapsulated particles and the effectiveness of nanoencapsulation are significantly influenced by the type and quantity of extractive chemicals present in the extract. Because of this, PPP extract has a high concentration of antioxidant chemicals, which can be found out by closely examining the optimal extraction | [27] |
| Cocoa shell | UAE at 55°C for 45 min with 60% ethanol | Phenolics and flavonoids | Ratio between the extract and mixture of Arabic gum and maltodextrin (40% w/v) was 2:1 (w/w) | Spray drying | 59.92% | 34.4 μm | The optimized extraction of bioactive compounds from cocoa shell using UAE resulted in microcapsules that exhibited antioxidant and antibacterial activities | [30] |
| Date pit | Conventional extraction with water–ethanol solvent (25%:75%) at 25° stirred for 5 h in an incubator with shaker at 280 rpm | Phenolics | Three-layercoating consists of 20% (w/v) of maltodextrin, 15% (w/w) medium-chain triglycerides (MCT oil), and 20% (w/v) Alhagi maurorum gum | Fluidized-bed dryer | Specific numerical values are not available | Specific numerical values are not available | The combination of ethanol and water demonstrated a superior capacity for extracting phenolic compounds compared to individual solvents, which may affect the microcapsules’ physicochemical properties | [31] |
| Rosemary | Optimized high-voltage electrical discharge (HVED parameters were of frequency of 100 Hz, high- voltage current of 30 mA, pulse width of 0.4 μs, and voltage of 25 kV using nitrogen as a reaction gas, with the gap between electrodes of 15 mm, 9 min, and ratio mass to solvent of 1:50 (w/v) | Phenolics and flavonoids | All coating materials were dissolved in the rosemary extract, 1.0% sodium alginate + 0.2% zein + 0.3% hydroxypropyl methylcellulose (HPMC) and CaCl2 | Ionic gelation | 120.59% | 651.29 to 1,087.37 μm | Rosemary extract obtained through HVED has demonstrated significant potential as a source of bioactive compounds, particularly polyphenols, which facilitated the successful microencapsulation of the aqueous extract. This microencapsulation was achieved using the ionic gelation method, employing calcium alginate, zein, and HPMC as coating materials | [32] |
| Lemon | High-hydrostaticpressure and UAE with 80% methanol | Phenolics and flavonoids | The ratio between lemon extract and 30% maltodextrin solution (w/v) was 1:2 (w/w) | Freeze drying | 93.2% | 48.9 μm | High-hydrostatic-pressure and UAE affected the relative bio-accessibility and total phenolic content as well as total flavonoid content in microencapsulated lemon extract, which were higher than those obtained by the conventional methods. Additionally, the choice of solvent in the extraction process can impact the solubility and stability of the encapsulated material, which can, in turn, affect its release properties and bioavailability | [19] |
| Herbal plant leaves (Cannabis sativa L., Cannabis indica L., and Mitragyna speiosa K.) | Microwave extraction with deionized water at 100 W for 35 min (final temperature ≤ 60°C) | Phenolic and flavonoid | 2% chitosan (w/v) was dissolved in 1% (v/v) acetic acid combined with surfactant (2% (v/v) Tween 80). Wall material and extract ratio was 1:1 (v/v) | Spray drying | Cannabis sativa L. was 54.6%, Cannabis indica L. was 84.3%, and Mitragyna speiosa K. was 99.7% | 1.45 to 11 μm | The application of microwave treatment prior to chitosan encapsulation of leaf extracts led to an increased recovery of bioactive compounds within the encapsulation process | [33] |
| Blueberry pomace | Conventional extraction with acidic water (pH 2.0) at 60°C for 100 min, and the liquid to solid ratio was 10 mL/g | Anthocyanins | Volume to weight ratios between the extract and maltodextrin was 50:60 (mL/g) | Spray drying | 98.98% | 5.5 μm | The choice of the extraction method can impact the solubility of the microencapsulated compounds. The water extracts showed a high encapsulation yield and encapsulation efficiency | [34] |
| Red cabbage | Conventional extraction with acidic 70% ethanol (pH 3.3–3.5) in continuous agitation for 4 h, and the liquid to solid ratio was 1:1 | Polyphenols | CAPSUL modified starch at a final concentration of 15% (w/v) | Spray drying | 79% | 1 – 30 μm | Different solvents used for extraction affected the stability and color properties of polyphenols in red cabbage extract and microencapsulated extract. The ethanolic extract showed the highest thermal stability compared to methanolic and water extract | [35] |
The papers collectively suggest that the extraction methods used for microencapsulated bioactive compound extracts may exhibit a significant impact on the properties and bioaccessibility of the extracts. Giovagnoli-Vicuña et al. [19] found that high hydrostatic pressure and ultrasound-assisted extraction (UAE) methods resulted in lemon extracts with higher levels of bioactive compounds and improved bioaccessibility and total phenolic content as well as total flavonoid content in microencapsulated lemon extract, which were higher than those found using the conventional method. A previous study discussed the advantages of emergent non-thermal extraction technologies, such as high hydrostatic pressure, ultrasounds, pulsed electric fields, and supercritical fluids, in enhancing extraction yields and preserving the structure of bioactive compounds [36]. The choice of solvent in the extraction process can impact the solubility and stability of the encapsulated material, which can, in turn, affect its release. Moreover, the extraction method also affects the physical properties of microcapsules such as the particle size [19].
For example, Jafari et al. [30] optimized the extraction of bioactive compounds from cocoa shell using UAE and found that the resulting microcapsules exhibited antioxidant and antibacterial activities. Similarly, Zahed et al. [27] extracted pomegranate residues, which are peels and seeds, with supercritical CO2 extraction and microencapsulated the extract and reported that the extraction process influences the encapsulation efficiency by affecting the concentration and composition of the core material to be encapsulated. In summary, the extraction methods employed for microencapsulated bioactive compound extracts can influence the composition, bioaccessibility, and functional properties of the microcapsules.
2.1.1 Influence of solvent polarity
The varying polarities of bioactive compounds within intricate food matrices may hinder their concurrent extraction. The choice of solvent affected the morphology of the microparticles and microcapsules [37]. Mehta et al. [38] found that drug incorporation, matrix porosity, and solvent residues are some of the microparticle features that are impacted by the polymers’ solubilities in organic solvents, which in turn influence the pace of polymer solidification during microparticle formation (Figure 2). The influence of solvent polarity on the structure of microcapsules has been investigated in several studies. A study reported that the polarity of solvent can significantly impact the crystallinity and physicochemical properties of microcapsules. For instance, reducing solvent polarity may enhance the crystallinity of specific components within the microcapsules, potentially influencing their digestibility and functionality in various applications [39]. Moreover, in the encapsulation of gallic acid using yeast cells revealed a notable influence of the solvent type in the encapsulation media (H2O or EtOH:H2O) on both the encapsulation efficiency and bioactive properties of the resulting microcapsules. At higher concentrations, the effectiveness of yeast cell encapsulation was greatly improved by the solvent choice. The gallic acid loading and bioactive performance of distilled water were better than those of the ethanol–water mixture (EtOH:H2O) [40]. Similarly, in the preparation of bovine hemoglobin-loaded nanoparticles, an aqueous system with surfactants achieved encapsulation efficiencies exceeding 97%, demonstrating the suitability of aqueous media for hydrophilic compounds [41]. A separate investigation examined alginate microcapsules for yeast in sparkling winemaking, utilizing an aqueous system with calcium chloride as the crosslinker, resulting in a high encapsulation efficiency (∼97%) and excellent structural stability, thereby illustrating the efficacy of aqueous alginate systems for hydrophilic or biologically active agents [42]. In contrast, alcohol-based solvents exhibited improvement in solubility and encapsulation of hydrophobic compounds, such as risperidone, and allowed greater control over the particle size and porosity. Due to their greater solubility in alcohol-based solvents, the encapsulation efficiency increased from 62 to 78% or 69 to 80% [43]. A study on propolis-alginate microcapsules indicated low encapsulation efficiency when sodium alginate was dissolved in water; however, altering the solvent solution to incorporate alcohol markedly enhanced encapsulation efficiency to ∼99% [44]. This indicated that alcohol-based solvents may enhance the incorporation of hydrophobic substances such as propolis in alginate microcapsules, possibly owing to greater solubility and interaction with the active chemicals in comparison to only aqueous systems [44]. These findings suggest that solvent polarity plays a significant role in determining the structure and properties of microcapsules. Quantitative comparisons demonstrate that aqueous solvents are generally more advantageous for hydrophilic and biologically active compounds, particularly when stability and gelation through ionic crosslinking are necessary, whereas alcohol-based systems are more efficacious for hydrophobic bioactives due to enhanced solubility and diminished polarity mismatch.
![Figure 2
Factors influencing encapsulation efficiency (redrawn from Yeo and Park [20]).](/document/doi/10.1515/ntrev-2025-0222/asset/graphic/j_ntrev-2025-0222_fig_002.jpg)
Factors influencing encapsulation efficiency (redrawn from Yeo and Park [20]).
2.1.2 Effect of solvent pH
The pH of the solvent can significantly impact the structure and properties of microcapsules. Besides its influence on the yield of bioactive compounds during extraction, it also impacts the pH in the microencapsulation system. Guo et al. [45] reported how pH levels impact the microstructure and characteristics of self-assembled graphene microcapsules and revealed the presence of core material in the shells, with the pH values significantly affecting the deposition of the core material onto the shells, which leads to changes in the morphology, size, and shell thickness. The surface morphologies and core–shell structures of microcapsules are affected by the pH of the reaction medium. A different study indicated that the characteristics and degree of interactions among microcapsule components, affected by pH, affect the overall stability of the structure [46].
In addition, pH plays a crucial role in determining the charge characteristics of encapsulating polymers such as alginate, chitosan, and proteins, which in turn impact their interactions with bioactive compounds. The changes in pH influence the ionization states of functional groups, such as carboxyl or amino groups, which in turn affect electrostatic interactions and the formation of complexes. For example, alginate shows larger pores and diminished mechanical strength in acidic environments (such as pH 2.5), which may enhance the release rate of encapsulated bioactives. At a neutral pH of approximately 6.0, alginate microspheres exhibit enhanced structural stability and decreased porosity, leading to improved retention of bioactive compounds [47].
Chitosan, as a polycation, possesses a positive charge under acidic conditions, which promotes electrostatic interactions with negatively charged polymers such as alginate, leading to the creation of polyelectrolyte complexes [48]. The encapsulation efficiency of chitosan–alginate systems reaches its peak when chitosan is introduced initially at lower pH levels [49]. In a similar way, wall materials derived from proteins exhibit responses to changes in pH relative to their isoelectric point, influencing their solubility, surface activity, and capacity to interact with active compounds.
Additionally, pH-responsive synthetic polymers, like poly(acrylic acid), demonstrate charge-switching behavior that can be adjusted for controlled encapsulation and release. For instance, carboxyl-containing polymers that exhibit weak ionization may shift from extended to globular conformations in response to pH changes, thereby improving their interaction with membranes and encapsulation capabilities [50–52]. Although research regarding the influence of the pH solvent on the microcapsule structures and properties is still limited, existing studies clearly demonstrate that the solvent pH influences polymer ionization, molecular interactions, and ultimately the structural integrity and release behavior of encapsulated bioactives (Figure 3).
General factors that affect microencapsulation efficiency.
2.2 Microencapsulation techniques
2.2.1 Encapsulation through fluidized-bed spray coating
Fluidization and spray techniques can be combined to agglomerate particles into larger granules or apply coatings to agglomerate particles or apply coatings, a process often referred to as fluid bed coating or granulation (Figure 4). This includes spray drying, cooling, and chilling, where the process air both fluidizes the particles and promotes solidification, either by congealing molten materials (e.g., waxes, hydrogenated oils) or evaporating solvents. In fluidized-bed spray coating, core particles are suspended and sprayed with an encapsulating solution or molten material. The encapsulating material may adhere to the core surface through various mechanisms, which vary based on the material used and specific encapsulation techniques, including physical adsorption such as electrostatic forces and van der Waals forces in cases where the surface is rough or irregular, chemical bonding such as covalent bonds and hydrogen bonding, coating techniques such as coacervation and in situ polymerization, mechanical interlocking, and capillary forces [11,13,53]. Since it is a coating process, the resulting encapsulation typically showcases a core–shell or mononuclear microstructure [54]. In the process of encapsulation using fluidized-bed spray coating, solidification takes place through a complex combination of fluidization, droplet spraying, adhesion, and the swift drying facilitated by circulating hot air. The interplay of these processes results in the formation of uniform microcapsules that exhibit controlled release characteristics, while effectively reducing the risks of agglomeration [55,56].
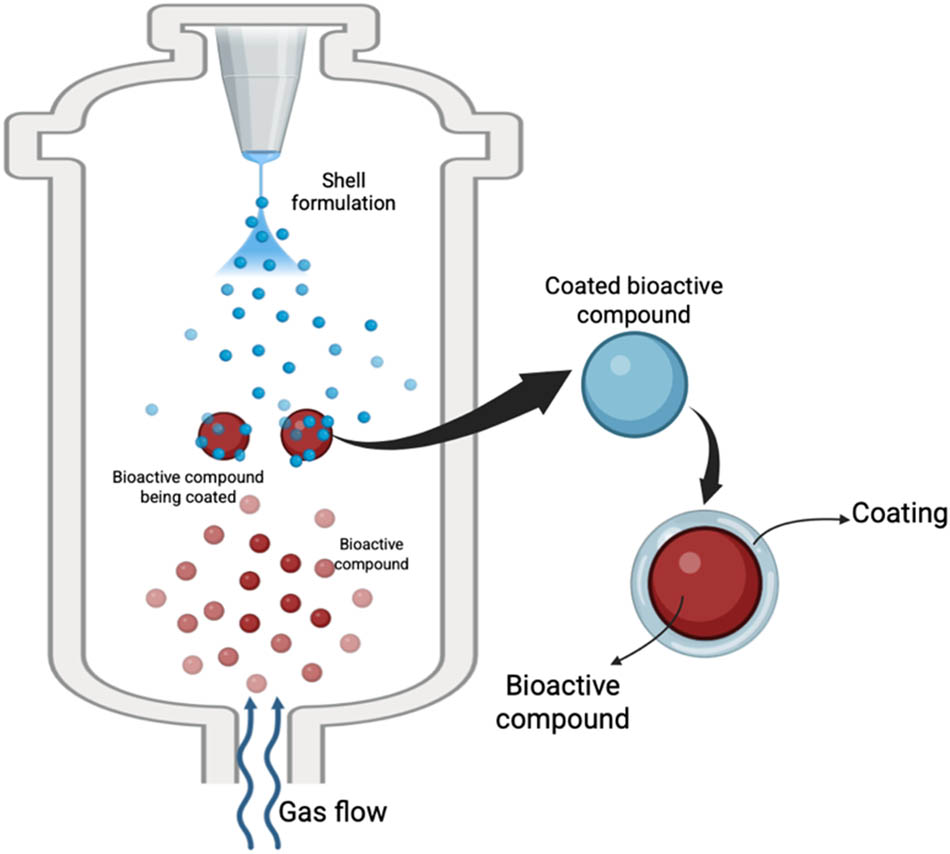
Illustration of the fluidized-bed spray coating process.
The physical properties of microcapsules produced via fluidized-bed spray coating are strongly influenced by process parameters and have a direct impact on the coating performance and final product stability. Particle sizes typically range from 0.1 mm to several millimeters, with specific applications reporting size distributions between 20 and 120 μm, and about 80% of the particles below 100 μm [57,58]. Sphericity is critical for uniform coating – systems like polyamide powders used in fluidized-bed sintering exhibit at least 75% spherical particles, enhancing coating adhesion and flow behavior [59,60]. Density and porosity also vary depending on particle size and coating materials. For example, smaller particles (50–75 μm) generally result in lower porosity, but may compromise the mechanical strength and hardness [61]. These characteristics must be carefully optimized to ensure functional encapsulation tailored to specific applications (Figure 3).
An encapsulant solution is characterized by two critical properties: surface tension and viscosity. The efficiency of encapsulation can be considerably impacted if the viscosity is either too low or too high, resulting in imperfect microstructures such as pores, cracks, or uneven thickness[62]. The atomization of coating solutions may be limited by high viscosity. The production of uniformly coated particles in fluidized-bed systems requires effective atomization of fine droplets. An excessively viscous solution may lead to inadequate dispersion and uneven coating on the substrate, preventing it from forming small droplets [63]. To attain more refined structures, uniform wall thickness, and improved encapsulation efficiency, various studies indicate the utilization of encapsulants characterized by reduced viscosity. In general, encapsulation efficiency for fluidized-bed spray coating ranges from 75 to 95%, depending on the coating thickness and substrate type. When applied to sensitive materials such as biocontrol bacteria (e.g., Collimonas arenae), this method achieved about 6 log[CFU/g coated solids], which is slightly lower than spray drying under similar conditions (7 log[CFU/g solids]) [64]. Compared to other encapsulation methods, spray drying often yields higher encapsulation efficiency for hydrophilic ingredients, typically in the 85–99% range. Extrusion and emulsion techniques using alginate systems show efficiencies of 80–97%, especially for probiotics like Lactiplantibacillus plantarum [65].
To achieve the desired microstructure and increased encapsulation efficiency in spray coating, careful selection of the encapsulant solution's concentration, surface tension, and viscosity, as well as the temperature during the drying process, is essential. Lowering the inlet drying air temperatures below 75°C has been observed to yield high encapsulation efficiencies, reaching up to 99%. It is recommended to use a material with lower viscosity for improved results [63]. The most frequently utilized coating agents for achieving the desired microstructure of capsules include polymer-based components, specifically acrylic polymers, cellulose esters, and copolymers. Enhanced solubility, decreased viscosity and permeability, along with the ability to generate more compact films, represent some of the recognized advantages of these coating agents [66].
In terms of release profiles, fluidized-bed spray coatings offer controlled release times ranging from less than 1 h to more than 22 h, depending on the coating thickness and material properties [67]. Thicker and uniform coatings favor slower, sustained release, whereas thinner or porous coatings may lead to faster “burst” release. In contrast, spray-dried capsules typically have thinner walls and higher porosity, resulting in faster release, often within minutes to a few hours [64]. Extrusion-based alginate beads also allow for prolonged release depending on the crosslinking density and bead size, with smaller beads releasing faster due to higher surface area, and multilayered beads mimicking the slow-release behavior of fluidized-bed capsules [64,68].
It is important to note that adjusting the flow rate of the coating solution, along with factors such as glass transition temperature, atomization pressure, core/encapsulant ratio, and fluidizing air velocity, is crucial for optimal results.
2.2.2 Emulsion technique encapsulation
An emulsion is a mixture of at least two immiscible liquids – liquids that do not dissolve into each other. One of these liquids makes up the dispersed phase, which is distributed as tiny droplets within the other liquid, referred to as the continuous phase (Figure 5). The continuous phase may consist of an organic solvent, oil, a meltable solid, or any substance that is soluble in a solvent. The polymer functioning as the encapsulant is typically dissolved in the solution or within the continuous phase of a suspension or emulsion, such as sorbitans, polysorbates, sucrose esters, sodium caseinate, sodium carboxymethyl cellulose, gelatine, or natural surfactants such as soybean lecithin and saponins [69–71]. After being homogenized, the liquid core forms small droplets in the continuous phase [72].
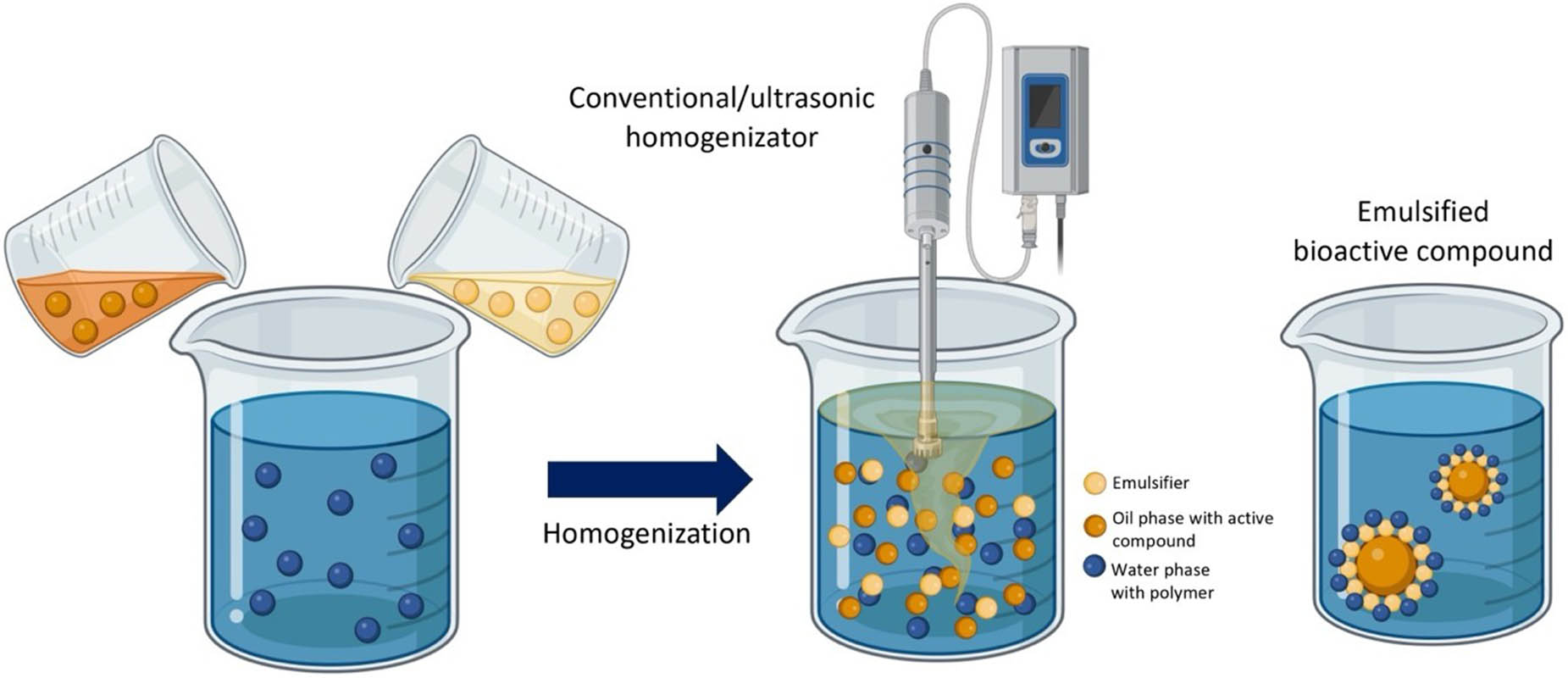
Illustration of the emulsion technique encapsulation.
Commonly, oil and water are used as the two immiscible liquids to create an emulsion. Emulsions are categorized into two primary forms: single emulsions and double emulsions. Single emulsions, particularly oil-in-water (O/W) emulsions, are widely used for oil-soluble core materials [73]. In practical food applications, O/W emulsions are employed to encapsulate flavor oils, enhancing sensory properties and masking undesirable tastes [74]. They are also used to deliver nutraceuticals – such as essential vitamins and minerals – to improve the health value of food products [75], and can contribute to novel textures through gel formation, improving the overall eating experience [76].
Particles produced through emulsion encapsulation techniques exhibit a wide range of sizes and morphologies, depending on the formulation and processing parameters. Colloidosomes, for example, vary from 5 nm to several microns and often show broad size distributions due to the use of polydisperse emulsions [77]. Chitosan-based microcapsules typically display uniform spherical shapes with micron-sized, monomodal distributions, as confirmed by laser diffraction [78]. Polymeric nanoparticles produced for pharmaceutical use are generally smaller than 500 nm, offering advantages for controlled drug release [79]. The mechanical strength, permeability, and encapsulation efficiency of these particles depend heavily on the emulsion type, stabilizers, and polymer matrix, with challenges such as size uniformity and structural integrity during processing remaining critical for optimized performance.
Double emulsions (W1/O/W2), on the other hand, may be employed for specific purposes, referring to emulsions in which oil globules (O) containing small aqueous droplets (W1) are dispersed in a continuous aqueous phase (W2) [70]. In the process of incorporation, cores that are either oil-soluble or water-soluble may first be dissolved in their respective mediums – oil or water – prior to the introduction of an encapsulant or emulsifier, followed by the homogenization of the resultant mixture. Homogenization is essential in the production of emulsions, as it adeptly minimizes droplet size, amplifies surface area, boosts stability, and ensures an even distribution of core materials throughout the solution [80]. In the process of emulsion production, small droplets are effectively coated by emulsifiers, utilizing a blend of electrostatic forces, hydrogen bonding, steric stabilization, and the intricate dance of hydrophobic and hydrophilic interactions. The interplay of these mechanisms promotes a stable emulsion, effectively inhibiting droplet coalescence and ensuring a consistent dispersion throughout the continuous phase [81].
Various types of emulsions, including conventional, layer-by-layer or multilayer, microemulsions, nanoemulsions, emulsion gel, and pickering emulsion, have been developed to enhance their performance [69,82–84]. The process of creating multilayer emulsions typically commences with the creation of a main emulsion with a charged emulsifier. This comprises the homogenization of an oil and aqueous phase, wherein the ionized hydrophilic emulsifier rapidly adsorbs to the surface of the droplets generated during homogenization, yielding small charged droplets [85]. The addition of a biopolymer, electrolyte, or another oppositely charged polymer to the mixture leads to adsorption onto the surfaces of the droplets, which facilitates the formation of a secondary emulsion. This technique can be employed repeatedly to construct multiple layers. The pH of an emulsion requires meticulous regulation, as the charged state of the biopolymer may fluctuate with changes in pH [86].
The zeta potential (ζ-potential) is a critical factor in predicting emulsion stability, as it reflects the net surface charge of dispersed droplets. Stable emulsions are characterized by zeta potential values exceeding 30 mV, irrespective of whether the charge is positive or negative. The stability of these emulsions is primarily maintained through electrostatic repulsions within the colloidal system [87]. While high ζ-potential generally promotes stability, excessively high values can lead to emulsion instability due to over-repulsion, causing droplet breakup or flocculation [88–90]. On the other hand, flocculation can occur when the δ-potential is close to zero since there is not enough repulsive force within the system. Therefore, maintaining a balanced zeta potential is key to ensuring long-term emulsion stability, especially in functional food formulations.
2.2.3 Emulsion solidification process
Various atomization techniques, including spray drying, spray cooling, spray chilling, and spray-freeze drying, are frequently employed to solidify emulsions. The most common technique of this process is spray drying, which employs hot air to facilitate the evaporation of water from atomized droplets [91]. Spray cooling and chilling, on the other hand, use temperatures below the encapsulant material's melting point to solidify atomized droplets. Spray–freeze drying is a method that utilizes the properties of spray drying, which entails the atomization of a liquid to produce smaller particles, alongside freeze-drying, which is particularly beneficial for drying thermally sensitive materials, to generate dry powders with controlled size and improved stability [92].
The physical characteristics of particles produced by solidification of emulsion encapsulation techniques – such as spray drying, spray cooling, spray chilling, and freeze drying – are highly dependent on the processing method and encapsulating materials (Figure 6). Spray-dried powders typically have particle sizes ranging from 20 to 120 μm, with approximately 80% of particles measuring less than 100 μm in diameter [57]. Spray-freeze drying tends to yield spherical particles, although some aggregation may occur depending on the properties of the encapsulation agents [93]. The morphology of spray-dried particles generally falls into three categories: crystalline structures, skin-forming shells, and loose agglomerates [94]. In particular, spray-dried flavor encapsulates are known for their low surface area to volume ratio, resulting in high bulk density and good flowability [95]. Bulk density is significantly influenced by the composition of the encapsulant; for instance, milk fat with higher lipid content tends to produce powders with lower bulk density [57]. Flowability, as indicated by the angle of repose, ranges between 37 and 46° and is closely linked to the particle size and morphology. While spray drying often provides powders with desirable flow and encapsulation characteristics, freeze-drying techniques can be more effective in preserving the solubility and stability of thermally sensitive bioactive compounds, highlighting the need to carefully balance physical attributes with functional requirements [96].
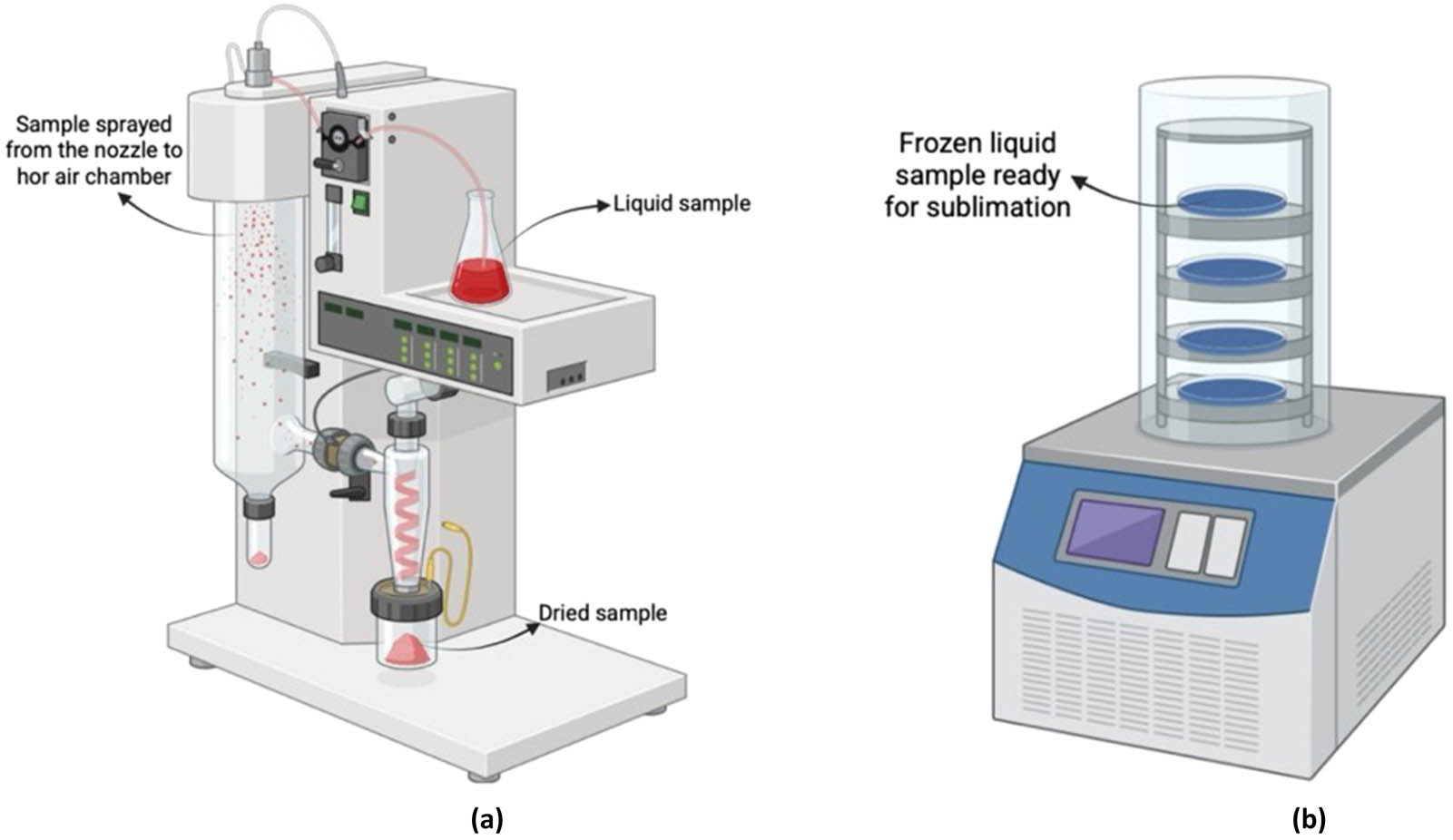
Illustration of the emulsion technique encapsulation. (a) Spray drying illustration and (b) freeze drying illustration.
The microstructure of matrix encapsulates can exhibit considerable variation in characteristics such as surface morphology, which refers to the external structure; it may be rough, porous, hollow, fractured, or shrinking [16]. The shape may be spherical, but it can also exhibit heterogeneity in size and form based on the encapsulants employed [97]. This can be influenced by variables such as the inlet temperature, the encapsulant's properties (such as molecular weight), and the drying medium's characteristics [54]. Encapsulates with a compact microstructure, often referred to as perfect-microstructure encapsulates, are reported to have the highest encapsulation efficiency according to several studies. Conversely, encapsulates with microstructures such as holes, dents, or shrinkage may result in reduced encapsulation efficiency. This is because these features have the ability to push the core material beyond the outer surface [98]. Additionally, hollow encapsulates are prone to breakage during handling, resulting in the loss of volatile core materials. Microcapsules with structural defects such as cracks or blow holes exhibit reduced encapsulation efficiency [99]. This is primarily because these defects create pathways that allow environmental factors, such as moisture and oxygen, to penetrate and interact with the core material. Consequently, the protective function of the microcapsule is compromised, leading to a more rapid release of the encapsulated substance and diminished stability. Consequently, these types of microstructures generally lead to lower encapsulation efficiencies.
Encapsulates with a porous structure are typically achieved through processes such as spray–freeze drying or freeze drying [100]. While spray–freeze or freeze-dried encapsulates exhibit elevated encapsulation efficiencies initially, which is related to reduced drying temperatures [101], the pronounced porosity associated with these structures becomes a drawback when considering the abilities to preserve and promote sustained release of a core material. Increased porosity also implies a larger surface area, making them more susceptible to oxidative degradation for oxygen-sensitive core materials.
Incorporating high-molecular-weight encapsulants such as maltodextrin into an emulsion prior to spray drying is advisable to enhance both the efficiency of the process and the quality of the resultant powder. Low-molecular-weight sugars in food powders can induce stickiness during spray drying, which can be reduced by employing high-molecular-weight encapsulants [102]. Employing suitable coating materials, such as carbohydrates and proteins, is crucial for food-grade applications and preserves the core materials from external influences [103]. To prevent the occurrence of cracks, blow holes, and hollow structures, it is essential to meticulously regulate the temperature of the drying process. Moreover, regulating the exit air temperature can indirectly control the moisture levels, hence affecting the final particle output [104]. The inclusion of substances with a high molecular weight can result in smaller droplet sizes in emulsions [105]. Smaller droplets generally yield reduced pore diameters in the final dried products, as they facilitate a more uniform structure during the drying process. Subsequently, it is advisable for the drying process to be conducted at a temperature lower than the glass transition temperature of the resultant emulsion.
2.2.4 Liposome encapsulation technique
Liposomes are colloidal carriers characterized by their spherical structure composed of lipid bilayers that encapsulate an aqueous core (Figure 7). This structural versatility makes them highly effective in food technology applications, particularly for improving the stability, solubility, and bioavailability of sensitive bioactive compounds [106]. The ability of liposomes to encapsulate both hydrophilic and hydrophobic drugs allows for versatile therapeutic applications, including in pharmaceuticals, cosmetics, and food industries [107]. In the food industry, liposomes are used for nutrient enrichment (e.g., vitamins and antioxidants), controlled delivery of flavors and natural colorants, and protection of volatile or oxidation-prone ingredients. By shielding encapsulated materials from environmental stressors such as heat, oxygen, and light, liposomes help preserve sensory quality and extend shelf life [108,109]. Their biocompatibility and ability to target delivery make them a valuable encapsulation method in functional and fortified food products [110].
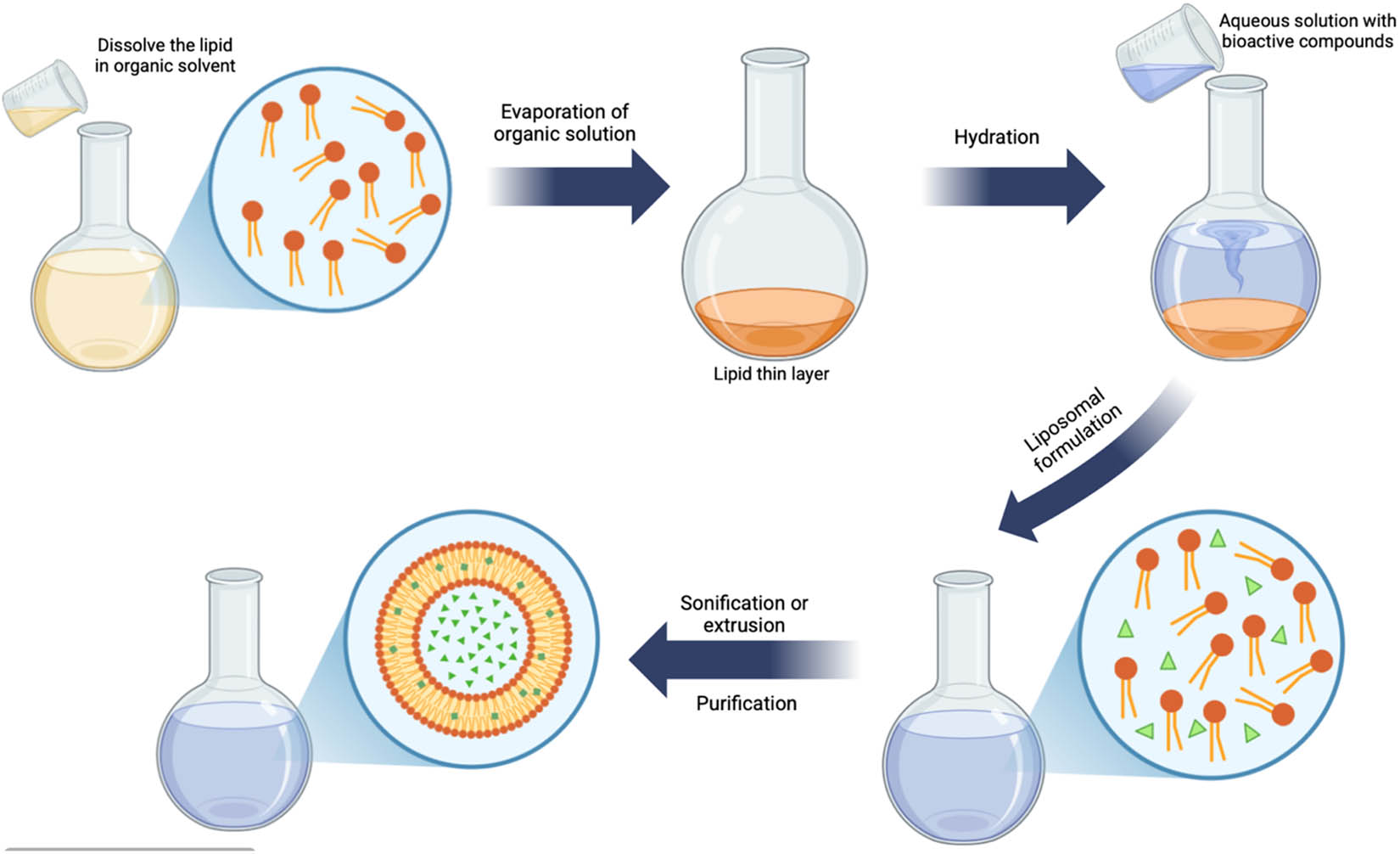
Illustration of the liposome encapsulation technique.
The primary classifications of liposomes structures are small unilamellar vesicles (SUVs), characterized by a single lipid bilayer and a size typically under 100 nm; large unilamellar vesicles (LUVs), which possess a single bilayer exceeding 100 nm; multilamellar vesicles (MLVs), consisting of several lipid bilayers arranged in an onion-like configuration; and multivesicular vesicles (MVVs), which feature a multilamellar structure with concentric phospholipid spheres formed by multiple unilamellar vesicles within larger liposomes [111]. MLVs generally vary in size from 500 nm to 5 μm. MLVs possess an onion-like architecture, consisting of concentric bilayer membranes [112]. MLVs and MVVs demonstrate enhanced stability compared to unilamellar liposomes [113]. MLVs and UVs, despite being similar in size, exhibit distinct physicochemical characteristics such as permeability, stability, elasticity, and toughness, resulting in varied applications [114]. LUV, possessing an extensive hydrophilic zone, adeptly encapsulates a substantial quantity of hydrophilic substances, whereas MLV successfully entraps hydrophobic materials owing to its lipophilic region. Liposomes can concurrently encapsulate both hydrophilic and hydrophobic core substances.
The internal microstructure of liposomes is determined by their composition and can be classified according to their size and the quantity of bilayers present [111], such as phospholipids, cholesterol molecules, position, and vesicle types. Phospholipids act as the primary structural element of liposomes, characterized by a hydrophilic head and a hydrophobic tail, which facilitates the development of an amphiphilic architecture. Cholesterol plays a key role in liposome formation by positioning its hydroxyl group toward the aqueous core and its hydrophobic part in the bilayer's hydrocarbon region. It enhances bilayer rigidity, strengthening its mechanical stability [115].
Liposomes' internal microstructure is predominantly determined by their distinctive composition and organization. The internal microstructures are unique and do not include mononuclear, polynuclear, or matrix components. Liposomes typically demonstrate a significant capacity for encapsulating hydrophobic core materials effectively. The encapsulation efficiency of liposomes for hydrophilic substances improves with liposome size and decreases with the number of bilayers [111]. The application of biopolymers like chitosan, pectin, and alginate to coat liposomes has demonstrated enhancements in their stability and encapsulation efficiency. The application of these coatings serves to protect the liposomes against environmental degradation while minimizing the leakage of active components [116].
Nevertheless, previous studies have suggested that liposomes generally demonstrate a lower encapsulation efficiency for hydrophilic core materials than for hydrophobic ones. Many studies have reported that encapsulation efficiencies of hydrophilic materials, e.g., Gonzalez Gomez et al. [117] have reported that the ability of liposomes to integrate hydrophobic drugs into their lipid bilayer offers them an optimal encapsulant for such drugs. Conversely, hydrophilic drugs are typically less effectively retained due to their propensity to diffuse from the liposomal aqueous core. Substances that are challenging to encapsulate in liposomes include hydrophilic antibiotics such as vancomycin, which has an encapsulation effectiveness of 33.4%. One possible explanation is that the twisted structure of liposomes acts as a barrier for certain water-soluble core components with molecular weights below 500 Da [117]. Liposome encapsulation efficiency varies based on core material properties. While larger molecules like peptides and proteins may encapsulate more effectively, high core loading can compromise liposome stability, leading to leakage. Hydrophobic cores with lower molecular weights achieve high encapsulation efficiency, whereas hydrophilic cores below 500 Da exhibit reduced efficiency.
2.2.5 Encapsulation by coacervation and ionic gelation
Two significant techniques for microencapsulation are coacervation and ionic gelation, commonly employed across multiple domains, including pharmaceuticals, food technology, and cosmetics (Figure 8). Both techniques leverage the physicochemical properties of polymers to encapsulate bioactive compounds, enhancing their stability and bioavailability.
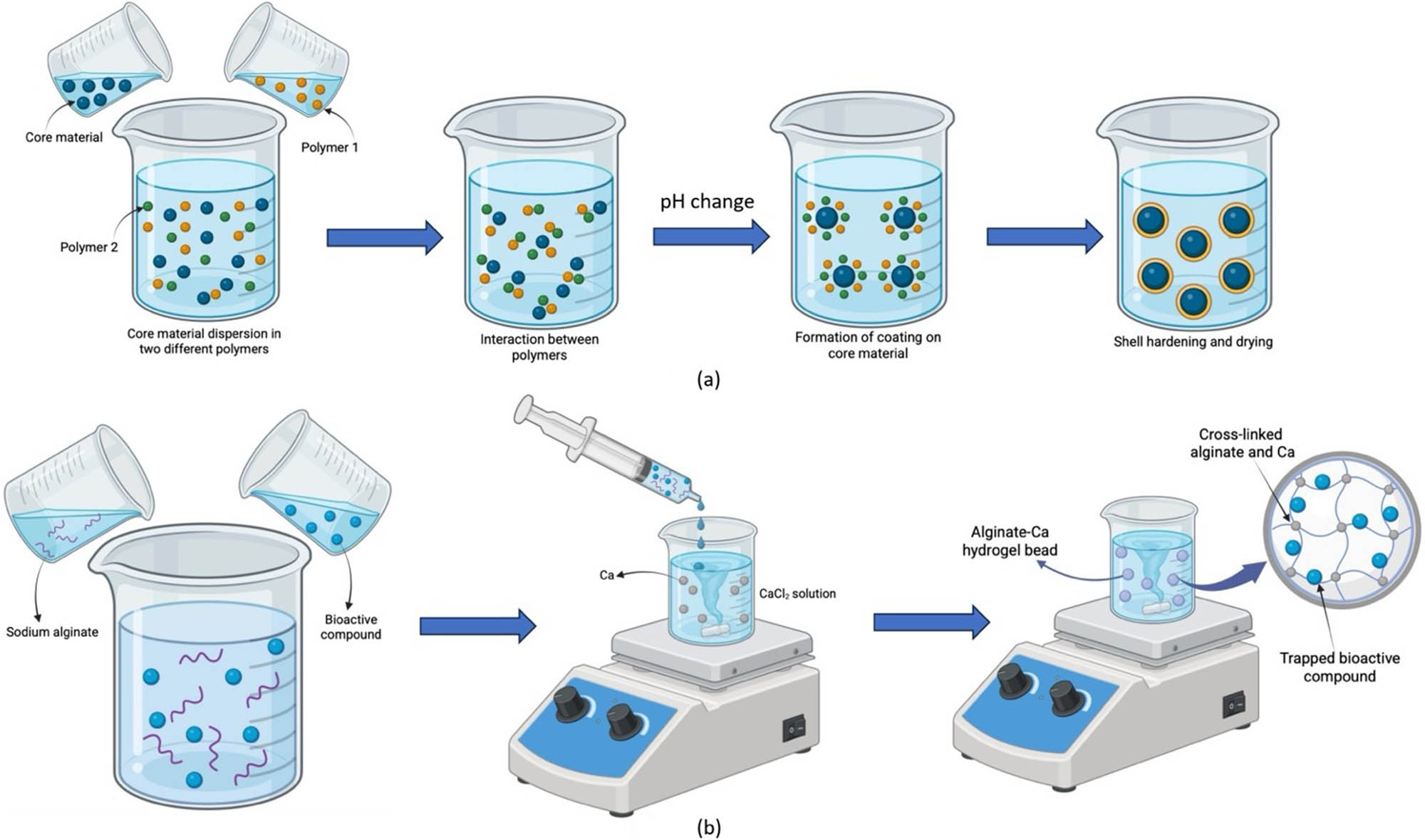
Illustration of coacervation (a) and ionic gelation (b) encapsulation.
Coacervation is a microencapsulation technique that involves the separation of a colloidal solution into two liquid phases: one rich in colloid (coacervate) and the other poor in colloid. This process can be categorized into two types: simple and complex coacervation [118]. In simple coacervation, a single polymer, such as sodium alginate, is dissolved in water, and upon the addition of a core material, droplets form. Introducing these droplets into a gel-forming medium (e.g., calcium chloride) leads to the creation of calcium alginate microcapsules [118,119]. This method is particularly effective for encapsulating lipophobic molecules but has limitations due to its sensitivity to pH and electrolyte concentrations. Complex coacervation involves two or more polymers, such as alginate and gelatine, which are solubilized in water at different pH levels [118,119].
Electrostatic interactions between the polymers promote coacervate formation around the core material. This method provides improved stability for sensitive compounds like anthocyanins but requires careful control of pH and charge conditions. When the ζ-potential between biopolymers approaches zero, charge neutralization facilitates coacervation and the formation of compact microcapsules [120,121]. However, a near-neutral ζ-potential alone does not guarantee full interaction or efficient encapsulation – maintaining electrostatic balance and suitable environmental conditions is essential for stable capsule formation [122].
Ionic gelation is another encapsulation technique that has gained attention for its simplicity and effectiveness. This method relies on the ability of ionic polymers to crosslink with counter ions to form hydrogels. The mechanism of ionic gelation entails the establishment of complexes between anionic salts and charged biopolymers [118]. Ionotropic gelation typically occurs when two molecules possessing opposing charges interact with each other. In a chemical reaction, negatively charged divalent or multivalent ions interact with positively charged polymer chains. The creation of microstructured particles featuring interconnected nanofibrillar networks results from the electrostatic response. This can be achieved through one of three techniques: internal, external, or inverse gelation (Table 2) [123].
Comparison of three ionic gelation techniques
| Gelation type | Mechanism | Pros | Cons | Common applications |
|---|---|---|---|---|
| External gelation | Crosslinking ions (e.g., Ca²⁺) diffuse from outside into polymer droplets (dropwise addition) | Simple to control | Heterogeneous structure | Probiotic alginate beads [127], enzyme delivery [128], antioxidant [129]. |
| Rapid bead formation | Higher porosity at the core | |||
| Internal gelation | Insoluble salts (e.g., CaCO₃) inside polymer matrix dissolve in acid bath, releasing ions gradually | More uniform gelation | Requires optimization of acid–salt ratio | Vitamin delivery [130], antioxidant [129] |
| Improved core retention | Larger pores, lower density | |||
| Inverse gelation | Gelling ions are added to polymer in oil-based emulsion (W/O or O/W) | Soft, thin-shelled capsules | Fragile structure | Antioxidant [131], flavor oil encapsulation [132,133] |
| Minimal polymer required | Less suited for harsh conditions |
The external method is the most prevalent method employed in ionotropic gelation. This technique is also referred to as controlled diffusion [124]. The crosslinking solution is administered incrementally, dropwise, in conjunction with the polysaccharide solution. The formation of the bead matrix occurs as crosslinking agents migrate from the external continuous phase of the polymer into its internal structure. The droplets conducting the sol–gel transition are located within the outermost layer of the produced hydrogel bead. The outer layer of the produced hydrogel bead exhibits a rapid sol–gel transition, resulting in immediate gel formation. Rapidly, counter-ions start forming in the subsequent phases, and the process of gel formation initiates immediately. The subsequent steps entail the penetration of counter-ions into the particle, leading to a heterogeneous gelation profile. This profile indicates that the interaction between the ions and polymer functional groups is maximized at the surface and diminishes to zero at the core [125].
Internal and in situ gelling are interchangeable. An alternative to this procedure is polymer particle preparation. An insoluble calcium salt (CaCO3 or CaSO4) is incorporated into the polymer solution and extruded into an acidic crosslinking bath. Various conditions enhance the solubility of the calcium salt, facilitating its release and the formation of a gel network with the polymer. This regulates the gelling mechanism and uniformly exposes the polymer to cations, resulting in the formation of a gel network. The primary disadvantage of this strategy is significant, which generates matrices with larger pore sizes and reduced densities compared to external gelling, enhancing permeability but diminishing entrapment efficiency and elevating release rates [125,126].
The third method is reverse gelation, which involves the gradual addition of a medium containing gelling agents to the polymer solution. This approach is commonly employed for the production of polymer-based soft-shell microcapsules containing oil-in-emulsions. This technique employs minimal quantities of biopolymer, leading to the formation of a soft molecular shell. The unique characteristics of the produced microcapsules, such as mechanical qualities and pharmaceutical active compounds release, may differ based on the emulsion type employed (water-in-oil or oil-in-water) [123,125].
The outcomes of inverse gelation, internal gelation, and external gelation consistently yield equivalent levels of encapsulation efficiency. The final product's microstructures can exhibit compact, rough, or cracked characteristics, influenced by the concentrations of biopolymers and anionic salts, the degree of cross-linkage, and the specific drying processes and conditions employed. Sufficient concentrations and effective complexation between biopolymers and anionic salts yield compact complexes. Insufficient concentrations, complex formation, or cross-linking may result in diminished, uneven, or porous structures, hence decreasing encapsulation efficiency [54]. Encapsulation efficiency improves with reduced core loads. Consequently, an optimally optimized ionic gelation technique utilizing a reduced core load, adequate charged biopolymers and anionic salts, along with enhanced cross-linking, can attain very high encapsulation efficiencies.
The physical characteristics of particles formed through coacervation and ionic gelation encapsulation techniques are significantly shaped by the composition and process parameters. In ionic gelation, particles such as chitosan-based nanoparticles generally exhibit sizes in the nanometer range, with their dimensions governed by the concentrations of chitosan and crosslinking agents like tripolyphosphate, as well as by the reaction time [134,135]. The morphology is typically spherical, as confirmed by scanning electron microscopy, and remains stable even with the addition of substances like sodium fluoride, unless used at high concentrations [136]. These nanoparticles also exhibit positive zeta potential values that increase with higher chitosan concentrations, contributing to their colloidal stability, which is essential for pharmaceutical and food delivery systems [135]. In contrast, coacervation produces larger particles, generally below 1,000 μm, with morphologies ranging from spherical to elongated ellipsoids. These particles are typically mononuclear in structure and maintain integrity without aggregation, even when encapsulating heat-sensitive compounds [137]. Structural analyses using gas chromatography–mass spectrometry and infrared spectroscopy confirm their stable architecture. While both methods allow for the encapsulation of sensitive bioactive materials, ionic gelation offers enhanced control over particle stability, whereas coacervation is particularly suited for producing larger, structurally robust particles for applications requiring thermal protection.
3 Impact of microcapsule structures on the bioavailability of bioactive compounds
Microcapsule structures play a crucial role in determining the bioavailability of encapsulated compounds. The behavior of microcapsules within the body can be influenced by various aspects of their structures, encompassing the size, shape, and wall composition [13]. The properties such as permeability, porosity, and the existence of multiple concentric coatings may play a role in determining the release of the encapsulated substance and subsequently affect its bioavailability.
In conjunction with the microcapsule structure, the concept of bioaccessibility adds a crucial dimension to understanding ingredient bioavailability. Bioaccessibility, defined as the release of components or their fractions during digestion in the gastrointestinal tract (GIT) to make them available for absorption, marks the initial stage in releasing an ingredient bioavailable. Nutritionally speaking, bioaccessibility is the fraction of substances that are released from the food matrix during the digestive process and are subsequently available for tissue distribution [138]. Besides bioaccessibility, the concept of bioavailability encompasses the utilization of ingredients and their ensuing bioactivity, which pertains to the impact exerted by the absorbed components [17]. To elaborate, bioavailability includes processes such as gastrointestinal digestion, absorption, entry into the bloodstream, distribution within tissues, and, finally, the bioactivity. While these terms are closely linked, they are not always directly correlated, and improving one does not guarantee enhancement of the other.
Microencapsulation has been shown to improve both parameters in various applications. For instance, liposoluble vitamin B1 microcapsules prepared with β-cyclodextrin demonstrated a high bioaccessibility of 82.81% in oil-based systems, indicating enhanced solubility and stability (Tian et al., 2023). Similarly, iron-peptide microparticles showed bioaccessibility and bioavailability of 49% and 56%, respectively, outperforming traditional iron salts [139]. However, despite these successes, the relationship between bioaccessibility and bioavailability remains complex. For instance, high bioaccessibility may not translate into high bioavailability if the compound is poorly absorbed or rapidly metabolized after release. Factors such as the physicochemical properties of the core material (e.g., hydrophobicity), the type of encapsulating polymer, gastrointestinal transit time, and interactions with food matrices significantly affect this relationship [133,140]. Furthermore, the release profile of microcapsules under varying pH or enzyme conditions can be inconsistent, leading to partial or premature release before absorption. Thus, while microencapsulation is a promising tool to enhance the delivery of functional compounds, it requires careful design and optimization to align release kinetics with the absorption window of the target bioactive.
3.1 Microcapsule structures and their influence on release mechanisms
The design of microcapsules, particularly their structural features, significantly influences the mechanisms governing the release of encapsulated compounds. The microcapsule matrix may not change throughout the release, but it occasionally occurs because of fragmentation, shrinkage, or swelling behaviors. Apart from the microcapsules’ structural characteristics, other factors may influence the release of the bioactive material. These include the composition of the bioactive compounds and the physicochemical characteristics of the encapsulating material, such as the degree of solubility of the compound in the microcapsules’ internal core, the rheological characteristics of the material inside the polymer capsule, the size of the pores in biopolymer microcapsules, the potential interaction between the microcapsule network and properties of the encapsulated substance in microcapsules (like shape, size, and structure), and the gradient of bioactive material concentration between the microcapsules’ wall and the surrounding environment [141].
A study emphasized the significance of food microstructure in influencing the bioavailability of nutrients. It is suggested that encapsulating bioactive molecules in carefully designed matrices can provide protection during the process of digestion [142]. Yang et al. [143] discussed the relationship between protein encapsulation in microcapsules and the bioavailability of astaxanthin esters. They stated that protein encapsulation, especially with whey protein, improves the stability, water solubility, and bioavailability of astaxanthin esters. Differently, previous studies discussed in the study of Lin et al. [144] indicated that the bioaccessibility of β-carotene is influenced by both simulated digestive conditions and the features (composition and structure) of lipid-based microcapsules. These factors affect the structural stability of delivery systems, the digestion of lipids, and the transfer of β-carotene to mixed micelles. Building on these insights, the ability to adjust microcapsule composition and structure presents a significant approach to regulating the release kinetics of encapsulated substances, which is essential for enhancing bioavailability and functional efficacy in food systems.
3.1.1 Control over release rates
One of the key factors influencing release mechanisms is the composition of the microcapsule shell. Shell materials such as alginate, chitosan, and lipids contribute to the mechanical strength and permeability of the microcapsule, thus dictating the controlled diffusion and release rates of encapsulated substances [141]. The morphology of microcapsules, including regular or irregular shapes, mononuclear, polynuclear, and matrix types, also affects the release properties. Different microstructures resulting from encapsulation methods or systems, such as spray coating, co-extrusion, emulsion-based, and ionic gelation encapsulation, influence the encapsulation efficiency and retention of core materials [145]. The size of the pores in the microcapsule shell determines the release mechanism, with smaller pores leading to slower release rates [54]. Additionally, the thickness and permeability of the microcapsule shell can be adjusted to control the release kinetics of the encapsulated compounds [146]. In one study, it was observed that the size of the pores in the Ca-alginate network has an impact on the release of encapsulated bioactive compounds. The release kinetics of encapsulated compounds within the core of Ca-alginate microcapsules were noticeably influenced by the structure and physicochemical properties of the bioactive substance [141]. Jurić et al. [147] further demonstrated that the surface morphology and structure of alginate microparticles, influenced by factors such as calcium concentration and the presence of Trichoderma viride spores, can impact the release behavior of bioactive agents.
Another study stated that spray-coated encapsulates are recognized as a slow-release system, primarily attributed to the presence of additional layer(s) covering the core particles [72]. The outer layer(s) plays a crucial role in regulating the release of the encapsulated core. In order to extend the release duration of encapsulated cores, low-solubility materials are frequently employed in conjunction with fluidized-bed coating. As an illustration, ethylcellulose has been observed to significantly prolong the release of a coated drug for up to 10 h in demineralized water at 37°C [148]. Hence, the outer layer's role is crucial in determining the duration and pace of the release process. Incorporating low-solubility materials in the formulations can be a consideration to achieve desired controlled-release characteristics for various drugs.
3.1.2 pH-responsive system
The pH of the microencapsulation matrix has a significant impact on the release of the encapsulated material. In one study, pectin matrices were used to encapsulate gallic acid (GA) via spray drying. The pH-responsive release mechanism was monitored, and optimal results were obtained at a pH value of 7, indicating that the release of GA was influenced by the pH of the matrix [149]. Similarly, Baghi et al. [150] examined the ability of pea protein isolate and soybean lecithin to encapsulate trans-cinnamaldehyde (TC) at pH 3 and 7. Better thermal stability was demonstrated by the powders generated at pH 3, indicating that the matrix's pH had an impact on the TC release properties. Furthermore, one study found that the pH of the coacervation and crosslinking processes influenced the size, morphology, and release properties of geraniol-containing microcapsules. Optimal conditions for pH resulted in longer-lasting retention of geraniol, indicating that pH affected the release of the encapsulated material [151]. Moreover, Lavelli and Sri Harsha [152] also stated that the pH of the microencapsulation matrix affects the release of the encapsulated material. At pH 1.4, only 13% of the total phenolic compounds were released, while at pH 7.4, the microbeads dissolved and released the encapsulated material. By controlling the rate at which the microcapsule shells dissolve, the pH of the microencapsulation matrix influences the release of the substance that has been encapsulated. Microcapsules that react to either basic or acidic conditions are made using various polymers with varying pH values. Once exposed to a trigger pH, the shells dissolve steadily, releasing the contents in the process. By adjusting the ratios of pH-responsive and pH-unresponsive polymers in the shell composition, the rate of release can be independently adjusted [153].
3.2 Impact of coating materials
The substances that are enclosed are often referred to as active, core, payload, internal phase, encapsulate, or filling, whereas the materials that surround the core are typically termed the outer layer, protective shell, coating, external phase, supportive layer, or barrier. The coating materials can create a protective and unified layer around the core, ensuring its stability and enhancing the capsules’ durability. It is typically unable to dissolve and is unreactive with the core, inert, and does not impart any distinct flavor to the product [17]. Moreover, it is impermeable and able to release the core under specific conditions at a predetermined time and place [6,154,155]. In the context of application within the food sector, it is imperative that the coating material employed for encapsulation attains the status of generally recognized as safe. The critical first step in the encapsulation process is the identification of the suitable material to coat the substance to be microencapsulated. The choice of this coating material is contingent upon the specific properties of the active ingredient and the desired attributes of the final product. The final microcapsule properties are influenced by the structure and composition of the coating material [156]. The chemical as well as physical properties of the final microcapsules or microspheres are determined by the choice of a suitable coating material. The needs of the product, such as stability, decreased volatility, release characteristics, environmental factors, bioavailability, etc., should be taken into account while determining a coating material [6,11]. Previous studies have investigated how the coating material influences the biological activity and bioavailability of the bioactive compounds after microencapsulation, as depicted in Table 3.
List of studies reporting the impact of coating agent(s) on bioavailability
| Coating agent(s) | Microencapsulation method | Bioactive compound(s) | Core to wall concentration/ratio | Encapsulation efficiency (%) | Bioaccessibility/ bioavailability gain (%) | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Pectin, chitosan, and alginate | Emulsification and ionic gelation | Microalgae Haematococcus pluvialis (H.p.) astaxanthin | Astaxhantin concentration to be 1.3 mg g−1 in oleoresin soybean oil and combined with the polymer solutions at a polymer:oleoresin ratio of 6:1 (w:w). | 87% | Alginate: 58%, Pectin: 46–48% (compared by payload) | By the time the beads with alginate finished digesting, 58% of the astaxanthin had been released, compared to 46–48% for the beads made of pectin. The higher the concentration of pectin, the greater the stability of the carotenoid, but the lower its bioaccessibility | [157] |
| Polyvinylpyrrolidone K30 (PVP) and phospholipid | Electrospray | Capsaicin | Ratio of capsaicin/PVP/phospholipid is 1:6:0.5 | 97.3% | 219.8% increase in oral bioavailability vs free capsaicin (AUC) >80% release in 24 h vs 24–37% from control in vitro | Electrosprayed microcapsules were significantly enhanced both in vitro release and in vivo absorption; excellent IVIVC (r ≥ 0.981) confirmed the predictive power of dissolution data | [158] |
| β-Cyclodextrin, maltodextrin, and Arabic gum | Spray drying | Polyphenols | Leaf extract:carrier ratio was 1:2, with carrier composition β-cyclodextrin/maltodextrin 50:50 | 80.43% | Bioaccessibility in gastric: ∼150%; intestinal: ∼80% vs extract ∼60% | Spray drying microencapsulation with carrier mixtures increased the bioaccessibility of laurel flavonols by approximately 50% in the gastric phase and 10% in the intestinal phase, compared to the non-encapsulated extract | [159] |
| Sodium alginate and soluble potato starch | Ionic gelation | Iron | Alginate, starch, ferrous bisglycinate powder, and water in a ratio of 2:1:2:100 (w:w:w:v) | 82.3% | At the end of simulated oral-gastric-intestinal digestion, the beads heated to 180°C released 1.22-fold more bioaccessible ferrous ions than the corresponding bisglycinate powder | The alginate-starch-iron beads have potential as carriers for oral delivery of iron, as they can enhance the bioaccessibility of ferrous ions during digestion | [160] |
| Sodium carboxymethyl and maltodextrin | Freeze drying and spray drying | Phenolic acids and flavonols | Maltodextrin 10% and 12% in extract, and carboxymethyl 0.70 and 0.75% in extract | N.A | All treatments lost flavonols: 16.58–28.90%; GABA: 34.91–51.14%; 1-deoxynojirimycin: 17.56–20.42%; Phenolic acids: 0.53–0.67% (during the gastric phase) | Encapsulation was found to enhance bioaccessibility but had negative effects on bioefficiency and bioavailability. Carriers had a major effect on the digestibility and antioxidative activity of the compounds. Sodium carboxymethyl cellulose was found to reduce the losses of biocompounds during intestinal digestion, and spray-drying resulted in lesser losses of biomolecules compared to freeze-drying | [161] |
| High methyl pectin (HMP), whey protein isolates (WPI), and soy protein isolates (SPI) | Spray drying | Anthocyanins | Blueberry anthocyanin powder/wall materials (4% SPI + 2% HMP) ratio was 1:4 | 93.5% | Microencapsulated blueberry anthocyanin powder <50% anthocyanins vs. blueberry anthocyanin powders alone (control) ∼100%, indicating controlled release and increased bioaccessibility | Microcapsules created with a combination of 4% SPI and 2% HMP (MBAPc) showed superior anthocyanin release behavior and antioxidant stability compared to those produced with 4% SPI alone (MBAPs). Both MBAPc and MBAPs exhibited continuous release of anthocyanins throughout simulated gastrointestinal digestion and followed two first-order kinetics | [162] |
| Enzymatic cyclodextrin synthesis product, maltodextrin, and β-cyclodextrin | Spray drying | Tributyrin | Tributyrin/maltodextrin ratio was 1:1 | 90.90% | 69.06% released in stomach (SGF); 100% release within 1 h in the intestine. Moderate to low protection; reduced bioavailability | Tributyrin microcapsules prepared using the method of encapsulating tributyrin during enzymatic cyclodextrin synthesis (CGT) showed superior controlled release of their tributyrin content in a model of the mammalian intestine, indicating improved bioavailability | [163] |
| Maillard reaction products (MRP) and OSA starch | Spray drying | Docosahexaenoic acid (DHA) | Not available | Not available | Significant increase in blood DHA and Omega-3 index by week 2; low fecal DHA; superior bioavailability vs non-encapsulated oil | The results demonstrated enhanced bioavailability with significantly greater concentrations of blood DHA levels in formulas with microencapsulated powders. The bioavailability of DHA was assessed through blood and fecal fatty acid levels, and the results indicated improved bioavailability with the use of microencapsulated powders | [164] |
3.2.1 Hydrophilic coating agents
The application of hydrophilic encapsulants facilitates the effective encapsulation of micro- or nanosized hydrophilic compounds. The utilization of the internal aqueous pockets within the capsules enhances encapsulation efficiency by maintaining the stability of the active components and inhibiting their premature release into the surrounding environment [165]. The encapsulation effectiveness of hydrophilic substances can be improved using hydrophilic encapsulants such as chitosan and alginate. Microcapsules containing alginate-coated chitosan, produced from poly (dl-lactide-co-glycolide) (PLGA), exhibited superior encapsulation rates and reduced early burst release compared to conventional methods, facilitating a more regulated and extended-release profile [166]. The choice of coating material has a significant impact on the kinetics of chemical release. As an example, chitosan coatings allow hydrophilic drugs to be released slowly, reducing the initial burst release significantly [166]. This is crucial for reaching and maintaining therapeutic levels in the bloodstream while minimizing the risk of undesirable effects caused by rapid drug release. The ability of various hydrophilic encapsulants to respond to pH variations renders them optimal for application in biological contexts as drug delivery systems. Certain tissues or pathological conditions may lead to the degradation of biodegradable nanocarriers made from poly(thiourethane-urethane). Encapsulated hydrophilic compounds can be selectively released upon reaching certain regions of the body [167].
A different study found that eugenol, an antimicrobial compound that has been encapsulated and dried by spray-drying, demonstrated a significant reduction in the populations of both Gram-negative (Escherichia coli) and Gram-positive (Listeria innocua) bacteria, decreasing from 5 to 6 log CFU/mL to 0 log CFU/mL within a time frame of just 30 min [86]. The implementation of low-solubility encapsulants facilitates the achievement of a slower, controlled release over a duration ranging from hours to days from spray-dried encapsulates. When combined with suitable encapsulation technology, this approach leads to extended-release periods. In such cases, an initial rapid release may occur, attributed to the presence of some core droplets on the surface of the encapsulates, followed by a slower and sustained release. Additionally, manipulating the release rate of the encapsulated material can be achieved by varying the core-encapsulant ratio. Decreasing the core load, for instance, contributes to a reduced release rate due to the resulting larger shell thickness of the encapsulates.
3.2.2 Hydrophobic coating agents
Lipid carriers are essential in improving the bioavailability of microencapsulated compounds. Lipid-based carriers, including nanoemulsions, liposomes, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs), have been successfully developed to enhance the solubility, stability, and release profiles of bioactive compounds within the food industry [168,169]. Lipid-based carriers improve the bioavailability of microencapsulated compounds by offering heightened chemical and physical stability, extended release duration, and protection against external influences [169].
The development of NLCs aimed to address certain issues and constraints associated with other lipid-based carriers like SLNs. NLCs were designed to overcome the limitations of SLNs, which exhibit a lower capacity to accommodate bioactive compounds compared to NLCs. Additionally, SLNs tend to have high water content, ranging from approximately 70% to 99.9%. This particular drawback renders SLNs unsuitable for delivering certain food ingredients and limits their application as a delivery system in certain food-related contexts [170]. Shishir et al. [171] stated that the distribution of solid and liquid lipid constituents in NLCs significantly impacts the transport and release rates of bioactive compounds. Due to the great mobility and solubility of bioactives inside the liquid fraction channels, it is probable that their path transpires through these channels instead of the denser, crystalline solid lipids. Consequently, an increase in oil fractions is anticipated to elevate the rate of bioactive release. The transport and release kinetics of bioactive molecules are profoundly influenced by the features of liquid fractions, including fatty acid profile, compositional heterogeneity, saturation level, and chain length [172].
There is some inconsistency in the data collected about the rate of bioactive release from NLCs. Bioactive release rates varied among experiments; in some, they increased as oil fractions increased, while in others, they declined as fractions increased. Release rates of bioactives were shown to vary over time apart from changes in the oil fraction mass, according to certain research that failed to find a correlation between the two. In essence, lipid-based encapsulation provides a range of significant advantages and proves effective in enhancing encapsulation efficiency, ensuring improved stability, elevating the bioavailability of hydrophobic bioactive compounds, and mitigating potential toxicity concerns.
3.3 Studies of bioavailability assessments
The bioavailability and bioaccessibility of bioactives and nutraceuticals have been evaluated and quantified using a variety of models. A summary of each model's primary benefits and drawbacks is provided in Table 4.
Advantages and disadvantages of the different models to evaluate bioavailability [173]
| Model | Advantages | Disadvantages |
|---|---|---|
| In vitro model | Rapid, affordable, and poses no ethical concern | Can only evaluate bioaccessibility and not bioavailability |
| Cell models | Rapid and present no ethical issues. Assessment of the actual permeation of epithelial cells | Excludes considerations of gastrointestinal tract conditions. Limited to evaluating absorption |
| Animal models | More cost-effective than clinical trials | Correlating results from animal models with human trials is not always possible. The process is labor-intensive and incurs higher expenses compared to in vitro or cell studies, and ethical concerns may arise |
| Option to collect organs | ||
| Capability to carry out toxicology investigations | ||
| Clinical trials | Most precise method | Labor-intensive, |
| higher cost, | ||
| ethical concerns, | ||
| time-consuming, and | ||
| not suitable for toxicology studies |
3.3.1 In vitro bioavailability evaluation
In vitro digestion evaluation is extensively employed to assess the bioaccessibility of bioactive compounds. These procedures are convenient cost-effective, rapid, and do not present the same ethical concerns as clinical trials. In vitro models replicate gastrointestinal conditions by modifying ionic strength and pH, introducing enzymes, bile salts, and even fermentation reactions to emulate colon conditions. Static, semi-dynamic, and dynamic in vitro digestion models are used to study the digestion behavior of various substances. These models simulate different aspects of the human gastrointestinal system to better understand the digestion process. In static digestion models, the digestion behavior is observed without considering the dynamic variations of the gastrointestinal conditions [174]. Semi-dynamic digestion models introduce some dynamic aspects, such as pH variations, but still have limitations in replicating physiological conditions [175]. Dynamic digestion models, on the other hand, take into account parameters like gastric juice secretion, gastric emptying rate, intestinal juice secretion, and pH variations, providing a more realistic representation of the digestion process [176]. These models have been used to study the digestion of lipids, proteins, and starch [177,178]. Although static models are widely used due to their simplicity and standardization, they lack dynamic physiological elements such as gastric emptying and enzyme flow, which limits their predictive power for real-life digestion. Semi-dynamic models offer partial improvements, but they still fall short in replicating complex gastrointestinal conditions. In contrast, dynamic models provide a more physiologically relevant environment, yet their complexity and cost limit their accessibility and scalability for routine screening. Thus, the choice of model must balance realism with practicality, depending on the research goal.
Among these, the INFOGEST protocol has emerged as the most widely accepted in vitro digestion model due to its standardized, physiologically relevant design. It simulates human digestion across three stages – oral, gastric, and intestinal – and has been widely adopted in academic and industry research [179]. Its reproducibility and comprehensive simulation of digestive conditions allow for inter-laboratory consistency and more accurate evaluation of nutrient release and food matrix interactions. However, despite their utility, in vitro models face critical limitations when extrapolating to in vivo outcomes, particularly in the context of protein–polyphenol interactions. In vitro systems often apply fixed pH and lack dynamic enzyme activities that occur naturally during digestion, leading to potentially inaccurate estimates of bioaccessibility [180,181]. Moreover, complex formation mechanisms – both covalent and non-covalent – are influenced in vivo by fluctuating ionic strength, protein conformation, and compartmentalized absorption pathways that are rarely captured in vitro [182]. These discrepancies can result in over- or underestimation of bioavailability. For example, polyphenols may exhibit high antioxidant activity in vitro, yet this can be masked in vivo due to binding with proteins, altering their nutritional functionality [183]. Therefore, while in vitro digestion models are invaluable for early-stage screening, critical interpretation is necessary when translating findings to real physiological scenarios. Improvements in model complexity and physiological mimicry, especially concerning gut microbiota metabolism and compound–protein interactions, remain essential for accurate predictions.
In vitro models have been used to assess the bioaccessibility of microencapsulated bioactive compounds. Based on the simulated digestion system, the protective effect of encapsulation against environmental conditions was assessed by Vergara et al. [184], where the bioaccessibility of anthocyanin from the microencapsulated extract with maltodextrin was found to be 20% higher than that of the non-encapsulated extract, indicating the protective effect of encapsulation against environmental conditions. The rate of bioactive compounds degradation, like anthocyanins, and how the coating agents slow it down, were also observed during in vitro digestion [185]. Both increase the thermal stability of blackberry anthocyanins and reduce the rate at which these pigments degrade in simulated gastrointestinal settings. Furthermore, an in vitro enzymatic model was able to analyze the potential of the anti-hyperglycemic effect of encapsulated compound, and the results suggest that encapsulated ferulic acid has a potential anti-hyperglycemic effect [186]. In the release study model, the duration of encapsulated bioactive compound retention was prolonged by the outer coating of the particles. Gomes et al. [187] observed that the duration of encapsulated catechin retention is prolonged by the lipid outer coating of the particles. This is because the use of these lipid/particle structures is intended for techniques involving cell absorption under physiological settings, as well as the regulated release of EGCG at the lower digestive tract. Other in vitro assessments of microcapsules are presented in Table 5.
In vitro assessments of microcapsules
| Microencapsulated compound(s) | Study model | Microencapsulation method | Bioavailability/biological effects | Ref. |
|---|---|---|---|---|
| Prickly pear betalain | Simulated gastrointestinal digestion | Freeze drying | The bioaccessibility of encapsulated betalain is higher than the unencapsulated conventional extract | [28] |
| Red propolis phenolics formononetin | Simulated gastrointestinal digestion | Spray drying, spray chilling, and combining both techniques | The gastrointestinal release study showed distinct release profiles across phases. Spray-dried particles released formononetin mainly in the oral phase, spray-chilled particles in the intestinal phase, and coated particles are gradually released, peaking in the intestinal phase | [188] |
| Microalgae Haematococcus pluvialis (H.p.) astaxanthin | Simulated gastrointestinal digestion | Emulsification and ionic gelation | The alginate-containing beads have a faster release rate, which could be attributed to either a weaker interaction between the polymer and astaxanthin or a less tightly packed matrix structure that promotes carotenoid release | [157] |
| Vaccinium spp. leaves phenolic compounds | Simulated gastrointestinal digestion | Spray drying | Although the microencapsulated forms exhibited a targeted release at the intestinal level, the phenolic content decreased following gastrointestinal digestion. Interestingly, the bioaccessibility of the microencapsulated extracts demonstrated higher values compared to their non-encapsulated counterparts | [189] |
| Mesona chinensis polyphenols | Simulated gastrointestinal digestion | Ionic gelation | Alginate encapsulation of M. chinensis extract may be a method to increase the bioaccessibility and biological activity of polyphenols | [190] |
| Blueberry anthocyanins | Simulated gastrointestinal digestion | Spray drying | Microencapsulation of anthocyanins can increase their stability and bioaccessibility by entrapping them in coating materials, preventing direct exposure to adverse environments | [162] |
| Purple potato anthocyanins | Simulated mouth, gastric, and gut digestion | Spray drying | The bioaccessibility of anthocyanin from the microencapsulated extract with maltodextrin was found to be 20% higher than that of the non-encapsulated extract, indicating the protective effect of encapsulation against environmental conditions | [184] |
| Black rice anthocyanins | Simulated gastric and intestinal digestion | Double emulsifications | After 2 h of incubation in simulated gastric fluid (SGF), the double emulsion was able to control the release of anthocyanins in the stomach. In the simulated intestinal fluid, a total release of anthocyanins in the double emulsion was observed within the first 20 min | [191] |
| Trans-resveratrol | In vitro release, digestion, and intestinal permeability | Electrospraying | The encapsulated compounds showed sustained release profiles and improved permeability in an ex vivo dynamic engineered small intestinal system, indicating enhanced bioavailability | [192] |
| Quercetin | Release study | Spray drying | The release studies of quercetin-loaded casein nanoparticles showed zero-order kinetics | [193] |
| Ferulic acid | Enzymatically | Ionic gelation | The results suggest that encapsulated ferulic acid has a potential anti-hyperglycemic effect | [186] |
| Blackberry anthocyanin | Simulated gastric and small intestine digestion | Molecular inclusion | The anthocyanins degraded quickly within the first several minutes of the simulated intestine digestion, although this was slowed down by complexation with β-cyclodextrin. The observed results show that β-cyclodextrin can both increase the thermal stability of blackberry anthocyanins and reduce the rate at which these pigments degrade in simulated gastrointestinal settings | [194] |
Notably, studies using static in vitro models may overestimate encapsulation stability, as they do not simulate mechanical stresses or gradual pH shifts encountered in vivo. For instance, anthocyanin retention [184] and ferulic acid functionality [186] were demonstrated under simplified conditions, which may not fully capture degradation patterns seen in dynamic digestion. Moreover, the use of lipid-based encapsulants [187] shows promise for targeted intestinal release, but further validation in dynamic systems is needed to confirm controlled release kinetics. Comparing across studies, it becomes clear that while encapsulation enhances bioaccessibility and functionality, outcomes can vary significantly depending on the model employed. This underlines the importance of selecting appropriate in vitro systems based on the physicochemical characteristics of the encapsulated compound and intended application.
3.3.2 In vivo bioavailability assessment
Currently, it remains unfeasible to completely substitute in vivo studies with in vitro models, regardless of their advancements. The inherent complexity of the human body has led to a growing recognition of animal models as a viable alternative to in vitro research. Given fundamental differences in metabolic processes between animals and humans, the findings derived from animal studies may be subject to debate. In order to gain a deeper insight into the health benefits of bioactive compounds (CBAs), in vitro studies and animal experiments remain valuable methodologies for assessing human in vivo processes such as digestion, absorption, metabolism, and tissue distribution, among others [195]. Consequently, findings from both in vitro and in vivo research involving animals or other non-human models can provide supportive evidence. It is essential to note that human clinical trials remain irreplaceable, as emphasized in the EFSA scientific and technical guidelines for health claim authorization [196]. Human intervention studies should ideally complement nutritional research after in vitro and in vivo (animal experimental) investigations have yielded strong findings supporting the proposed theory. This is considered the most effective way to evaluate the genuine significance of foods or bioactive components concerning their health benefits [195]. Human intervention studies provide insights into how these elements interact with the human body and contribute to overall health, ensuring a more comprehensive understanding of their impact.
In vivo animal models are able to examine the delivery of bioactive compounds both in digestive and non-digestive tissues, such as in Tong et al. [197], where anthocyanins encapsulated with amylopectin nanoparticles showed improved stability and delivery to the stomach and lungs, respectively. The level of microencapsulated active compound in plasma was able to be detected [193]. In that study, animals treated with quercetin-loaded casein nanoparticles displayed higher plasma levels of quercetin compared to animals receiving the solution of the flavonoid (control). Moreover, the effect of microencapsulated bioactive compounds on the blood profile can be detected [186,198]. Panwar et al. [186] reported that encapsulated ferulic acid was found to attenuate the diabetes-associated symptoms in the rats. It showed an enhancement in body weight but a decrease in blood glucose levels, and also had a regulatory effect on the blood lipid profile of the diabetic rats. Moreover, Cian et al. [199] stated that the pro-oxidant and prothrombotic effects of a diet high in sucrose on rats were prevented by microencapsulated peptides. Other in vivo assessments of microcapsules are presented in Table 6.
In vivo assessments of microcapsules
| Microencapsulated compound(s) | Study model | Microencapsulation method | Bioavailability/biological effects | Ref. |
|---|---|---|---|---|
| Aronia melanocarpa anthocyanins | Animal model on rats, analysis of compound distribution and bioactivity in nine tissues | Gelation | Anthocyanins encapsulated with amylopectin nanoparticles showed improved stability and delivery to both digestive and non-digestive tissues, such as the stomach and lungs, respectively | [197] |
| Brewers’ spent grain peptides | Antioxidant and antithrombotic activity in rats | Spray drying | The pro-oxidant and prothrombotic effects of a diet high in sucrose on rats were prevented by microencapsulated peptides | [198] |
| Quercetin | Pharmacokinetic studies in rats | Spray drying | Animals treated with quercetin-loaded casein nanoparticles displayed higher plasma levels of quercetin compared to animals receiving the solution of the flavonoid (control) | [193] |
| Quercetin | Biodistribution study, anti-tumor activity | Ionic gelation | In vivo evaluations using tumor xenograft mice showed that treatment with encapsulated quercetin resulted in a significant reduction in tumor volume compared to disease control groups. Additionally, it led to a marked increase in serum antioxidant enzyme superoxide dismutase (SOD) levels in tumor-bearing mice, indicating enhanced antioxidant activity | [200] |
| Ferulic acid | A pharmacokinetics study, administered orally to rats | Ionic gelation | Encapsulated ferulic acid was found to attenuate the diabetes-associated symptoms in the rats. It showed an enhancement in body weight but a decrease in blood glucose levels and also had a regulatory effect on the blood lipid profile of the diabetic rats | [186] |
4 Future trends and application prospects of microencapsulation in the food industry
Various bioactive compounds play a significant role in preventing various diseases in the human body, according to numerous studies. Several established microencapsulation processes, including spray drying, coacervation, extrusion, and spray cooling, find widespread application in the food industry. These processes are versatile and can be employed to encapsulate various active food ingredients such as flavors, polyunsaturated fatty acids, probiotics, antioxidants, colors, and vitamins. The key advantage of microencapsulation lies in the uniformity it achieves, resulting in enhanced encapsulation efficiency and improved physical and chemical properties. In essence, microencapsulation holds the potential to bring about a transformative impact on the food industry by delivering effective protective systems for the controlled release of bioactive substances.
Microencapsulation emerges as a highly promising technology within the food industry, offering considerable future potential and diverse application possibilities. This technique entails enclosing active ingredients within a matrix to enhance both their stability and bioaccessibility [201]. Leveraging microcapsules as carriers in the food sector holds promise of enhancing the shelf life of food products and maintaining the stability of bioactive compounds over extended periods. Additionally, microencapsulation opens opportunities for the development of functional foods with protective and preservative attributes, thereby contributing to valuable health effects.
Microencapsulation has become a practical and widely adopted technology across diverse food sectors, offering solutions for ingredient protection, controlled release, and product enhancement. In the beverage industry, it is employed to protect volatile flavors and sensitive nutrients such as vitamins and antioxidants, improving shelf life and sensory quality [202,203].
Microencapsulation plays a pivotal role in protecting sensitive ingredients in beverages. For instance, Habibi et al. [204] successfully microencapsulated fish oil using complex coacervation (gelatin–gum Arabic) and incorporated the capsules into pomegranate juice. The microcapsules enhanced the sensory properties and allowed for the controlled release of omega-3 fatty acids, demonstrating how encapsulation can enrich aqueous-based beverages with lipophilic nutraceuticals without compromising product quality during storage. Similarly, Shehata et al. [205] explored the use of alginate microcapsules enriched with plant extracts such as moringa and green tea to improve the stability and survival of probiotic strains in fruit juices and drinkable yoghurt. This highlights microencapsulation's value in maintaining probiotic viability during shelf life and simulated digestion – a key challenge in functional beverage development.
In bakery products, microencapsulation has been used to stabilize polyunsaturated fatty acids and enhance the nutritional profile. For example, polyunsaturated fatty acids are microencapsulated to reduce oxidative degradation, while probiotics are encapsulated to maintain viability through baking and storage [201,206]. In another example, Papillo et al. [210] encapsulated anthocyanin-rich extracts from Artemide black rice using spray-drying and freeze-drying methods. When added to model biscuits, the enriched powders significantly increased antioxidant capacity and polyphenol content, demonstrating the potential of microencapsulation to introduce functional ingredients into thermally processed products without major degradation.
Microencapsulation is also being applied to improve the quality and health impact of dairy products. Dairy products benefit from the taste masking of functional ingredients and the controlled release of bioactive compounds to enhance functionality and consumer appeal [201]. In the study by Shehata et al. [205], probiotic-loaded alginate capsules fortified with plant extracts enhanced the survival of bacteria in drinkable yogurt for up to 30 days of refrigerated storage, offering a functional and commercial advantage in probiotic dairy formulation.
These examples underscore microencapsulation's expanding role in improving nutritional quality, stability, and product innovation across the food industry, although ongoing challenges such as optimizing encapsulation efficiency and material compatibility remain [206].
Several successful microencapsulation technologies have been commercialized in the functional food sector. One prominent example is OMEGAWATER, a ready-to-drink functional beverage that incorporates omega-3 fatty acids (EPA/DHA) through nanoencapsulation. This technology enables the transformation of oil-based omega-3 into stable, water-soluble micelles, which not only enhance bioavailability but also effectively mask undesirable fishy odors and tastes. In addition, the encapsulation system improves the oxidative stability of omega-3, extending the product’s shelf life. A complementary product, Omega Squeeze, offers a more concentrated form of encapsulated omega-3, intended for use in smoothies, cereals, or as a direct nutritional supplement.
Other examples include microencapsulated lipid powders such as VegeLipi®, developed by HSF Biotech, and encapsulated DHA/EPA powders produced by the Custom Food Group in Malaysia. These ingredients are commonly used in infant formula, functional beverages, and dietary supplements. The microencapsulation of these sensitive lipophilic compounds protects them from degradation caused by environmental factors such as oxygen, heat, and light, thereby ensuring greater stability during processing and storage. These commercial applications clearly demonstrate the value of encapsulation technologies in enhancing the functional performance and consumer acceptance of bioactive ingredients in food systems.
However, to fully benefit from these compounds, it is crucial to ensure they are absorbed properly. Understanding their bioaccessibility (how easily they can be released) and bioavailability (how effectively they can be absorbed) is essential. Despite increased research in recent years, there are still many things unknown. Scientists commonly use in vitro simulated digestion tests to study how well the compounds are absorbed in the digestive system. These tests aim to provide insights into how effectively these compounds can be absorbed and utilized by the body. To support the findings from in vitro simulated digestion tests, it is recommended that future research should include in vivo studies on microcapsules. This would provide a more comprehensive understanding of how effectively these compounds are absorbed and utilized by the body in real-life conditions.
-
Funding information: This study was supported by the National Science Center of Poland (Preludium 23 Grant No. DEC-2024/53/N/NZ9/00445) and the statutory funds of the Department of Gastronomy Science and Functional Foods (Grant No. 506.751.03.00).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: Maciej Jarzębski, who is the co-author of this article, is a current Editorial Board member of Nanotechnology Reviews. This fact did not affect the peer-review process. The authors declare no other conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Ab Rashid S, Tong WY, Leong CR, Abdul Ghazali NM, Taher MA, Ahmad N, et al. Anthocyanin microcapsule from clitoria ternatea: potential bio-preservative and blue colorant for baked food products. Arab J Sci Eng. 2021;46(1):65–72.10.1007/s13369-020-04716-ySearch in Google Scholar
[2] Dhia F, Karim A. Microencapsulation of clitoria ternatea, curcuma longa, brassica oleracea and hibiscus sabdariffa using thermal effect ionic gelation technique. 2022;4(1):57–65.Search in Google Scholar
[3] Lazăr AR, Pușcaș A, Tanislav AE, Mureșan V. Bioactive compounds delivery and bioavailability in structured edible oils systems. Compr Rev Food Sci Food Saf. 2024;23(6):e70020.10.1111/1541-4337.70020Search in Google Scholar PubMed
[4] Chai J, Jiang P, Wang P, Jiang Y, Li D, Bao W, et al. The intelligent delivery systems for bioactive compounds in foods: Physicochemical and physiological conditions, absorption mechanisms, obstacles and responsive strategies. Trends Food Sci Technol. 2018;78:144–54, https://www.sciencedirect.com/science/article/pii/S0924224417306465.10.1016/j.tifs.2018.06.003Search in Google Scholar
[5] Shishir MRI, Gowd V, Suo H, Wang M, Wang Q, Chen F, et al. Advances in smart delivery of food bioactive compounds using stimuli‐responsive carriers: Responsive mechanism, contemporary challenges, and prospects. Compr Rev Food Sci Food Saf. 2021;20(6):5449–88.10.1111/1541-4337.12851Search in Google Scholar PubMed
[6] Multisona RR, Shirodkar S, Arnold M, Gramza-Michalowska A. Clitoria ternatea flower and its bioactive compounds: potential use as microencapsulated ingredient for functional foods. Appl Sci. 2023;13(4):2134.10.3390/app13042134Search in Google Scholar
[7] Daliri EBM, Lee BH. Current trends and future perspectives on functional foods and nutraceuticals. Beneficial microorganisms in food and nutraceuticals. Cham, Switzerland: Springer; 2015. p. 221–44.10.1007/978-3-319-23177-8_10Search in Google Scholar
[8] Jędrusek‐Golińska A, Górecka D, Buchowski M, Wieczorowska‐Tobis K, Gramza‐Michałowska A, Szymandera‐Buszka K. Recent progress in the use of functional foods for older adults: A narrative review. Compr Rev Food Sci Food Saf. 2020;19(2):835–56.10.1111/1541-4337.12530Search in Google Scholar PubMed
[9] Pistorio E, Chinnici G, Zarbà C, Bellia C, Pappalardo G. The revolution of functional food: a market analysis. Int Multidiscip Sci Geo. 2023;23(6.2):433–41.10.5593/sgem2023V/6.2/s25.53Search in Google Scholar
[10] Tsoupras A, Zabetakis I, Lordan R. Chapter 14 - Functional foods: growth, evolution, legislation, and future perspectives. In: Zabetakis I, Tsoupras A, Lordan R, Ramji D, editors. Functional foods and their implications for health promotion. Academic Press; 2023. 367–77. https://www.sciencedirect.com/science/article/pii/B9780128238110000031.10.1016/B978-0-12-823811-0.00003-1Search in Google Scholar
[11] Singh MN, Hemant KSY, Ram M, Shivakumar HG. Microencapsulation: A promising technique for controlled drug delivery. Res Pharm Sci. 2010;5(2):65–77.Search in Google Scholar
[12] Giro-Paloma J, Martínez M, Cabeza LF, Fernández. AITypes. methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew Sustain Energy Rev. Elsevier Ltd 2016;53:1059–75.10.1016/j.rser.2015.09.040Search in Google Scholar
[13] Choudhury N, Meghwal M, Das K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Front. John Wiley and Sons Inc 2021;2:426–42.10.1002/fft2.94Search in Google Scholar
[14] Jayanudin J, Heriyanto H. A review of encapsulation using emulsion crosslinking method. World Chem Eng J. 2021;5(2):37–43.10.48181/wcej.v5i2.12312Search in Google Scholar
[15] Nizori A, Bui LTT, Jie F, Small DM. Spray‐drying microencapsulation of ascorbic acid: impact of varying loading content on physicochemical properties of microencapsulated powders. J Sci Food Agric. 2020;100(11):4165–71.10.1002/jsfa.10455Search in Google Scholar PubMed
[16] Mehta N, Kumar P, Verma AK, Umaraw P, Kumar Y, Malav OP, et al. Microencapsulation as a noble technique for the application of bioactive compounds in the food industry: a comprehensive review. Appl Sci. 2022;12(3):1–34.10.3390/app12031424Search in Google Scholar
[17] Grgić J, Šelo G, Planinić M, Tišma M, Bucić-Kojić A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants. 2020;9(10):1–36.10.3390/antiox9100923Search in Google Scholar PubMed PubMed Central
[18] Hu Y, Lin Q, Zhao H, Li X, Sang S, McClements DJ, et al. Bioaccessibility and bioavailability of phytochemicals: Influencing factors, improvements, and evaluations. Food Hydrocoll. 2023;135:108165. https://www.sciencedirect.com/science/article/pii/S0268005X22006853 10.1016/j.foodhyd.2022.108165Search in Google Scholar
[19] Giovagnoli-Vicuña C, Briones-Labarca V, Romero MS, Giordano A, Pizarro S. Effect of extraction methods and in vitro bio-accessibility of microencapsulated lemon extract. Molecules. 2022;27(13):1–12.10.3390/molecules27134166Search in Google Scholar PubMed PubMed Central
[20] Yeo Y, Chen AU, Basaran OA, Park K. Solvent exchange method: a novel microencapsulation technique using dual microdispensers. Pharm Res. 2004;21(8):1419–27.10.1023/B:PHAM.0000036916.96307.d8Search in Google Scholar
[21] Łętocha A, Miastkowska M, Sikora E. Preparation and characteristics of alginate microparticles for food, pharmaceutical and cosmetic applications. Polymers. 2022;14(18):3834.10.3390/polym14183834Search in Google Scholar PubMed PubMed Central
[22] Thach NA. Investigation of the effects of extraction temperature and time on bioactive compounds content from garlic (Allium sativum L.) husk. Front Sustain Food Syst. 2022;6:1004281.10.3389/fsufs.2022.1004281Search in Google Scholar
[23] Clímaco GN, de Sousa LCS, de Cássia Bergamasco R. Evaluation of time, temperature and concentration of solvent, in the extraction of bioactive compounds from carrot (daucus carota). Res Soc Dev. 2020;9(8):e922986130.10.33448/rsd-v9i8.6130Search in Google Scholar
[24] Tsvetov N, Pasichnik E, Korovkina A, Gosteva A. Extraction of bioactive components from Chamaenerion angustifolium (L.) Scop. with choline chloride and organic acids natural deep eutectic solvents. Molecules. 2022;27(13):4216.10.3390/molecules27134216Search in Google Scholar PubMed PubMed Central
[25] Plawgo M, Kocira S, Bohata A. Multi-objective optimization of the green extraction conditions of bio-active compounds from a levisticum officinale WDJ Koch: Pareto optimality and compromise solutions for process management. Agric Eng. 2024;28:137–65.10.2478/agriceng-2024-0010Search in Google Scholar
[26] Ferreira RM, Ramalho Ribeiro A, Patinha C, Silva AMS, Cardoso SM, Costa R. Water extraction kinetics of bioactive compounds of Fucus vesiculosus. Molecules. 2019;24(18):3408.10.3390/molecules24183408Search in Google Scholar PubMed PubMed Central
[27] Zahed N, Esmaeilzadeh Kenari R, Farahmandfar R. Effect of different extraction methods on antioxidant properties and encapsulation efficiency of anthocyanin of pomegranate peel. Food Sci Nutr. 2023;11(7):3780–7.10.1002/fsn3.3362Search in Google Scholar PubMed PubMed Central
[28] Mehta D, Kuksal K, Yadav K, Kumar Yadav S, Zhang Y, Hariram Nile S. Ultrasound-assisted extraction and encapsulation of betalain from prickly pear: Process optimization, in-vitro digestive stability, and development of functional gummies. Ultrason Sonochem. 2024;108:106975.10.1016/j.ultsonch.2024.106975Search in Google Scholar PubMed PubMed Central
[29] Carpentieri S, Ferrari G, Donsì F. All-natural wheat gliadin-gum arabic nanocarriers for encapsulation and delivery of grape by-products phenolics obtained through different extraction procedures. Food Chem. 2023;424(December 2022):136385.10.1016/j.foodchem.2023.136385Search in Google Scholar PubMed
[30] Jafari S, Karami Z, Shiekh KA, Kijpatanasilp I, Worobo RW, Assatarakul K. Ultrasound-assisted extraction of bioactive compounds from cocoa shell and their encapsulation in gum arabic and maltodextrin: a technology to produce functional food ingredients. Foods. 2023;12(2):412.10.3390/foods12020412Search in Google Scholar PubMed PubMed Central
[31] Afshari K, Javanmard Dakheli M, Ramezan Y, Bassiri A, Ahmadi Chenarbon H. Physicochemical and control releasing properties of date pit (Phoenix dactylifera L.) phenolic compounds microencapsulated through fluidized‐bed method. Food Sci Nutr. 2023;11(3):1367–82.10.1002/fsn3.3173Search in Google Scholar PubMed PubMed Central
[32] Nutrizio M, Jurić S, Kucljak D, Švaljek SL, Vlahoviček-Kahlina K, Režek Jambrak A, et al. Encapsulation of rosemary extracts using high voltage electrical discharge in calcium alginate/zein/hydroxypropyl methylcellulose microparticles. Foods. 2023;12(8):1570.10.3390/foods12081570Search in Google Scholar PubMed PubMed Central
[33] Phupaboon S, Matra M, Prommachart R, Totakul P, Supapong C, Wanapat M. Extraction, characterization, and chitosan microencapsulation of bioactive compounds from cannabis sativa L., cannabis indica L., and mitragyna speiosa K. Antioxidants. 2022;11(11):2103.10.3390/antiox11112103Search in Google Scholar PubMed PubMed Central
[34] Lu Y, Liang X, Cheng L, Fang S. Microencapsulation of pigments by directly spray-drying of anthocyanins extracts from blueberry pomace: chemical characterization and extraction modeling. Int J Food Eng. 2020;16(3):1–10.10.1515/ijfe-2019-0247Search in Google Scholar
[35] Zanoni F, Primiterra M, Angeli N, Zoccatelli G. Microencapsulation by spray-drying of polyphenols extracted from red chicory and red cabbage: Effects on stability and color properties. Food Chem. 2020;307:125535.10.1016/j.foodchem.2019.125535Search in Google Scholar PubMed
[36] Moreira SA, Alexandre EMC, Pintado M, Saraiva JA. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res Int. 2019;115:177–90.10.1016/j.foodres.2018.08.046Search in Google Scholar PubMed
[37] Pu M, Liu K, Zhang M, Yuan P, Cai J. Microparticles and Microcapsules from the Solvent Extraction of Deep Eutectic Solvent-Based Emulsion. Ind Eng Chem Res. 2020;59(7):2892–8.10.1021/acs.iecr.9b05061Search in Google Scholar
[38] Mehta RC, Thanoo BC, Deluca PP. Peptide containing microspheres from low molecular weight and hydrophilic poly (d, l-lactide-co-glycolide). J controlled Rel. 1996;41(3):249–57.10.1016/0168-3659(96)01332-6Search in Google Scholar
[39] Zhi J, Huang S, Zhu X, Joy Adra H, Luo K, Kim YR. Impact of solvent polarity on the morphology, physicochemical properties, and digestibility of A-type resistant starch particles. Food Chem. 2023;418:135942. https://www.sciencedirect.com/science/article/pii/S0308814623005599.10.1016/j.foodchem.2023.135942Search in Google Scholar PubMed
[40] Karaman K. Fabrication of gallic acid loaded yeast (Saccharomyces cerevisiae) microcapsules: Effect of plasmolysis treatment and solvent type on bioactivity and release kinetics. LWT. 2021;148:111640.10.1016/j.lwt.2021.111640Search in Google Scholar
[41] Zhang X, Yuan Y, Shan X, Sheng Y, Xu F, Liu C. Effects of surfactant and solvent on the encapsulation efficiency and size in using double emulsion method for preparing bovine hemoglobin loaded nanoparticles as blood substitutes. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009;26(1):116–21.Search in Google Scholar
[42] Assilova M, Mutaliyeva B, Tleukeyeva A. Design and assessment of microencapsulation systems for biologically active agents in homemade sparkling winemaking. Int J Innovative Res Sci Stud. 2025;8(1):1429–39.10.53894/ijirss.v8i1.4667Search in Google Scholar
[43] Kias F, Bodmeier R. Control of encapsulation efficiency and morphology of poly (lactide-co-glycolide) microparticles with a diafiltration-driven solvent extraction process. Eur J Pharm Biopharm. 2024;204:114515.10.1016/j.ejpb.2024.114515Search in Google Scholar PubMed
[44] Keskin M, Keskin Ş, Kolayli S. Preparation of alcohol free propolis-alginate microcapsules, characterization and release property. LWT. 2019;108:89–96, https://www.sciencedirect.com/science/article/pii/S0023643819302257.10.1016/j.lwt.2019.03.036Search in Google Scholar
[45] Guo YD, Su JF, Mu R, Wang XY, Zhang XL, Xie XM, et al. Microstructure and properties of self-assembly graphene microcapsules: Effect of the pH value. Nanomaterials. 2019;9(4):587.10.3390/nano9040587Search in Google Scholar PubMed PubMed Central
[46] Lombardo S, Villares A. Engineered multilayer microcapsules based on polysaccharides nanomaterials. Molecules. MDPI AG 2020;25(19):4420.10.3390/molecules25194420Search in Google Scholar PubMed PubMed Central
[47] Meskelis L F, Agondi R, Duarte LGR, de Carvalho MD, Sato ACK, Picone CSF. New approaches for modulation of alginate-chitosan delivery properties. Food Res Int. 2024;175:113737.10.1016/j.foodres.2023.113737Search in Google Scholar PubMed
[48] Simsek-Ege FA, Bond GM, Stringer J. Polyelectrolye complex formation between alginate and chitosan as a function of pH. J Appl Polym Sci. 2003;88(2):346–51.10.1002/app.11989Search in Google Scholar
[49] Brahmi M, Essifi K, Bakirhan NK, EL Bachiri A, Ouldriane SD, Tahani A. New insights into physicochemical aspects involved in the formation of chitosan@ alginate biobased polyelectrolyte complexes on natural montmorillonite clay surface. J Mol Liq. 2023;387:122635.10.1016/j.molliq.2023.122635Search in Google Scholar
[50] Guo Y, Van Ravensteijn BGP, Evers CHJ, Kegel WK. pH reversible encapsulation of oppositely charged colloids mediated by polyelectrolytes. Langmuir. 2017;33(18):4551–8.10.1021/acs.langmuir.7b00845Search in Google Scholar PubMed PubMed Central
[51] Liechty WB, Chen R, Farzaneh F, Tavassoli M, Slater NKH. Synthetic pH-responsive polymers for protein transduction. Adv Mater. 2009;21(38–39):3910.10.1002/adma.200901733Search in Google Scholar PubMed PubMed Central
[52] Singh J, Nayak P. pH‐responsive polymers for drug delivery: trends and opportunities. J Polym Sci. 2023;61(22):2828–50.10.1002/pol.20230403Search in Google Scholar
[53] Podgornik BB, Šandric S, Kert M. Microencapsulation for functional textile coatings with emphasis on biodegradability—a systematic review. Coatings. 2021;11(11):1371.10.3390/coatings11111371Search in Google Scholar
[54] Yun P, Devahastin S, Chiewchan N. Microstructures of encapsulates and their relations with encapsulation efficiency and controlled release of bioactive constituents: A review. Compr Rev Food Sci Food Saf. 2021;20(2):1768–99.10.1111/1541-4337.12701Search in Google Scholar PubMed
[55] Teunou E, Poncelet D. Batch and continuous fluid bed coating–review and state of the art. J Food Eng. 2002;53(4):325–40.10.1016/S0260-8774(01)00173-XSearch in Google Scholar
[56] Reineccius G, Patil S, Anantharamkrishnan V. Encapsulation of orange oil using fluidized bed granulation. Molecules. 2022;27(6):1854.10.3390/molecules27061854Search in Google Scholar PubMed PubMed Central
[57] Onwulata C, Smith PW, Craig Jr JC, Holsinger VH. Physical properties of encapsulated spray‐dried milkfat. J Food Sci. 1994;59(2):316–20.10.1111/j.1365-2621.1994.tb06957.xSearch in Google Scholar
[58] Guignon B, Duquenoy A, Dumoulin ED. Fluid bed encapsulation of particles: principles and practice. Dry Technol. 2002;20(2):419–47.10.1081/DRT-120002550Search in Google Scholar
[59] Heinrich D, Scholten H. Polyamide fluidized bed coating powder for fluidized thin-layer coating; US Pat., 7,951,416 B2, 2011.Search in Google Scholar
[60] Donida MW, Rocha SCS, Bartholomeu F. Influence of polymeric suspension characteristics on the particle coating in a spouted bed. Dry Technol. 2005;23(9–11):1811–23.10.1080/07373930500209905Search in Google Scholar
[61] Sang P, Chen LY, Zhao C, Wang ZX, Wang H, Lu S, et al. Particle size-dependent microstructure, hardness and electrochemical corrosion behavior of atmospheric plasma sprayed NiCrBSi coatings. Metals. 2019;9(12):1342.10.3390/met9121342Search in Google Scholar
[62] Benelli L, Oliveira WP. Fluidized bed coating of inert cores with a lipid-based system loaded with a polyphenol-rich Rosmarinus officinalis extract. Food Bioprod Process. 2019;114:216–26.10.1016/j.fbp.2019.01.004Search in Google Scholar
[63] Zhang R, Hoffmann T, Tsotsas E. Novel technique for coating of fine particles using fluidized bed and aerosol atomizer. Processes. 2020;8(12):1–18.10.3390/pr8121525Search in Google Scholar
[64] Kawakita R, Leveau JHJ, Jeoh T. Comparing fluidized bed spray-coating and spray-drying encapsulation of non-spore-forming gram-negative bacteria. Ind Biotechnol. 2021;17(5):283–9.10.1089/ind.2021.0019Search in Google Scholar
[65] Bautista-Villarreal M, Hernández SLC, López Uriarte S, González MPB. Encapsulation of lactiplantibacillus plantarum and beetroot extract with alginate and effect of capsules on rheological properties and stability of an oil-in-water emulsion model food. Pol J Food Nutr Sci. 2023;73(3):242–52.10.31883/pjfns/169729Search in Google Scholar
[66] Havaić T, Đumbir AM, Gretić M, Matijašić G, Žižek K. Droplet impact phenomena in fluidized bed coating process with a wurster insert. Int J Chem Eng. 2018;2018(1):4546230.10.1155/2018/4546230Search in Google Scholar
[67] Nascimento RF, de França PRL, Ferreira MA, Kurozawa LE. Assessment of the protective potential of coated microparticles in a fluidized bed against the simulated digestion. Food Res Int. 2025;208:116273, https://www.sciencedirect.com/science/article/pii/S0963996925006106.10.1016/j.foodres.2025.116273Search in Google Scholar PubMed
[68] Namrata G, Piyush T. Formulation & development of pellets of tolterodine tartrate: a qualitative study on wurster based fluidized bed coating technology. J Drug Delivery Ther. 2011;2012(4):90–6, http://jddtonline.info.Search in Google Scholar
[69] Smułek W, Jarzȩbski M. Hemp seed oil nanoemulsion with Sapindus saponins as a potential carrier for iron supplement and vitamin D. Rev Adv Mater Sci. 2023;62(1):20220317.10.1515/rams-2022-0317Search in Google Scholar
[70] Beldarrain-Iznaga T, Villalobos-Carvajal R, Leiva-Vega J, Sevillano Armesto E. Influence of multilayer mi;croencapsulation on the viability of Lactobacillus casei using a combined double emulsion and ionic gelation approach. Food Bioprod Process. 2020;124:57–71. 10.1016/j.fbp.2020.08.009 Search in Google Scholar
[71] Belščak-Cvitanovic A, Bušić A, Barišić L, Vrsaljko D, Karlović S, Špoljarić I, et al. Emulsion templated microencapsulation of dandelion (Taraxacum officinale L.) polyphenols and β-carotene by ionotropic gelation of alginate and pectin. Food Hydrocoll. 2016;57:139–52. 10.1016/j.foodhyd.2016.01.020.Search in Google Scholar
[72] Đorđević V, Balanč B, Belščak-Cvitanović A, Lević S, Trifković K, Kalušević A, et al. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng Rev. 2015;7:452–90.10.1007/s12393-014-9106-7Search in Google Scholar
[73] Shekhar C, Mehandia V, Sabapathy M. Single-step generation of double emulsions in aqueous two-phase systems. Phys Fluids. 2023;35(7):073109.10.1063/5.0153788Search in Google Scholar
[74] Ghiasi F, Hashemi H, Esteghlal S, Hosseini SMH. An updated comprehensive overview of different food applications of W1/O/W2 and O1/W/O2 double emulsions. Foods. 2024;13(3):485.10.3390/foods13030485Search in Google Scholar PubMed PubMed Central
[75] Mehmood T, Kaleem M, Ahmad Z, Waseem M, Ahmad B, Rehman MA, et al. Applications of nanoemulsions in food manufacturing. Handbook of research on nanoemulsion applications in agriculture, food, health, and biomedical sciences. IGI Global Scientific Publishing; 2022. p. 448–65.10.4018/978-1-7998-8378-4.ch020Search in Google Scholar
[76] Donsì F. Chapter 11 - applications of nanoemulsions in foods. In: Jafari SM, McClements DJ, editors. Nanoemulsions. Cambridge, MA, USA: Academic Press; 2018. p. 349–77. https://www.sciencedirect.com/science/article/pii/B9780128118382000114.10.1016/B978-0-12-811838-2.00011-4Search in Google Scholar
[77] Lee D, Weitz DA. Double emulsion‐templated nanoparticle colloidosomes with selective permeability. Adv Mater. 2008;20(18):3498–503.10.1002/adma.200800918Search in Google Scholar
[78] Milenkova S, Marudova M, Zahariev N, Yovcheva T, Pilicheva B. Crosslinked chitosan-based particles obtained by water-in-oil emulsion technique. J Phys: Conf Ser. 2023;2436(1):012027.10.1088/1742-6596/2436/1/012027Search in Google Scholar
[79] Piñón-Segundo E, G Nava-Arzaluz M, Lechuga-Ballesteros D. Pharmaceutical polymeric nanoparticles prepared by the double emulsion-solvent evaporation technique. Recent Pat Drug Delivery Formulation. 2012;6(3):224–35.10.2174/187221112802652606Search in Google Scholar PubMed
[80] Juttulapa M, Piriyaprasarth S, Takeuchi H, Sriamornsak P. Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J Pharm Sci. 2017;12(1):21–7.10.1016/j.ajps.2016.09.004Search in Google Scholar PubMed PubMed Central
[81] Douliez JP, Arlaut A, Beven L, Fameau AL, Saint-Jalmes A. One step generation of single-core double emulsions from polymer-osmose-induced aqueous phase separation in polar oil droplets. Soft Matter. 2023;19(39):7562–9.10.1039/D3SM00970JSearch in Google Scholar PubMed
[82] Dai H, Wu J, Zhang H, Chen Y, Ma L, Huang H, et al. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci Technol. 2020;102:16–29.10.1016/j.tifs.2020.05.016Search in Google Scholar
[83] Tavassoli M, Khezerlou A, Punia Bangar S, Bakhshizadeh M, Haghi PB, Moghaddam TN, et al. Functionality developments of Pickering emulsion in food packaging: Principles, applications, and future perspectives. Trends Food Sci Technol. Elsevier Ltd 2023;132:171–87.10.1016/j.tifs.2023.01.007Search in Google Scholar
[84] Lu Y, Mao L, Hou Z, Miao S, Gao Y. Development of emulsion gels for the delivery of functional food ingredients: From structure to functionality. Food Eng Rev. 2019;11:245–58.10.1007/s12393-019-09194-zSearch in Google Scholar
[85] Bortnowska G. Multilayer oil-in-water emulsions: formation, characteristics and application as the carriers for lipophilic bioactive food components - a review. Pol J Food Nutr Sci Pol Acad Sci. 2015;65:157–66.10.2478/v10222-012-0094-0Search in Google Scholar
[86] Talón E, Lampi AM, Vargas M, Chiralt A, Jouppila K, González-Martínez C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019;295:588–98.10.1016/j.foodchem.2019.05.115Search in Google Scholar PubMed
[87] Smułek W, Makiej A, Jarzȩbski M, Zdarta A, Jeszka-Skowron M, Ciesielczyk F, et al. Nanoemulsions of essential oils stabilized with saponins exhibiting antibacterial and antioxidative properties. Rev Adv Mater Sci. 2023;62(1):20220337.10.1515/rams-2022-0337Search in Google Scholar
[88] Ding, Z, Jiang, Y, Liu, X. Nanoemulsions-based drug delivery for brain tumors. Nanotechnology-based targeted drug delivery systems for brain tumors. Amsterdam, Netherlands: Elsevier; 2018. p. 327–58.10.1016/B978-0-12-812218-1.00012-9Search in Google Scholar
[89] Krstić M, Medarević Đ, Đuriš J, Ibrić S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. Lipid nanocarriers for drug targeting. Amsterdam, Netherlands: Elsevier; 2018. p. 473–508.10.1016/B978-0-12-813687-4.00012-8Search in Google Scholar
[90] Li Z, Kuang H, Yang J, Hu J, Ding B, Sun W, et al. Improving emulsion stability based on ovalbumin-carboxymethyl cellulose complexes with thermal treatment near ovalbumin isoelectric point. Sci Rep. 2020;10(1):3456.10.1038/s41598-020-60455-ySearch in Google Scholar PubMed PubMed Central
[91] Taboada ML, Schäfer A, Karbstein HP, Gaukel V. Oil droplet breakup during pressure swirl atomization of food emulsions: Influence of atomization pressure and initial oil droplet size. J Food Process Eng. 2021;44(1):e13598.10.1111/jfpe.13598Search in Google Scholar
[92] Adali MB, Barresi AA, Boccardo G, Pisano R. Spray freeze-drying as a solution to continuous manufacturing of pharmaceutical products in bulk. Processes. 2020;8(6):709.10.3390/pr8060709Search in Google Scholar
[93] Isleroglu H, Turker I. Particle morphology of spray-freeze dried microencapsulation agents. Int J Sci Technol Res. 2019;5(2):199–206.Search in Google Scholar
[94] Walton DE, Mumford CJ. Spray dried products—characterization of particle morphology. Chem Eng Res Des. 1999;77(1):21–38.10.1205/026387699525846Search in Google Scholar
[95] Beetz C, Schlipf DM, Li JZ. Spray dried powders. International Application published under the patent cooperation treaty (PCT), WO 2020/198297 A1, 2020. Search in Google Scholar
[96] Yang M, Li L, Zhu X, Liang L, Chen J, Cao W, et al. Microencapsulation of fish oil by spray drying, spray freeze‐drying, freeze‐drying, and microwave freeze‐drying: Microcapsule characterization and storage stability. J Food Sci. 2024;89(6):3276–89.10.1111/1750-3841.17098Search in Google Scholar PubMed
[97] Jordán-Suárez O, Glorio-Paulet P, Vidal L. Microstructure of Annona muricata L. leaves extract microcapsules linked to physical and chemical characteristics. J Encapsulation Adsorpt Sci. 2018;8(3):178–93.10.4236/jeas.2018.83009Search in Google Scholar
[98] Sukri N, Multisona RR, Zaida, Saputra RA, Mahani, Nurhadi B. Effect of maltodextrin and arabic gum ratio on physicochemical characteristic of spray dried propolis microcapsules. Int J Food Eng. 2021;17(2):159–65.10.1515/ijfe-2019-0050Search in Google Scholar
[99] Ramos F de M, Silveira Júnior V, Prata AS. Impact of vacuum spray drying on encapsulation of fish oil: Oxidative stability and encapsulation efficiency. Food Res Int. 2021;143:110283, https://www.sciencedirect.com/science/article/pii/S0963996921001824.10.1016/j.foodres.2021.110283Search in Google Scholar PubMed
[100] Pudziuvelyte L, Marksa M, Sosnowska K, Winnicka K, Morkuniene R, Bernatoniene J. Freeze-drying technique for microencapsulation of elsholtzia ciliata Ethanolic extract using different coating materials. Molecules. 2020;25(9):2237.10.3390/molecules25092237Search in Google Scholar PubMed PubMed Central
[101] Stabrauskiene J, Pudziuvelyte L, Bernatoniene J. Optimizing encapsulation: comparative analysis of spray-drying and freeze-drying for sustainable recovery of bioactive compounds from citrus x paradisi l. peels. Pharmaceuticals. 2024;17(5):596.10.3390/ph17050596Search in Google Scholar PubMed PubMed Central
[102] Ramakrishnan P, Dutta S, Moses, JA CA. Effect of high molecular weight maltodextrin and spray drying conditions for developing an encapsulated noni juice powder. ETP Int J Food Eng. 2019;5(2):92–8, http://www.ijfe.org/index.php?m=content&c=index&a=show&catid=131&id=639.10.18178/ijfe.5.2.92-98Search in Google Scholar
[103] Mohammed NK, Tan CP, Manap YA, Muhialdin BJ, Hussin ASM. Spray drying for the encapsulation of oils—a review. Molecules. 2020;25(17):3873.10.3390/molecules25173873Search in Google Scholar PubMed PubMed Central
[104] Patil AK. A review: spray dryer design and control system. J Pharm Negat Results. 2022;13:2022.Search in Google Scholar
[105] Kalita U, Jafari VF, Ashokkumar M, Singha NK, Qiao GG. Synthesis of ultra-high molecular weight homo- and copolymers via an ultrasonic emulsion process with a fast rate. Commun Chem. 2024;7(1):113.10.1038/s42004-024-01191-6Search in Google Scholar PubMed PubMed Central
[106] Subramani T, Ganapathyswamy H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J Food Sci Technol Springer. 2020;57:3545–55.10.1007/s13197-020-04360-2Search in Google Scholar PubMed PubMed Central
[107] Mishra H, Chauhan V, Kumar K, Teotia D. A comprehensive review on liposomes: a novel drug delivery system. J Drug Delivery Ther. 2018;8(6):400–4.10.22270/jddt.v8i6.2071Search in Google Scholar
[108] Abd El Kader AE, Abu Hashish HM. Encapsulation techniques of food bioproduct. Egypt J Chem. 2020;63(5):1881–909.Search in Google Scholar
[109] Padma Ishwarya S, Anandharamakrishnan C. Chap. 8-Liposomal encapsulation of natural color, flavor, and additives for the food industry. Liposomal encapsulation in food science and technology. Cambridge, MA, USA: Academic Press; 2023.10.1016/B978-0-12-823935-3.00001-1Search in Google Scholar
[110] Aragão TH, Feitosa V, Adelnia H. Liposomes: methods and protocols. Liposomes funct foods nutraceuticals. Oakville, Canada; Waretown, USA.: Apple Academic Press; 2022. p. 1–40.10.1201/9781003277361-1Search in Google Scholar
[111] Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394.10.1016/j.heliyon.2022.e09394Search in Google Scholar PubMed PubMed Central
[112] Koehler JK, Gedda L, Wurster L, Schnur J, Edwards K, Heerklotz H, et al. Tailoring the lamellarity of liposomes prepared by dual centrifugation. Pharmaceutics. 2023;15(2):706.10.3390/pharmaceutics15020706Search in Google Scholar PubMed PubMed Central
[113] Giuliano CB, Cvjetan N, Ayache J, Walde P. Multivesicular vesicles: preparation and applications. ChemSystemsChem. 2021;3(2):e2000049.10.1002/syst.202000049Search in Google Scholar
[114] Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev. 2018;47(23):8572–610.10.1039/C8CS00162FSearch in Google Scholar
[115] Pande S. Liposomes for drug delivery: review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artificial cells, nanomedicine and biotechnology. Vol. 51, Taylor and Francis Ltd 2023. p. 428–40.10.1080/21691401.2023.2247036Search in Google Scholar PubMed
[116] Pasarin D, Ghizdareanu AI, Enascuta CE, Matei CB, Bilbie C, Paraschiv-Palada L, et al. Coating materials to increase the stability of liposomes. Polymers. 2023;15(3):782.10.3390/polym15030782Search in Google Scholar PubMed PubMed Central
[117] Gonzalez Gomez A, Syed S, Marshall K, Hosseinidoust Z. Liposomal nanovesicles for efficient encapsulation of staphylococcal antibiotics. ACS Omega. 2019;4(6):10866–76.10.1021/acsomega.9b00825Search in Google Scholar PubMed PubMed Central
[118] Azad AK, Lai J, Sulaiman WMAW, Almoustafa H, Alshehade SA, Kumarasamy V, et al. The fabrication of polymer-based curcumin-loaded formulation as a drug delivery system: an updated review from 2017 to the present. Pharmaceutics. 2024;16(2):160.10.3390/pharmaceutics16020160Search in Google Scholar PubMed PubMed Central
[119] Detsi A, Kavetsou E, Kostopoulou I, Pitterou I, Pontillo ARN, Tzani A, et al. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics. MDPI AG 2020;12:1–68.10.3390/pharmaceutics12070669Search in Google Scholar PubMed PubMed Central
[120] Napiórkowska A, Szpicer A, Wojtasik-Kalinowska I, Perez MDT, González HD, Kurek MA. Microencapsulation of juniper and black pepper essential oil using the coacervation method and its properties after freeze-drying. Foods. 2023;12(23):4345.10.3390/foods12234345Search in Google Scholar PubMed PubMed Central
[121] Rawat K. Coacervation in Biopolymers. J Phys Chem Biophys. 2014;4(6):15610.4172/2161-0398.1000165Search in Google Scholar
[122] Napiórkowska A, Kurek M. Coacervation as a novel method of microencapsulation of essential oils—a review. Molecules. 2022;27(16):5142.10.3390/molecules27165142Search in Google Scholar PubMed PubMed Central
[123] Auriemma G, Russo P, Del Gaudio P, García-González CA, Landín M, Aquino RP. Technologies and formulation design of polysaccharide-based hydrogels for drug delivery. Molecules. 2020;25(14):3156.10.3390/molecules25143156Search in Google Scholar PubMed PubMed Central
[124] Lavrentev FV, Shilovskikh VV, Alabusheva VS, Yurova VY, Nikitina AA, Ulasevich SA, et al. Diffusion-limited processes in hydrogels with chosen applications from drug delivery to electronic components. Molecules. 2023;28(15):5931.10.3390/molecules28155931Search in Google Scholar PubMed PubMed Central
[125] Gadziński P, Froelich A, Jadach B, Wojtyłko M, Tatarek A, Białek A, et al. Ionotropic gelation and chemical crosslinking as methods for fabrication of modified-release gellan gum-based drug delivery systems. Pharmaceutics. 2023;15(1):108.10.3390/pharmaceutics15010108Search in Google Scholar PubMed PubMed Central
[126] Jeoh T, Wong DE, Strobel SA, Hudnall K, Pereira NR, Williams KA, et al. How alginate properties influence in situ internal gelation in crosslinked alginate microcapsules (CLAMs) formed by spray drying. PLoS One. 2021;16(2):e0247171.10.1371/journal.pone.0247171Search in Google Scholar PubMed PubMed Central
[127] Song H, Yu W, Gao M, Liu X, Ma X. Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydr Polym. 2013;96(1):181–9, https://www.sciencedirect.com/science/article/pii/S0144861713003184.10.1016/j.carbpol.2013.03.068Search in Google Scholar PubMed
[128] Ferraraccio LS, Di Lisa D, Pastorino L, Bertoncello P. Enzymes encapsulated within alginate hydrogels: bioelectrocatalysis and electrochemiluminescence applications. Anal Chem. 2022;94(46):16122–31.10.1021/acs.analchem.2c03389Search in Google Scholar PubMed PubMed Central
[129] Shu J, McClements DJ, Luo S, Ye J, Liu C. Effect of internal and external gelation on the physical properties, water distribution, and lycopene encapsulation properties of alginate-based emulsion gels. Food Hydrocoll. 2023;139:108499.10.1016/j.foodhyd.2023.108499Search in Google Scholar
[130] Dima C, Dima S. Bioaccessibility study of calcium and vitamin D3 co-microencapsulated in water-in-oil-in-water double emulsions. Food Chem. 2020;303:125416.10.1016/j.foodchem.2019.125416Search in Google Scholar PubMed
[131] Celli GB, Teixeira AG, Duke TG, Brooks MSL. Encapsulationof lycopene from watermelon in calcium-alginate microparticles using an optimised inverse-gelation method by response surface methodology. Int J Food Sci Technol. 2016;51(6):1523–9.10.1111/ijfs.13114Search in Google Scholar
[132] Palamutoğlu R, Kasnak C, Özen B. Encapsulation of black cumin seed (Nigella sativa) oil by using inverse gelation method. Food Hydrocoll Health. 2022;2:100089.10.1016/j.fhfh.2022.100089Search in Google Scholar
[133] Sy C, Gleize B, Dangles O, Landrier JF, Veyrat CC, Borel P. Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood and tissue concentrations. Mol Nutr Food Res. 2012;56(9):1385–97.10.1002/mnfr.201200041Search in Google Scholar PubMed
[134] Vaezifar S, Razavi S, Golozar MA, Karbasi S, Morshed M, Kamali M. Effects of some parameters on particle size distribution of chitosan nanoparticles prepared by ionic gelation method. J Clust Sci. 2013;24(3):891–903.10.1007/s10876-013-0583-2Search in Google Scholar
[135] Alehosseini E, Shahiri Tabarestani H, Kharazmi MS, Jafari SM. Physicochemical, thermal, and morphological properties of chitosan nanoparticles produced by ionic gelation. Foods. 2022;11(23):3841.10.3390/foods11233841Search in Google Scholar PubMed PubMed Central
[136] Furtado GTF da S, Fideles TB, Cruz R de CAL, Souza JW de L, Rodriguez Barbero MA, Fook MVL. Chitosan/NaF particles prepared via ionotropic gelation: evaluation of particles size and morphology. Mater Res. 2018;21(4):e20180101.10.1590/1980-5373-mr-2018-0101Search in Google Scholar
[137] Moawad S, El-Kalyoubi MH, Khallaf MF, Gawad RA, Saed B, Farouk A. Microencapsulation by coacervation: Physicochemical and sensory properties of food flavorings. Foods Raw Mater. 2025;13(1):73–81.10.21603/2308-4057-2025-1-624Search in Google Scholar
[138] Peanparkdee M, Iwamoto S. Encapsulation for Improving in vitro Gastrointestinal Digestion of Plant Polyphenols and Their Applications in Food Products. Food Rev Int. 2022;38(4):335–53.10.1080/87559129.2020.1733595Search in Google Scholar
[139] Gaigher B, do Nascimento da Silva E, Lacerda Sanches V, Fernanda Milani R, Galland F, Cadore S, et al. Formulations with microencapsulated Fe–peptides improve in vitro bioaccessibility and bioavailability. Curr Res Food Sci. 2022;5:687–97.10.1016/j.crfs.2022.03.007Search in Google Scholar PubMed PubMed Central
[140] Dima C, Assadpour E, Dima S, Jafari SM. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr Rev Food Sci Food Saf. 2020;19(3):954–94.10.1111/1541-4337.12547Search in Google Scholar PubMed
[141] Essifi K, Brahmi M, Berraaouan D, Ed-Daoui A, El Bachiri A, Fauconnier ML, et al. Influence of sodium alginate concentration on microcapsules properties foreseeing the protection and controlled release of bioactive substances. J Chem. 2021;2021(1):5531479.10.1155/2021/5531479Search in Google Scholar
[142] Parada J, Aguilera JM. Food microstructure affects the bioavailability of several nutrients. J Food Sci. 2007;72(2):R21–32.10.1111/j.1750-3841.2007.00274.xSearch in Google Scholar PubMed
[143] Yang L, Li F, Cao X, Qiao X, Xue C, Xu J. Stability and bioavailability of protein matrix-encapsulated astaxanthin ester microcapsules. J Sci Food Agric. 2022;102(5):2144–52.10.1002/jsfa.11556Search in Google Scholar PubMed
[144] Lin Q, Liang R, Williams PA, Zhong F. Factors affecting the bioaccessibility of β-carotene in lipid-based microcapsules: Digestive conditions, the composition, structure and physical state of microcapsules. Food Hydrocoll. 2018;77:187–203.10.1016/j.foodhyd.2017.09.034Search in Google Scholar
[145] Vasisht, N. Chapter 4- Applications of mass and heat transfer in microencapsulation processes. Sobel RBTM in the FI. Cambridge, MA, USA: Academic Press; 2023. p. 39–46.10.1016/B978-0-12-821683-5.00006-6Search in Google Scholar
[146] Wang Y, Cheng Q, Liu J, Tariq Z, Zheng Z, Li G, et al. Tuning microcapsule shell thickness and structure with silk fibroin and nanoparticles for sustained release. ACS Biomater Sci Eng. 2020;6(8):4583–94.10.1021/acsbiomaterials.0c00835Search in Google Scholar PubMed
[147] Jurić S, Šegota S, Vinceković M. Influence of surface morphology and structure of alginate microparticles on the bioactive agents release behavior. Carbohydr Polym. 2019;218(May):234–42.10.1016/j.carbpol.2019.04.096Search in Google Scholar PubMed
[148] Hacene YC, Singh A, Van den Mooter G. Drug loaded and ethylcellulose coated mesoporous silica for controlled drug release prepared using a pilot scale fluid bed system. Int J Pharm. 2016;506(1–2):138–47.10.1016/j.ijpharm.2016.04.047Search in Google Scholar PubMed
[149] Nájera-Martínez EF, Flores-Contreras EA, Araújo RG, Iñiguez-Moreno M, Sosa-Hernández JE, Iqbal HMN, et al. Microencapsulation of gallic acid based on a polymeric and ph-sensitive matrix of pectin/alginate. Polymer. 2023;15(14):3014.10.3390/polym15143014Search in Google Scholar PubMed PubMed Central
[150] Baghi F, Ghnimi S, Dumas E, Gharsallaoui A. Microencapsulation of antimicrobial trans-cinnamaldehyde: effect of emulsifier type, pH, and drying technique. Appl Sci. 2023;13(10):6184.10.3390/app13106184Search in Google Scholar
[151] Ogilvie-Battersby JD, Nagarajan R, Mosurkal R, Orbey N. Microencapsulation and controlled release of insect repellent geraniol in gelatin/gum arabic microcapsules. Colloids Surf A Physicochem Eng Asp. 2022;640:128494.10.1016/j.colsurfa.2022.128494Search in Google Scholar
[152] Lavelli V, Sri Harsha PSC. Microencapsulation of grape skin phenolics for pH controlled release of antiglycation agents. Food Res Int. 2019;119:822–8.10.1016/j.foodres.2018.10.065Search in Google Scholar PubMed
[153] Abbaspourrad A, Datta SS, Weitz DA. Controlling release from pH-responsive microcapsules. Langmuir. 2013;29(41):12697–702.10.1021/la403064fSearch in Google Scholar PubMed
[154] Gaonkar A, Vasisht N, Kharre A, Sobel R. Microencapsulation in the Food Industry: A Practical Implementation Guide. San Diego, USA: Academic Press; 2014. 10.1016/C2012-0-00852-6.Search in Google Scholar
[155] Gibbs BF, Kermasha S, Alli I, Mulligan CN. Encapsulation in the food industry: a review. Int J Food Sci Nutr. 1999;50(3):213–24.10.1080/096374899101256Search in Google Scholar PubMed
[156] Kłosowska A, Wawrzyńczak A, Feliczak-Guzik A. Microencapsulation as a route for obtaining encapsulated flavors and fragrances. Cosmetics. 2023;10(1):1–18.10.3390/cosmetics10010026Search in Google Scholar
[157] Vakarelova M, Zanoni F, Donà G, Fierri I, Chignola R, Gorrieri S, et al. Microencapsulation of astaxanthin by ionic gelation: effect of different gelling polymers on the carotenoid load, stability and bioaccessibility. Int J Food Sci Technol. 2023;58(5):2489–97.10.1111/ijfs.16389Search in Google Scholar
[158] Zhu Y, Li S, Wang H, Adu-Frimpong M, Xue Y, Gu Z, et al. Enhanced oral bioavailability of capsaicin-loaded microencapsulation complex via electrospray technology: Preparation, in vitro and in vivo evaluation. Biopharm Drug Dispos. 2023;44(2):137–46.10.1002/bdd.2355Search in Google Scholar PubMed
[159] Dobroslavić E, Elez Garofulić I, Zorić Z, Pedisić S, Roje M, Dragović-Uzelac V. Physicochemical properties, antioxidant capacity, and bioavailability of laurus nobilis l. leaf polyphenolic extracts microencapsulated by spray drying. Foods. 2023;12(9):1923.10.3390/foods12091923Search in Google Scholar PubMed PubMed Central
[160] Mihaly Cozmuta A, Purbayanto MAK, Jastrzębska A, Peter A, Nicula C, Uivarasan A, et al. Thermal stability and in vitro digestion of alginate–starch–iron beads for oral delivery of iron. Food Hydrocoll. 2023;142:108808.10.1016/j.foodhyd.2023.108808Search in Google Scholar
[161] Tchabo W, Kaptso GK, Ngolong Ngea GL, Wang K, Bao G, Ma Y, et al. In vitro assessment of the effect of microencapsulation techniques on the stability, bioaccessibility and bioavailability of mulberry leaf bioactive compounds. Food Biosci. 2022;47(November 2021):101461.10.1016/j.fbio.2021.101461Search in Google Scholar
[162] Pan LH, Chen LP, Wu CL, Wang JF, Luo SZ, Luo JP, et al. Microencapsulation of blueberry anthocyanins by spray drying with soy protein isolates/high methyl pectin combination: Physicochemical properties, release behavior in vitro and storage stability. Food Chem. 2022;395(July):133626.10.1016/j.foodchem.2022.133626Search in Google Scholar PubMed
[163] Li C, Li Z, Liu T, Gu Z, Ban X, Tang X, et al. Encapsulating tributyrin during enzymatic cyclodextrin synthesis improves the solubility and bioavailability of tributyrin. Food Hydrocoll. 2021;113:106512.10.1016/j.foodhyd.2020.106512Search in Google Scholar
[164] Fard SG, Loh SP, Turchini GM, Wang B, Elliott G, Sinclair AJ. Microencapsulated tuna oil results in higher absorption of DHA in toddlers. Nutrients. 2020;12(1):1–15.10.3390/nu12010248Search in Google Scholar PubMed PubMed Central
[165] Li Q, Li X, Zhao C. Strategies to obtain encapsulation and controlled release of small hydrophilic molecules. Front Bioeng Biotechnol. 2020;8:437.10.3389/fbioe.2020.00437Search in Google Scholar PubMed PubMed Central
[166] Ryu S, Park S, Lee HY, Lee H, Cho CW, Baek JS. Biodegradable nanoparticles-loaded plga microcapsule for the enhanced encapsulation efficiency and controlled release of hydrophilic drug. Int J Mol Sci. 2021;22(6):1–11.10.3390/ijms22062792Search in Google Scholar PubMed PubMed Central
[167] Pramanik SK, Seneca S, Peters M, D’Olieslaeger L, Reekmans G, Vanderzande D, et al. Morphology-dependent pH-responsive release of hydrophilic payloads using biodegradable nanocarriers. RSC Adv. 2018;8(64):36869–78.10.1039/C8RA07066KSearch in Google Scholar PubMed PubMed Central
[168] Abbasi S, Sabahi A, Shahbazi N, Karimi A, Barkhordari H. Nano lipid carrier in augmenting the bioavailability of functional bio-compounds. Biointerface Res Appl Chem. 2023;13:1–14.10.33263/BRIAC131.051Search in Google Scholar
[169] Perera KY, Pradhan D, Sharma S, Jaiswal AK. Jaiswal S. Design and Use of Lipid‐Based Systems for Food Component Encapsulation. Materials Science and Engineering in Food Product. Development. New York, United States: John Wiley & Sons Inc.; 2023; p. 117–38. 10.1002/9781119860594.ch7.Search in Google Scholar
[170] Barroso L, Viegas C, Vieira J, Ferreira-Pêgo C, Costa J, Fonte P. Lipid-based carriers for food ingredients delivery. J Food Eng. 2021;295:110451.10.1016/j.jfoodeng.2020.110451Search in Google Scholar
[171] Shishir MRI, Xie L, Sun C, Zheng X, Chen W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci Technol. 2018;78(May):34–60.10.1016/j.tifs.2018.05.018Search in Google Scholar
[172] Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomedicine Pharmacother. 2018;103:598–613.10.1016/j.biopha.2018.04.055Search in Google Scholar PubMed
[173] Jones D, Caballero S, Davidov-pardo G. Bioavailability of nanotechnology- based bioactives and nutraceuticals. Food Applications of Nanotechnology. vol. 88, 1st edn. Amsterdam, Netherlands: Elsevier Inc; 2019. p. 235273.10.1016/bs.afnr.2019.02.014Search in Google Scholar PubMed
[174] Wang Y, Liu K, Lu M, Shi J, Xu Y, Liu Y. Comparative evaluation of static and dynamic simulated digestion models. J Sci Food Agric. 2023.;103(12):5893–903.10.1002/jsfa.12692Search in Google Scholar PubMed
[175] Morzel M, Ramsamy S, Le Feunteun S. Feasibility of using a realistic food bolus for semi-dynamic in vitro gastric digestion of hard cheese with pH-stat monitoring of protein hydrolysis. Food Res Int. 2023;169:112818.10.1016/j.foodres.2023.112818Search in Google Scholar PubMed
[176] Pereira E, Fernandes JM, Gonçalves R, Pinheiro AC, Salomé Duarte M, Madalena Alves M, et al. Evaluating the in vitro digestion of lipids rich in medium-chain fatty acids (MCFAs) using dynamic and static protocols. Food Chem. 2023;406:135080.10.1016/j.foodchem.2022.135080Search in Google Scholar PubMed
[177] Li Y, Xu R, Xiu H, Feng J, Park HJ, Prabhakar H, et al. Effect of cinnamon on starch hydrolysis of rice pudding: Comparing static and dynamic in vitro digestion models. Food Res Int. 2022;161:111813.10.1016/j.foodres.2022.111813Search in Google Scholar PubMed
[178] Verkempinck SHE, Duijsens D, Michels D, Guevara-Zambrano JM, Infantes-Garcia MR, Pälchen K, et al. Studying semi-dynamic digestion kinetics of food: Establishing a computer-controlled multireactor approach. Food Res Int. 2022;156:111301.10.1016/j.foodres.2022.111301Search in Google Scholar PubMed
[179] Chernukha IM, Meliashchenia AV, Kaltovich IV, Vasilevskaya ER, Aryzina MA, Smaliak TM, et al. Evolution of in vitro digestibility techniques: A systematic review. Teopия и пpaктикa пepepaбoтки мяca. 2021;6(4):300–10.10.21323/2414-438X-2021-6-4-300-310Search in Google Scholar
[180] Chima B, Mathews P, Morgan S, Johnson SA, Van Buiten CB. Physicochemical characterization of interactions between blueberry polyphenols and food proteins from dairy and plant sources. Foods. 2022;11(18):2846.10.3390/foods11182846Search in Google Scholar PubMed PubMed Central
[181] Alminger M, Aura A, Bohn T, Dufour C, El SN, Gomes A, et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr Rev Food Sci Food Saf. 2014;13(4):413–36.10.1111/1541-4337.12081Search in Google Scholar PubMed
[182] Zhang K, Huang J, Wang D, Wan X, Wang Y. Covalent polyphenols-proteins interactions in food processing: formation mechanisms, quantification methods, bioactive effects, and applications. Front Nutr. 2024;11:1371401.10.3389/fnut.2024.1371401Search in Google Scholar PubMed PubMed Central
[183] Czubinski J, Dwiecki K. A review of methods used for investigation of protein–phenolic compound interactions. Int J Food Sci Technol. Blackwell Publishing Ltd 2017;52:573–85.10.1111/ijfs.13339Search in Google Scholar
[184] Vergara C, Pino MT, Zamora O, Parada J, Pérez R, Uribe M, et al. Microencapsulation of anthocyanin extracted from purple flesh cultivated potatoes by spray drying and its effects on in vitro gastrointestinal digestion. Molecules. 2020;25(3):722.10.3390/molecules25030722Search in Google Scholar PubMed PubMed Central
[185] Fernandes A, Rocha MAA, Santos LMNBF, Brás J, Oliveira J, Mateus N, et al. Blackberry anthocyanins : β -Cyclodextrin forti fi cation for thermal and gastrointestinal stabilization. 2018;245(September 2017):426–31.Search in Google Scholar
[186] Panwar R, Raghuwanshi N, Srivastava AK, Sharma AK, Pruthi V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater Sci Eng C. 2018;92(June):381–92.10.1016/j.msec.2018.06.055Search in Google Scholar PubMed
[187] Gomes JFPS, Rocha S, Pereira M do C, Peres I, Moreno S, Toca-Herrera J, et al. Lipid/particle assemblies based on maltodextrin–gum arabic core as bio-carriers. Colloids Surf B Biointerfaces. 2010;76(2):449–55.10.1016/j.colsurfb.2009.12.004Search in Google Scholar PubMed
[188] Gomes Sá SH, Chalella Mazzocato M, Saliba ASMC, Alencar SM, Sílvia Favaro-Trindade C. Evaluation of the release, stability and antioxidant activity of Brazilian red propolis extract encapsulated by spray-drying, spray-chilling and using the combination of both techniques. Food Res Int. 2023;164:112423, https://www.sciencedirect.com/science/article/pii/S0963996922014818.10.1016/j.foodres.2022.112423Search in Google Scholar PubMed
[189] Ștefănescu BE, Nemes SA, Teleky BE, Călinoiu LF, Mitrea L, Martău GA, et al. Microencapsulation and Bioaccessibility of phenolic compounds of vaccinium leaf extracts. Antioxidants. 2022;11(4):674.10.3390/antiox11040674Search in Google Scholar PubMed PubMed Central
[190] Wongverawattanakul C, Suklaew PO, Chusak C, Adisakwattana S, Thilavech T. Encapsulation of mesona chinensis benth extract in alginate beads enhances the stability and antioxidant activity of polyphenols under simulated gastrointestinal digestion. Foods. 2022;11(15):2378.10.3390/foods11152378Search in Google Scholar PubMed PubMed Central
[191] Huang Y, Zhou W. Microencapsulation of anthocyanins through two-step emulsification and release characteristics during in vitro digestion. Food Chem. 2019;278(November 2018):357–63.10.1016/j.foodchem.2018.11.073Search in Google Scholar PubMed
[192] Jayan H, Maria Leena M, Sivakama Sundari SK, Moses JA, Anandharamakrishnan C. Improvement of bioavailability for resveratrol through encapsulation in zein using electrospraying technique. J Funct Foods. 2019;57(April):417–24.10.1016/j.jff.2019.04.007Search in Google Scholar
[193] Peñalva R, Esparza I, Morales-Gracia J, González-Navarro CJ, Larrañeta E, Irache JM. Casein nanoparticles in combination with 2-hydroxypropyl-β-cyclodextrin improves the oral bioavailability of quercetin. Int J Pharm. 2019;570(August):118652.10.1016/j.ijpharm.2019.118652Search in Google Scholar PubMed
[194] Fernandes A, Rocha MAA, Santos LMNBF, Brás J, Oliveira J, Mateus N, et al. Blackberry anthocyanins: β-Cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018;245:426–31.10.1016/j.foodchem.2017.10.109Search in Google Scholar PubMed
[195] Motilva MJ, Serra A, Rubió L. Nutrikinetic studies of food bioactive compounds: From in vitro to in vivo approaches. Int J Food Sci Nutr. 2015;66:S41–52.10.3109/09637486.2015.1025721Search in Google Scholar PubMed
[196] EFSA NDA. Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA)), 2011. Scientific Opinion on the safety of’Glavonoid®’, an extract derived from the roots or rootstock of Glycyrrhiza glabra L., as a Novel Food ingredient. EFSA Journal 2011; 9 (7): 2287, 27 pp. 2011.10.2903/j.efsa.2011.2287Search in Google Scholar
[197] Tong Y, Li L, Meng X. Anthocyanins from aronia melanocarpa bound to amylopectin nanoparticles: tissue distribution and in vivo oxidative damage protection. J Agric Food Chem. 2022;71(1):430–42.10.1021/acs.jafc.2c06115Search in Google Scholar PubMed
[198] Cian RE, Oliva ME, Garzón AG, Ferreira M del R, Alessandro D´, Drago ME, SR. In vitro and in vivo antithrombotic and antioxidant properties of microencapsulated brewers’ spent grain peptides. Int J Food Sci Technol. 2022;57(6):3872–9.Search in Google Scholar
[199] Cian E, Oliva E, Garz AG, Alessandro ED, Drago SR. In vitro and in vivo antithrombotic and antioxidant properties of microencapsulated brewers’ spent grain peptides. Int J Food Sci Technol. 2022;57(6):3872–9.10.1111/ijfs.15717Search in Google Scholar
[200] Baksi R, Singh DP, Borse SP, Rana R, Sharma V, Nivsarkar M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed Pharmacother. 2018;106(April):1513–26.10.1016/j.biopha.2018.07.106Search in Google Scholar PubMed
[201] Cui F, Zhang H, Wang D, Tan X, Li X, Li Y, et al. Advances in the preparation and application of microencapsulation to protect food functional ingredients. Food Funct. 2023;14(15):6766–83.10.1039/D3FO01077ESearch in Google Scholar PubMed
[202] Ayoub A, Sood M, Singh J, Bandral JD, Gupta N, Bhat A. Microencapsulation and its applications in food industry. J Pharmacogn Phytochem. 2019;8(3):32–7.10.22271/chemi.2020.v8.i3l.9317Search in Google Scholar
[203] Yan C, Kim SR, Ruiz DR, Farmer JR. Microencapsulation for food applications: A review. ACS Appl Bio Mater. 2022;5(12):5497–512.10.1021/acsabm.2c00673Search in Google Scholar PubMed
[204] Habibi A, Keramat J, Hojjatoleslamy M, Tamjidi F. Preparation of fish oil microcapsules by complex coacervation of gelatin–gum arabic and their utilization for fortification of pomegranate juice. J Food Process Eng. 2017;40(2):e12385.10.1111/jfpe.12385Search in Google Scholar
[205] Shehata MG, Abd-Rabou HS, El-Sohaimy SA. Plant Extracts in probiotic encapsulation: evaluation of their effects on strains survivability in juice and drinkable yogurt during storage and an in-vitro gastrointestinal model. J Pure Appl Microbiol. 2019;13(1).10.22207/JPAM.13.1.70Search in Google Scholar
[206] Peanparkdee M, Iwamoto S, Yamauchi R. Microencapsulation: a review of applications in the food and pharmaceutical industries. Rev Agric Sci. 2016;4:56–65.10.7831/ras.4.56Search in Google Scholar
[207] Papillo VA, Locatelli M, Travaglia F, Bordiga M, Garino C, Arlorio M, et al. Spray-dried polyphenolic extract from Italian black rice (Oryza sativa L., var. Artemide) as new ingredient for bakery products. Food Chem. 2018;269:603–9.10.1016/j.foodchem.2018.07.059Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- MHD radiative mixed convective flow of a sodium alginate-based hybrid nanofluid over a convectively heated extending sheet with Joule heating
- Experimental study of mortar incorporating nano-magnetite on engineering performance and radiation shielding
- Multicriteria-based optimization and multi-variable non-linear regression analysis of concrete containing blends of nano date palm ash and eggshell powder as cementitious materials
- A promising Ag2S/poly-2-amino-1-mercaptobenzene open-top spherical core–shell nanocomposite for optoelectronic devices: A one-pot technique
- Biogenic synthesized selenium nanoparticles combined chitosan nanoparticles controlled lung cancer growth via ROS generation and mitochondrial damage pathway
- Fabrication of PDMS nano-mold by deposition casting method
- Stimulus-responsive gradient hydrogel micro-actuators fabricated by two-photon polymerization-based 4D printing
- Physical aspects of radiative Carreau nanofluid flow with motile microorganisms movement under yield stress via oblique penetrable wedge
- Effect of polar functional groups on the hydrophobicity of carbon nanotubes-bacterial cellulose nanocomposite
- Review in green synthesis mechanisms, application, and future prospects for Garcinia mangostana L. (mangosteen)-derived nanoparticles
- Entropy generation and heat transfer in nonlinear Buoyancy–driven Darcy–Forchheimer hybrid nanofluids with activation energy
- Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
- A numerical analysis of heat and mass transfer in water-based hybrid nanofluid flow containing copper and alumina nanoparticles over an extending sheet
- Investigating the behaviour of electro-magneto-hydrodynamic Carreau nanofluid flow with slip effects over a stretching cylinder
- Electrospun thermoplastic polyurethane/nano-Ag-coated clear aligners for the inhibition of Streptococcus mutans and oral biofilm
- Investigation of the optoelectronic properties of a novel polypyrrole-multi-well carbon nanotubes/titanium oxide/aluminum oxide/p-silicon heterojunction
- Novel photothermal magnetic Janus membranes suitable for solar water desalination
- Green synthesis of silver nanoparticles using Ageratum conyzoides for activated carbon compositing to prepare antimicrobial cotton fabric
- Activation energy and Coriolis force impact on three-dimensional dusty nanofluid flow containing gyrotactic microorganisms: Machine learning and numerical approach
- Machine learning analysis of thermo-bioconvection in a micropolar hybrid nanofluid-filled square cavity with oxytactic microorganisms
- Research and improvement of mechanical properties of cement nanocomposites for well cementing
- Thermal and stability analysis of silver–water nanofluid flow over unsteady stretching sheet under the influence of heat generation/absorption at the boundary
- Cobalt iron oxide-infused silicone nanocomposites: Magnetoactive materials for remote actuation and sensing
- Magnesium-reinforced PMMA composite scaffolds: Synthesis, characterization, and 3D printing via stereolithography
- Bayesian inference-based physics-informed neural network for performance study of hybrid nanofluids
- Numerical simulation of non-Newtonian hybrid nanofluid flow subject to a heterogeneous/homogeneous chemical reaction over a Riga surface
- Enhancing the superhydrophobicity, UV-resistance, and antifungal properties of natural wood surfaces via in situ formation of ZnO, TiO2, and SiO2 particles
- Synthesis and electrochemical characterization of iron oxide/poly(2-methylaniline) nanohybrids for supercapacitor application
- Impacts of double stratification on thermally radiative third-grade nanofluid flow on elongating cylinder with homogeneous/heterogeneous reactions by implementing machine learning approach
- Synthesis of Cu4O3 nanoparticles using pumpkin seed extract: Optimization, antimicrobial, and cytotoxicity studies
- Cationic charge influence on the magnetic response of the Fe3O4–[Me2+ 1−y Me3+ y (OH2)] y+(Co3 2−) y/2·mH2O hydrotalcite system
- Pressure sensing intelligent martial arts short soldier combat protection system based on conjugated polymer nanocomposite materials
- Magnetohydrodynamics heat transfer rate under inclined buoyancy force for nano and dusty fluids: Response surface optimization for the thermal transport
- Fly ash and nano-graphene enhanced stabilization of engine oil-contaminated soils
- Enhancing natural fiber-reinforced biopolymer composites with graphene nanoplatelets: Mechanical, morphological, and thermal properties
- Performance evaluation of dual-scale strengthened co-bonded single-lap joints using carbon nanotubes and Z-pins with ANN
- Computational works of blood flow with dust particles and partially ionized containing tiny particles on a moving wedge: Applications of nanotechnology
- Hybridization of biocomposites with oil palm cellulose nanofibrils/graphene nanoplatelets reinforcement in green epoxy: A study of physical, thermal, mechanical, and morphological properties
- Design and preparation of micro-nano dual-scale particle-reinforced Cu–Al–V alloy: Research on the aluminothermic reduction process
- Spectral quasi-linearization and response optimization on magnetohydrodynamic flow via stenosed artery with hybrid and ternary solid nanoparticles: Support vector machine learning
- Ferrite/curcumin hybrid nanocomposite formulation: Physicochemical characterization, anticancer activity, and apoptotic and cell cycle analyses in skin cancer cells
- Enhanced therapeutic efficacy of Tamoxifen against breast cancer using extra virgin olive oil-based nanoemulsion delivery system
- A titanium oxide- and silver-based hybrid nanofluid flow between two Riga walls that converge and diverge through a machine-learning approach
- Enhancing convective heat transfer mechanisms through the rheological analysis of Casson nanofluid flow towards a stagnation point over an electro-magnetized surface
- Intrinsic self-sensing cementitious composites with hybrid nanofillers exhibiting excellent piezoresistivity
- Research on mechanical properties and sulfate erosion resistance of nano-reinforced coal gangue based geopolymer concrete
- Impact of surface and configurational features of chemically synthesized chains of Ni nanostars on the magnetization reversal process
- Porous sponge-like AsOI/poly(2-aminobenzene-1-thiol) nanocomposite photocathode for hydrogen production from artificial and natural seawater
- Multifaceted insights into WO3 nanoparticle-coupled antibiotics to modulate resistance in enteric pathogens of Houbara bustard birds
- Synthesis of sericin-coated silver nanoparticles and their applications for the anti-bacterial finishing of cotton fabric
- Enhancing chloride resistance of freeze–thaw affected concrete through innovative nanomaterial–polymer hybrid cementitious coating
- Development and performance evaluation of green aluminium metal matrix composites reinforced with graphene nanopowder and marble dust
- Morphological, physical, thermal, and mechanical properties of carbon nanotubes reinforced arrowroot starch composites
- Influence of the graphene oxide nanosheet on tensile behavior and failure characteristics of the cement composites after high-temperature treatment
- Central composite design modeling in optimizing heat transfer rate in the dissipative and reactive dynamics of viscoplastic nanomaterials deploying Joule and heat generation aspects
- Double diffusion of nano-enhanced phase change materials in connected porous channels: A hybrid ISPH-XGBoost approach
- Review Articles
- A comprehensive review on hybrid plasmonic waveguides: Structures, applications, challenges, and future perspectives
- Nanoparticles in low-temperature preservation of biological systems of animal origin
- Fluorescent sulfur quantum dots for environmental monitoring
- Nanoscience systematic review methodology standardization
- Nanotechnology revolutionizing osteosarcoma treatment: Advances in targeted kinase inhibitors
- AFM: An important enabling technology for 2D materials and devices
- Carbon and 2D nanomaterial smart hydrogels for therapeutic applications
- Principles, applications and future prospects in photodegradation systems
- Do gold nanoparticles consistently benefit crop plants under both non-stressed and abiotic stress conditions?
- An updated overview of nanoparticle-induced cardiovascular toxicity
- Arginine as a promising amino acid for functionalized nanosystems: Innovations, challenges, and future directions
- Advancements in the use of cancer nanovaccines: Comprehensive insights with focus on lung and colon cancer
- Membrane-based biomimetic delivery systems for glioblastoma multiforme therapy
- The drug delivery systems based on nanoparticles for spinal cord injury repair
- Green synthesis, biomedical effects, and future trends of Ag/ZnO bimetallic nanoparticles: An update
- Application of magnesium and its compounds in biomaterials for nerve injury repair
- Micro/nanomotors in biomedicine: Construction and applications
- Hydrothermal synthesis of biomass-derived CQDs: Advances and applications
- Research progress in 3D bioprinting of skin: Challenges and opportunities
- Review on bio-selenium nanoparticles: Synthesis, protocols, and applications in biomedical processes
- Gold nanocrystals and nanorods functionalized with protein and polymeric ligands for environmental, energy storage, and diagnostic applications: A review
- An in-depth analysis of rotational and non-rotational piezoelectric energy harvesting beams: A comprehensive review
- Advancements in perovskite/CIGS tandem solar cells: Material synergies, device configurations, and economic viability for sustainable energy
- Deep learning in-depth analysis of crystal graph convolutional neural networks: A new era in materials discovery and its applications
- Review of recent nano TiO2 film coating methods, assessment techniques, and key problems for scaleup
- Antioxidant quantum dots for spinal cord injuries: A review on advancing neuroprotection and regeneration in neurological disorders
- Rise of polycatecholamine ultrathin films: From synthesis to smart applications
- Advancing microencapsulation strategies for bioactive compounds: Enhancing stability, bioavailability, and controlled release in food applications
- Corrigendum
- Corrigendum to “Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer”
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part III
- Efficiency optimization of quantum dot photovoltaic cell by solar thermophotovoltaic system
- Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
- Electrochemical investigation of bismuth-doped anode materials for low‑temperature solid oxide fuel cells with boosted voltage using a DC-DC voltage converter
- Synthesis of HfSe2 and CuHfSe2 crystalline materials using the chemical vapor transport method and their applications in supercapacitor energy storage devices
- Special Issue on Green Nanotechnology and Nano-materials for Environment Sustainability
- Influence of nano-silica and nano-ferrite particles on mechanical and durability of sustainable concrete: A review
- Surfaces and interfaces analysis on different carboxymethylation reaction time of anionic cellulose nanoparticles derived from oil palm biomass
- Processing and effective utilization of lignocellulosic biomass: Nanocellulose, nanolignin, and nanoxylan for wastewater treatment
- Retraction
- Retraction of “Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation”
Articles in the same Issue
- Research Articles
- MHD radiative mixed convective flow of a sodium alginate-based hybrid nanofluid over a convectively heated extending sheet with Joule heating
- Experimental study of mortar incorporating nano-magnetite on engineering performance and radiation shielding
- Multicriteria-based optimization and multi-variable non-linear regression analysis of concrete containing blends of nano date palm ash and eggshell powder as cementitious materials
- A promising Ag2S/poly-2-amino-1-mercaptobenzene open-top spherical core–shell nanocomposite for optoelectronic devices: A one-pot technique
- Biogenic synthesized selenium nanoparticles combined chitosan nanoparticles controlled lung cancer growth via ROS generation and mitochondrial damage pathway
- Fabrication of PDMS nano-mold by deposition casting method
- Stimulus-responsive gradient hydrogel micro-actuators fabricated by two-photon polymerization-based 4D printing
- Physical aspects of radiative Carreau nanofluid flow with motile microorganisms movement under yield stress via oblique penetrable wedge
- Effect of polar functional groups on the hydrophobicity of carbon nanotubes-bacterial cellulose nanocomposite
- Review in green synthesis mechanisms, application, and future prospects for Garcinia mangostana L. (mangosteen)-derived nanoparticles
- Entropy generation and heat transfer in nonlinear Buoyancy–driven Darcy–Forchheimer hybrid nanofluids with activation energy
- Green synthesis of silver nanoparticles using Ginkgo biloba seed extract: Evaluation of antioxidant, anticancer, antifungal, and antibacterial activities
- A numerical analysis of heat and mass transfer in water-based hybrid nanofluid flow containing copper and alumina nanoparticles over an extending sheet
- Investigating the behaviour of electro-magneto-hydrodynamic Carreau nanofluid flow with slip effects over a stretching cylinder
- Electrospun thermoplastic polyurethane/nano-Ag-coated clear aligners for the inhibition of Streptococcus mutans and oral biofilm
- Investigation of the optoelectronic properties of a novel polypyrrole-multi-well carbon nanotubes/titanium oxide/aluminum oxide/p-silicon heterojunction
- Novel photothermal magnetic Janus membranes suitable for solar water desalination
- Green synthesis of silver nanoparticles using Ageratum conyzoides for activated carbon compositing to prepare antimicrobial cotton fabric
- Activation energy and Coriolis force impact on three-dimensional dusty nanofluid flow containing gyrotactic microorganisms: Machine learning and numerical approach
- Machine learning analysis of thermo-bioconvection in a micropolar hybrid nanofluid-filled square cavity with oxytactic microorganisms
- Research and improvement of mechanical properties of cement nanocomposites for well cementing
- Thermal and stability analysis of silver–water nanofluid flow over unsteady stretching sheet under the influence of heat generation/absorption at the boundary
- Cobalt iron oxide-infused silicone nanocomposites: Magnetoactive materials for remote actuation and sensing
- Magnesium-reinforced PMMA composite scaffolds: Synthesis, characterization, and 3D printing via stereolithography
- Bayesian inference-based physics-informed neural network for performance study of hybrid nanofluids
- Numerical simulation of non-Newtonian hybrid nanofluid flow subject to a heterogeneous/homogeneous chemical reaction over a Riga surface
- Enhancing the superhydrophobicity, UV-resistance, and antifungal properties of natural wood surfaces via in situ formation of ZnO, TiO2, and SiO2 particles
- Synthesis and electrochemical characterization of iron oxide/poly(2-methylaniline) nanohybrids for supercapacitor application
- Impacts of double stratification on thermally radiative third-grade nanofluid flow on elongating cylinder with homogeneous/heterogeneous reactions by implementing machine learning approach
- Synthesis of Cu4O3 nanoparticles using pumpkin seed extract: Optimization, antimicrobial, and cytotoxicity studies
- Cationic charge influence on the magnetic response of the Fe3O4–[Me2+ 1−y Me3+ y (OH2)] y+(Co3 2−) y/2·mH2O hydrotalcite system
- Pressure sensing intelligent martial arts short soldier combat protection system based on conjugated polymer nanocomposite materials
- Magnetohydrodynamics heat transfer rate under inclined buoyancy force for nano and dusty fluids: Response surface optimization for the thermal transport
- Fly ash and nano-graphene enhanced stabilization of engine oil-contaminated soils
- Enhancing natural fiber-reinforced biopolymer composites with graphene nanoplatelets: Mechanical, morphological, and thermal properties
- Performance evaluation of dual-scale strengthened co-bonded single-lap joints using carbon nanotubes and Z-pins with ANN
- Computational works of blood flow with dust particles and partially ionized containing tiny particles on a moving wedge: Applications of nanotechnology
- Hybridization of biocomposites with oil palm cellulose nanofibrils/graphene nanoplatelets reinforcement in green epoxy: A study of physical, thermal, mechanical, and morphological properties
- Design and preparation of micro-nano dual-scale particle-reinforced Cu–Al–V alloy: Research on the aluminothermic reduction process
- Spectral quasi-linearization and response optimization on magnetohydrodynamic flow via stenosed artery with hybrid and ternary solid nanoparticles: Support vector machine learning
- Ferrite/curcumin hybrid nanocomposite formulation: Physicochemical characterization, anticancer activity, and apoptotic and cell cycle analyses in skin cancer cells
- Enhanced therapeutic efficacy of Tamoxifen against breast cancer using extra virgin olive oil-based nanoemulsion delivery system
- A titanium oxide- and silver-based hybrid nanofluid flow between two Riga walls that converge and diverge through a machine-learning approach
- Enhancing convective heat transfer mechanisms through the rheological analysis of Casson nanofluid flow towards a stagnation point over an electro-magnetized surface
- Intrinsic self-sensing cementitious composites with hybrid nanofillers exhibiting excellent piezoresistivity
- Research on mechanical properties and sulfate erosion resistance of nano-reinforced coal gangue based geopolymer concrete
- Impact of surface and configurational features of chemically synthesized chains of Ni nanostars on the magnetization reversal process
- Porous sponge-like AsOI/poly(2-aminobenzene-1-thiol) nanocomposite photocathode for hydrogen production from artificial and natural seawater
- Multifaceted insights into WO3 nanoparticle-coupled antibiotics to modulate resistance in enteric pathogens of Houbara bustard birds
- Synthesis of sericin-coated silver nanoparticles and their applications for the anti-bacterial finishing of cotton fabric
- Enhancing chloride resistance of freeze–thaw affected concrete through innovative nanomaterial–polymer hybrid cementitious coating
- Development and performance evaluation of green aluminium metal matrix composites reinforced with graphene nanopowder and marble dust
- Morphological, physical, thermal, and mechanical properties of carbon nanotubes reinforced arrowroot starch composites
- Influence of the graphene oxide nanosheet on tensile behavior and failure characteristics of the cement composites after high-temperature treatment
- Central composite design modeling in optimizing heat transfer rate in the dissipative and reactive dynamics of viscoplastic nanomaterials deploying Joule and heat generation aspects
- Double diffusion of nano-enhanced phase change materials in connected porous channels: A hybrid ISPH-XGBoost approach
- Review Articles
- A comprehensive review on hybrid plasmonic waveguides: Structures, applications, challenges, and future perspectives
- Nanoparticles in low-temperature preservation of biological systems of animal origin
- Fluorescent sulfur quantum dots for environmental monitoring
- Nanoscience systematic review methodology standardization
- Nanotechnology revolutionizing osteosarcoma treatment: Advances in targeted kinase inhibitors
- AFM: An important enabling technology for 2D materials and devices
- Carbon and 2D nanomaterial smart hydrogels for therapeutic applications
- Principles, applications and future prospects in photodegradation systems
- Do gold nanoparticles consistently benefit crop plants under both non-stressed and abiotic stress conditions?
- An updated overview of nanoparticle-induced cardiovascular toxicity
- Arginine as a promising amino acid for functionalized nanosystems: Innovations, challenges, and future directions
- Advancements in the use of cancer nanovaccines: Comprehensive insights with focus on lung and colon cancer
- Membrane-based biomimetic delivery systems for glioblastoma multiforme therapy
- The drug delivery systems based on nanoparticles for spinal cord injury repair
- Green synthesis, biomedical effects, and future trends of Ag/ZnO bimetallic nanoparticles: An update
- Application of magnesium and its compounds in biomaterials for nerve injury repair
- Micro/nanomotors in biomedicine: Construction and applications
- Hydrothermal synthesis of biomass-derived CQDs: Advances and applications
- Research progress in 3D bioprinting of skin: Challenges and opportunities
- Review on bio-selenium nanoparticles: Synthesis, protocols, and applications in biomedical processes
- Gold nanocrystals and nanorods functionalized with protein and polymeric ligands for environmental, energy storage, and diagnostic applications: A review
- An in-depth analysis of rotational and non-rotational piezoelectric energy harvesting beams: A comprehensive review
- Advancements in perovskite/CIGS tandem solar cells: Material synergies, device configurations, and economic viability for sustainable energy
- Deep learning in-depth analysis of crystal graph convolutional neural networks: A new era in materials discovery and its applications
- Review of recent nano TiO2 film coating methods, assessment techniques, and key problems for scaleup
- Antioxidant quantum dots for spinal cord injuries: A review on advancing neuroprotection and regeneration in neurological disorders
- Rise of polycatecholamine ultrathin films: From synthesis to smart applications
- Advancing microencapsulation strategies for bioactive compounds: Enhancing stability, bioavailability, and controlled release in food applications
- Corrigendum
- Corrigendum to “Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer”
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part III
- Efficiency optimization of quantum dot photovoltaic cell by solar thermophotovoltaic system
- Exploring the diverse nanomaterials employed in dental prosthesis and implant techniques: An overview
- Electrochemical investigation of bismuth-doped anode materials for low‑temperature solid oxide fuel cells with boosted voltage using a DC-DC voltage converter
- Synthesis of HfSe2 and CuHfSe2 crystalline materials using the chemical vapor transport method and their applications in supercapacitor energy storage devices
- Special Issue on Green Nanotechnology and Nano-materials for Environment Sustainability
- Influence of nano-silica and nano-ferrite particles on mechanical and durability of sustainable concrete: A review
- Surfaces and interfaces analysis on different carboxymethylation reaction time of anionic cellulose nanoparticles derived from oil palm biomass
- Processing and effective utilization of lignocellulosic biomass: Nanocellulose, nanolignin, and nanoxylan for wastewater treatment
- Retraction
- Retraction of “Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation”

