Abstract
C23H24N3O4ReS, orthorhombic, Pbca (no. 61), a = 15.1264(4) Å, b = 15.1167(5) Å, c = 20.1500 Å, V = 4607.5(2) Å3, Z = 8, T = 150(2) K, R gt (F) = 0.0416, wR ref (F 2) = 0.0691.
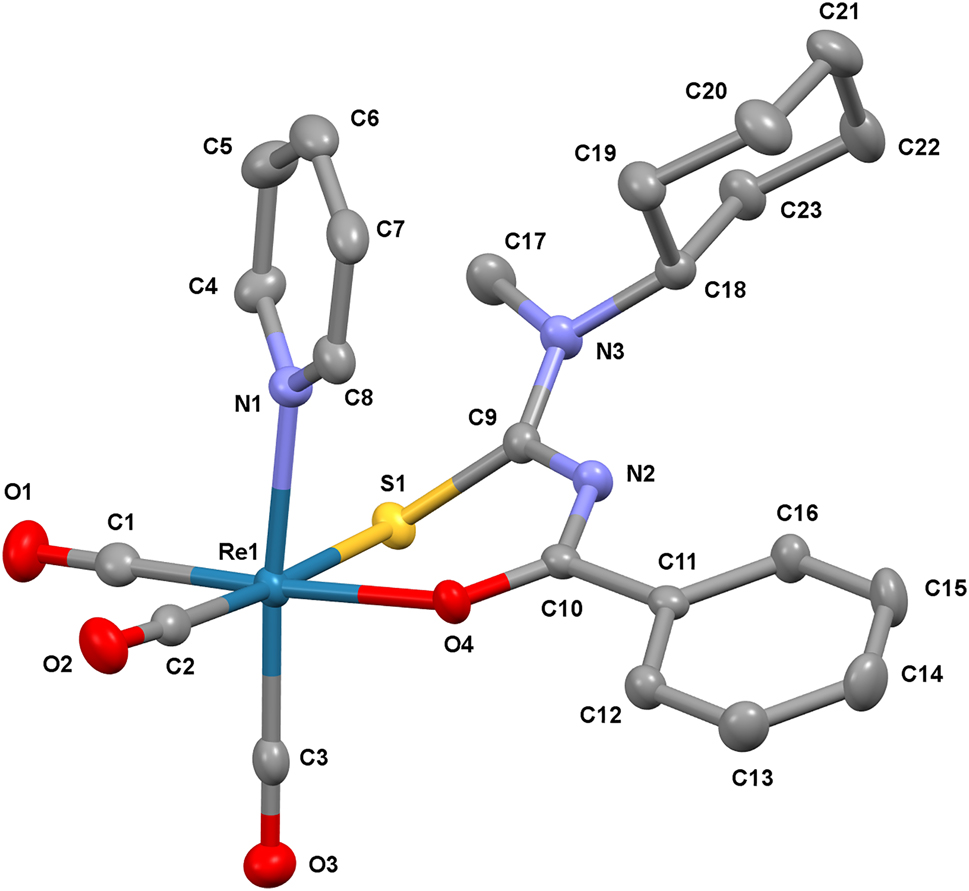
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.14 × 0.13 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 5.40 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy R, ω |
| θ max, completeness: | 31.1°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 73,180, 6553, 0.072 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 5438 |
| N(param)refined: | 290 |
| Programs: | CrysAlispro [1], Olex2 [2], WinGX [3], SHELX [4, 5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.9576 (3) | 0.3976 (3) | 0.6787 (2) | 0.0231 (8) |
| C2 | 0.9621 (3) | 0.3719 (3) | 0.5492 (2) | 0.0229 (8) |
| C3 | 1.0041 (3) | 0.5338 (3) | 0.60326 (19) | 0.0233 (8) |
| C4 | 0.7412 (3) | 0.3490 (3) | 0.6613 (2) | 0.0260 (9) |
| H4 | 0.766036 | 0.366106 | 0.702659 | 0.031* |
| C5 | 0.6657 (3) | 0.2981 (3) | 0.6615 (2) | 0.0326 (10) |
| H5 | 0.639454 | 0.280115 | 0.702166 | 0.039* |
| C6 | 0.6286 (3) | 0.2736 (3) | 0.6013 (2) | 0.0316 (9) |
| H6 | 0.576853 | 0.238062 | 0.600066 | 0.038* |
| C7 | 0.6678 (3) | 0.3015 (3) | 0.5436 (2) | 0.0281 (9) |
| H7 | 0.642954 | 0.286455 | 0.501773 | 0.034* |
| C8 | 0.7440 (3) | 0.3518 (3) | 0.5473 (2) | 0.0241 (8) |
| H8 | 0.771213 | 0.370281 | 0.507138 | 0.029* |
| C9 | 0.7247 (2) | 0.5736 (2) | 0.64389 (18) | 0.0190 (7) |
| C10 | 0.7691 (2) | 0.5767 (2) | 0.53014 (18) | 0.0182 (7) |
| C11 | 0.7454 (2) | 0.6206 (3) | 0.46589 (18) | 0.0183 (7) |
| C12 | 0.7991 (3) | 0.6077 (3) | 0.41046 (19) | 0.0232 (8) |
| H12 | 0.849996 | 0.571062 | 0.413620 | 0.028* |
| C13 | 0.7784 (3) | 0.6480 (3) | 0.3511 (2) | 0.0295 (9) |
| H13 | 0.815535 | 0.639597 | 0.313579 | 0.035* |
| C14 | 0.7041 (3) | 0.7006 (3) | 0.3456 (2) | 0.0318 (10) |
| H14 | 0.689942 | 0.727837 | 0.304476 | 0.038* |
| C15 | 0.6502 (3) | 0.7134 (3) | 0.4004 (2) | 0.0316 (10) |
| H15 | 0.598970 | 0.749429 | 0.396866 | 0.038* |
| C16 | 0.6712 (3) | 0.6735 (3) | 0.4604 (2) | 0.0250 (8) |
| H16 | 0.634333 | 0.682653 | 0.497998 | 0.030* |
| C17 | 0.6505 (3) | 0.5511 (3) | 0.7513 (2) | 0.0301 (9) |
| H17A | 0.707889 | 0.525691 | 0.763320 | 0.045* |
| H17B | 0.603777 | 0.507592 | 0.759450 | 0.045* |
| H17C | 0.639552 | 0.604068 | 0.778106 | 0.045* |
| C18 | 0.5643 (3) | 0.5947 (3) | 0.64901 (19) | 0.0201 (8) |
| H18 | 0.575368 | 0.639444 | 0.613448 | 0.024* |
| C19 | 0.5271 (3) | 0.5126 (3) | 0.6155 (2) | 0.0288 (9) |
| H19A | 0.513822 | 0.466907 | 0.649289 | 0.035* |
| H19B | 0.571255 | 0.488025 | 0.584254 | 0.035* |
| C20 | 0.4423 (3) | 0.5370 (4) | 0.5778 (2) | 0.0362 (11) |
| H20A | 0.456717 | 0.578844 | 0.541521 | 0.043* |
| H20B | 0.416414 | 0.483176 | 0.557647 | 0.043* |
| C21 | 0.3749 (3) | 0.5795 (4) | 0.6245 (2) | 0.0367 (11) |
| H21A | 0.322501 | 0.598294 | 0.598734 | 0.044* |
| H21B | 0.355365 | 0.535175 | 0.657608 | 0.044* |
| C22 | 0.4133 (3) | 0.6581 (3) | 0.6599 (2) | 0.0333 (10) |
| H22A | 0.369215 | 0.681432 | 0.691708 | 0.040* |
| H22B | 0.426136 | 0.705211 | 0.627159 | 0.040* |
| C23 | 0.4981 (3) | 0.6350 (3) | 0.6972 (2) | 0.0279 (9) |
| H23A | 0.523372 | 0.688959 | 0.717515 | 0.033* |
| H23B | 0.484801 | 0.592302 | 0.733145 | 0.033* |
| N1 | 0.7813 (2) | 0.3757 (2) | 0.60527 (16) | 0.0213 (6) |
| N2 | 0.7138 (2) | 0.5930 (2) | 0.57954 (16) | 0.0205 (7) |
| N3 | 0.6510 (2) | 0.5751 (2) | 0.68101 (16) | 0.0225 (7) |
| O1 | 0.9898 (2) | 0.3636 (2) | 0.72413 (15) | 0.0360 (7) |
| O2 | 0.99820 (19) | 0.3184 (2) | 0.51869 (15) | 0.0311 (7) |
| O3 | 1.0644 (2) | 0.5806 (2) | 0.60287 (15) | 0.0317 (7) |
| O4 | 0.83560 (17) | 0.52501 (17) | 0.52858 (13) | 0.0203 (5) |
| Re1 | 0.90396 (2) | 0.45652 (2) | 0.60597 (2) | 0.01838 (5) |
| S1 | 0.82605 (6) | 0.55705 (7) | 0.68298 (5) | 0.02222 (19) |
1 Source of materials
The title complex was synthesized using the starting precursor, fac-[NEt4]2 [Re(CO)3Br3], as explained by Albert et al. [6], Manicum et al. [7, 8]. fac-[NEt4]2 [Re (CO)3Br3], (201.1 mg, 0.336 mmol) was dissolved in 5 mL of acidic water (pH = 2.2) and stirred at room temperature for 10 min. AgNO3 (134.4 mg, 0.7912 mmol) was added to the solution and stirred for 24 h at room temperature. The precipitate, AgBr, was separated and weighed. N-(cyclohexyl(methyl)carbamothioyl) benzamide (N–CyHMCB) bidentate ligand (72.11 mg, 0.336 mmol) was added to the filtrate and the reaction mixture was stirred at room temperature for 24 h. The light-yellow precipitate (fac-[Re(N–CyHMCB)(CO)3(OH2)]) was filtered off and dried. fac-[Re(N–CyHMCB)(CO)3(OH2)], (74.5 mg, 0.132 mmol) was dissolved in methanol (5 mL), and pyridine (0.07 mL) was added. The solution was first stirred for 24 h at room temperature. The light-yellow solid (product) was obtained by evaporation of the solvent and the precipitate was recrystallized from ethylacetate:hexane (8:2). Yield = 71.52 mg, 86 %, IR (KBr, cm −1 ): v CO 2103, 2007, 1879.
2 Experimental details
All the hydrogen atoms were positioned geometrically and refined using a riding model with fixed C–HAromatic = 0.97 Å; C–Hmethyl = 0.96 Å. The H atoms isotropic displacement parameters were fixed; U iso(H) = 1.2U eq (C) for aromatic and U iso(H) = 1.5U eq (C) for methyl, allowing them to ride on the parent atom. The graphics were obtained by using the Mercury program with 50 % probability ellipsoids. All the hydrogen in the structure was omitted for clarity.
3 Comment
Organometallic rhenium(I) compounds have shown great promise for the development of new anticancer agents in recent years [8, 9]. A variety of tricarbonyl complexes containing mono- and bidentate ligands with nitrogen, oxygen, selenium, and phosphorus donors were discovered to have significant cytotoxic effects on various cancer cell lines [9], [10], [11], [12]. However, the tricarbonyl precursor is particularly appealing not only for its potential use in biological applications but also for its flexibility of M(CO)3 to accommodate different ligands, which has led to the development of several strategies to saturate the coordination sphere with ligands from a single ligand to a combination of two or more ligands [13], [14], [15].
The molecular structure of the title complex comprises three facial tricarbonyl ligands, a thiourea bidentate ligand (S,O) in the equatorial plane which is trans to the two of the carbonyl ligands and N-coordinated pyridine ligand (monodentate) in the axial position as shown in the Figure. The complex was obtained by using the [2 + 1] mixed ligand approach on fac-[M(CO)3(O2H)3]+ moiety. In particular, the [2 + 1] approach provides a combination strategy of a bidentate and a monodentate ligand to yield a unique asymmetric tailoring of the complex that affects its (bio)chemical properties such as charge, lipophilicity, and stability [16, 17]. The structure of the titled complex possesses a distorted octahedral geometry around the rhenium metal center as seen with the bond angles C1—Re—O4 and C3—Re—N1, reported as 175.27(14)° and 175.88(14)°, respectively. The bite angle distortion of the structure was 85.96(7)°, which is similar to that of other rhenium metals coordinated with thiourea ligands [18], [19], [20]. The bond length between the three carbons from the carbonyl ligands and rhenium metal, Re—C1, Re—C2, and Re—C3 were found as 1.897(4), 1.914(4), and 1.929(4) Å, respectively. The bond distances Re—S1, Re—N1, and Re—O4 were arranged in decreasing order 2.471(10), 2.220(3), and 2.138(3) Å, respectively. The bond length obtained in this study agrees with the other rhenium and rhodium structures that were reported previously [21–23]. There are three intramolecular hydrogen bonds in the titled complex that stabilizes the complex further from C16—H16⋯N2 which has the bond length of 2.768(5) Å, C17—H17A⋯S1 with a bond length of 2.992(5) Å, and C8—H8⋯O4 with a bond length of 2.986(5) Å.
Funding source: National Research Foundation of South Africa
Award Identifier / Grant number: 129468
Funding source: Tshwane University of Technology
Funding source: University of Pretoria
Funding source: NCP Chlorchem
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: We would like to thank the National Research Foundation of South Africa (Grant No. 129468), Tshwane University of Technology, and the University of Pretoria for institutional and financial support. The authors would like to express their gratitude towards NCP Chlorchem for financial assistance.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. System, C. S. Rigaku Oxford Diffraction. CrysAlis PRO; Software System: Yarnton, UK, 2021.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Farrugia, L. J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838; https://doi.org/10.1107/s0021889899006020.Search in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
6. Alberto, R., Schibli, R., Waibel, R., Abram, U., Schubiger, A. P. Basic aqueous chemistry of [M(OH2)3(CO)3]+ (M = Re, Tc) directed towards radiopharmaceutical application. Coord. Chem. Rev. 1999, 190, 901–919; https://doi.org/10.1016/s0010-8545(99)00128-9.Search in Google Scholar
7. Manicum, A. E., Schutte-Smith, M., Malan, F. P., Visser, H. G. Steric and electronic influence of Re(I) tricarbonyl complexes with various coordinated β-diketones. J. Mol. Struct. 2022, 1264, 133278; https://doi.org/10.1016/j.molstruc.2022.133278.Search in Google Scholar
8. Manicum, A. E., Schutte-Smith, M., Visser, H. G., Pretorius, C., Roodt, A. The crystal structure tetraethyl ammonium factricarbonyl (hexafluoroacatylacenato-κ2O,O′)-(nitrato-κO) rhenium(I), C16H21N2F6Re. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 263–266; https://doi.org/10.1515/ncrs-2015-0115.Search in Google Scholar
9. Collery, P., Desmaele, D., Vijaykumar, V. Design of rhenium compounds in targeted anticancer therapeutics. Curr. Pharm. Des. 2019, 25, 3306–3322; https://doi.org/10.2174/1381612825666190902161400.Search in Google Scholar PubMed
10. Bauer, E. B., Haase, A. A., Reich, R. M., Crans, D. C., Kühn, F. E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019, 393, 79–117; https://doi.org/10.1016/j.ccr.2019.04.014.Search in Google Scholar
11. Leonidova, A., Gasser, G. Underestimated potential of organometallic rhenium complexes as anticancer agents. ACS Chem. Biol. 2014, 9, 2180–2193; https://doi.org/10.1021/cb500528c.Search in Google Scholar PubMed
12. Murphy, B. L., Marker, S. C., Lambert, V. J., Woods, J. J., MacMillan, S. N., Wilson, J. J. Synthesis, characterization, and biological properties of rhenium(I) tricarbonyl complexes bearing nitrogen-donor ligands. J. Organomet. Chem. 2020, 901, 121064.10.1016/j.jorganchem.2019.121064Search in Google Scholar
13. Jurisson, S. S., Lydon, J. D. Potential technetium small molecule radiopharmaceuticals. Chem. Rev. 1999, 99, 2205–2218; https://doi.org/10.1021/cr980435t.Search in Google Scholar PubMed
14. Liu, G., Hnatowich, D. J. Labeling biomolecules with radiorhenium: a review of the bifunctional chelators. Anti Cancer Agents Med. Chem. 2007, 7, 367–377; https://doi.org/10.2174/187152007780618144.Search in Google Scholar PubMed PubMed Central
15. Papagiannopoulou, D. Radiopharmaceutical applications of organometallic technetium and rhenium complexes. Curr. Inorg. Chem. 2012, 2, 228–247; https://doi.org/10.2174/1877944111202020228.Search in Google Scholar
16. Abram, U., Abram, S., Alberto, R., Schibli, R. Ligand exchange reactions starting from [Re(CO)3Br3]2− synthesis, characterization and structures of rhenium(l) tricarbonyl complexes with thiourea and thiourea derivatives. Inorg. Chim. Acta 1996, 248, 193–202; https://doi.org/10.1016/0020-1693(96)05014-1.Search in Google Scholar
17. Hayes, T. R., Powell, A. S., Barnes, C. L., Benny, P. D. Synthesis and stability of 2+1 complexes of N,N-diethylbenzoylthiourea with [MI(CO)3]+ (M = Re, 99mTc). J. Coord. Chem. 2015, 68, 3432–3448; https://doi.org/10.1080/00958972.2015.1071801.Search in Google Scholar
18. Komane, W. K., Mokolokolo, P., Vatsha, B., Manicum, A. The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I)-methanol(1/1) C26H23O4N4SRe. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 767–769; https://doi.org/10.1515/ncrs-2021-0046.Search in Google Scholar
19. Ramoba, V. L., Alexander, T. O., Visser, H. G., Manicum, A. The crystal structure of fac-tricarbonyl (1,10-phenanthroline-κ2N,N′)-(pyrazole-κN) rhenium(I)nitrate, C18H12O3N4Re. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1203–1205; https://doi.org/10.1515/ncrs-2020-0249.Search in Google Scholar
20. Schmitt, B., Gerber, T. I. A., Hosten, E., Betz, R. Monomeric/dimeric complexes of fac-[Re(CO)3]+ with benzoylthiourea derivatives. Inorg. Chem. Commun. 2012, 24, 136–139; https://doi.org/10.1016/j.inoche.2012.08.008.Search in Google Scholar
21. Moremi, M. J., Alexander, O. T., Vatsha, B., Makgopa, K., Manicum, A. The crystal structure of fac-tricarbonyl(4,4-dimethyl 2,2-dipyridyl-κ2-N,N′)(pyrazole-κN)rhenium(I) nitrate, C18H12O3N4Re. Z. Kristallogr. N. Cryst. Struct. 2020, 236, 33–35; https://doi.org/10.1515/ncrs-2020-0458.Search in Google Scholar
22. Warsink, S., Riekert, K., Janse Van Rensburg, J. M., Venter, J. A., Otto, S., Botha, E., Roodt, A. Kinetic-mechanistic and solid-state study of the oxidative addition and migratory insertion of iodomethane to [Rhodium(S,O–BdiPT or N,O-ox)(CO)(PR1 R2 R3)] complexes. Eur. J. Inorg. Chem. 2018, 2018, 3615–3625; https://doi.org/10.1002/ejic.201800293.Search in Google Scholar
23. Wei, L., Zubieta, J. fac–Bromotricarbonyl[2-(2-pyridylmethyl)-2,3-dihydro-1H-isoindol-1-one]rhenium(I) methanol solvate. Acta Crystallogr. 2005, C61, m95–m97; https://doi.org/10.1107/s0108270104029592.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2

