Abstract
C22H22O7, orthorhombic, C2221 (no. 20), a = 8.891(2) Å, b = 17.938(5) Å, c = 25.996(6) Å, V = 4146.0(18) Å3, Z = 8, R gt (F) = 0.0461, wR ref (F 2) = 0.1189, T = 296 K.
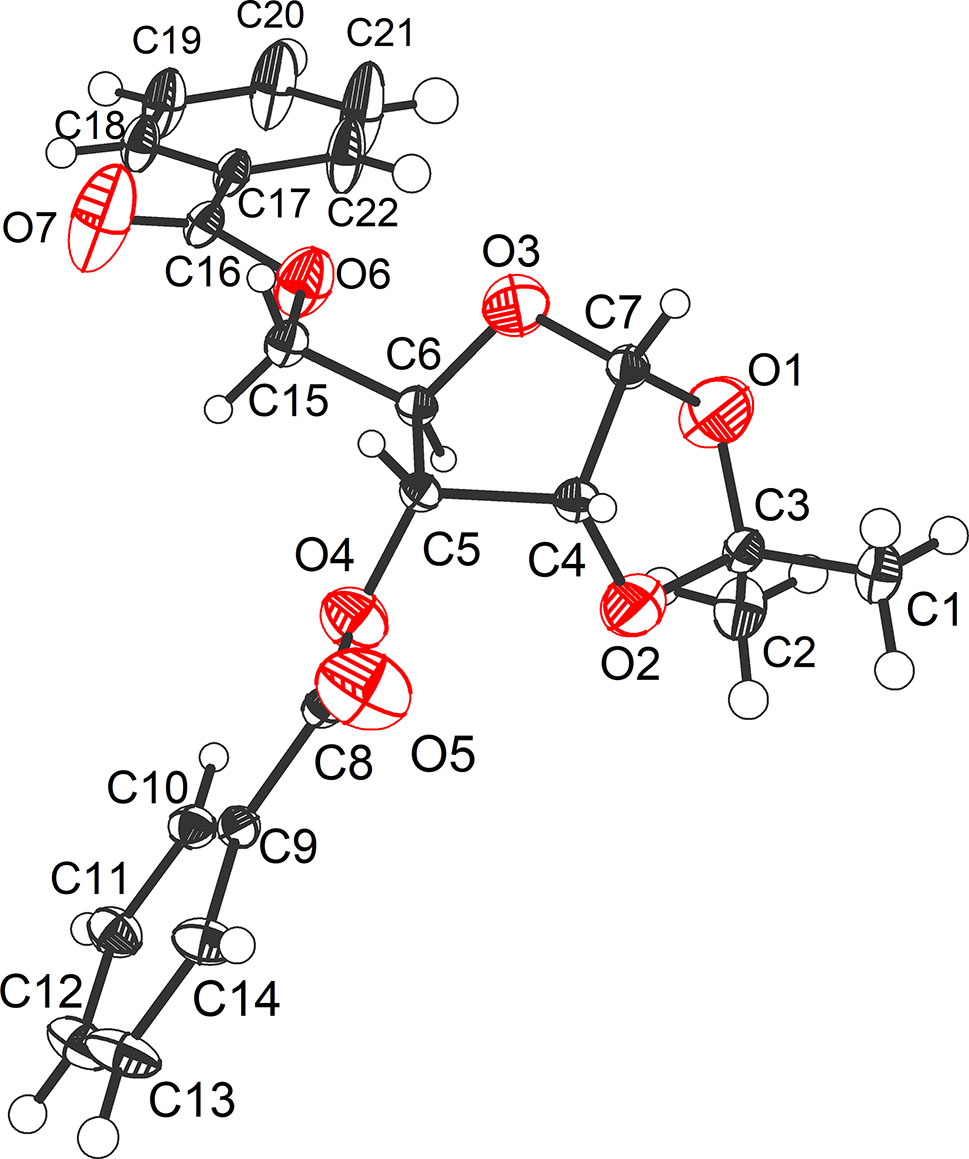
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block |
| Size: | 0.30 × 0.28 × 0.22 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | φ and ω |
| θ max, completeness: | 27.6°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 16,702, 4539, 0.039 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3164 |
| N(param)refined: | 262 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.1626 (5) | 0.0859 (2) | 0.38010 (14) | 0.0652 (10) |
| H1A | 0.116402 | 0.043860 | 0.396622 | 0.098* |
| H1B | 0.087305 | 0.114108 | 0.362154 | 0.098* |
| H1C | 0.236943 | 0.068727 | 0.356095 | 0.098* |
| C2 | 0.3521 (5) | 0.0935 (2) | 0.45118 (17) | 0.0773 (12) |
| H2A | 0.306689 | 0.050838 | 0.467146 | 0.116* |
| H2B | 0.432646 | 0.077580 | 0.429196 | 0.116* |
| H2C | 0.390962 | 0.126199 | 0.477237 | 0.116* |
| C3 | 0.2360 (3) | 0.13426 (15) | 0.41978 (11) | 0.0449 (7) |
| C4 | 0.2015 (3) | 0.25657 (16) | 0.39634 (10) | 0.0412 (7) |
| H4A | 0.155877 | 0.264618 | 0.362469 | 0.049* |
| C5 | 0.2739 (3) | 0.32682 (15) | 0.41806 (10) | 0.0364 (6) |
| H5A | 0.218325 | 0.370889 | 0.406401 | 0.044* |
| C6 | 0.2528 (3) | 0.31661 (15) | 0.47597 (10) | 0.0389 (6) |
| H6A | 0.324881 | 0.279962 | 0.489135 | 0.047* |
| C7 | 0.0852 (4) | 0.23637 (16) | 0.43776 (11) | 0.0439 (7) |
| H7A | −0.017381 | 0.237567 | 0.423974 | 0.053* |
| C8 | 0.4671 (3) | 0.35846 (15) | 0.36009 (11) | 0.0385 (7) |
| C9 | 0.6304 (3) | 0.37551 (16) | 0.35571 (11) | 0.0412 (7) |
| C10 | 0.7246 (3) | 0.37604 (16) | 0.39792 (12) | 0.0491 (8) |
| H10A | 0.687503 | 0.363802 | 0.430283 | 0.059* |
| C11 | 0.8743 (4) | 0.3948 (2) | 0.39188 (16) | 0.0634 (10) |
| H11A | 0.937685 | 0.395709 | 0.420315 | 0.076* |
| C12 | 0.9296 (5) | 0.4122 (2) | 0.34423 (17) | 0.0788 (13) |
| H12A | 1.030490 | 0.424649 | 0.340360 | 0.095* |
| C13 | 0.8372 (5) | 0.4113 (3) | 0.30249 (17) | 0.0803 (13) |
| H13A | 0.875577 | 0.422604 | 0.270146 | 0.096* |
| C14 | 0.6865 (4) | 0.3936 (2) | 0.30784 (13) | 0.0604 (9) |
| H14A | 0.623356 | 0.394020 | 0.279316 | 0.073* |
| C15 | 0.2631 (4) | 0.38726 (15) | 0.50629 (10) | 0.0456 (7) |
| H15A | 0.358821 | 0.411607 | 0.500152 | 0.055* |
| H15B | 0.182983 | 0.421154 | 0.496545 | 0.055* |
| C16 | 0.2560 (4) | 0.42233 (16) | 0.59383 (11) | 0.0487 (7) |
| C17 | 0.2418 (4) | 0.39580 (16) | 0.64724 (11) | 0.0499 (8) |
| C18 | 0.2857 (5) | 0.44208 (19) | 0.68670 (13) | 0.0673 (11) |
| H18A | 0.321579 | 0.489607 | 0.679348 | 0.081* |
| C19 | 0.2770 (7) | 0.4187 (2) | 0.73654 (13) | 0.0937 (16) |
| H19A | 0.310966 | 0.449345 | 0.762933 | 0.112* |
| C20 | 0.2182 (10) | 0.3498 (2) | 0.74772 (16) | 0.137 (3) |
| H20A | 0.209099 | 0.334167 | 0.781687 | 0.165* |
| C21 | 0.1737 (11) | 0.3048 (3) | 0.70877 (16) | 0.163 (4) |
| H21A | 0.134819 | 0.257896 | 0.716242 | 0.196* |
| C22 | 0.1848 (7) | 0.3272 (2) | 0.65851 (14) | 0.1032 (19) |
| H22A | 0.153551 | 0.295724 | 0.632184 | 0.124* |
| O1 | 0.1254 (3) | 0.16429 (12) | 0.45359 (9) | 0.0612 (7) |
| O2 | 0.3056 (2) | 0.19714 (11) | 0.39654 (8) | 0.0507 (6) |
| O3 | 0.1028 (2) | 0.28716 (11) | 0.47855 (7) | 0.0469 (5) |
| O4 | 0.4308 (2) | 0.33617 (10) | 0.40787 (7) | 0.0404 (5) |
| O5 | 0.3767 (3) | 0.36370 (14) | 0.32595 (8) | 0.0577 (6) |
| O6 | 0.2492 (2) | 0.36672 (10) | 0.55980 (7) | 0.0467 (5) |
| O7 | 0.2735 (4) | 0.48615 (12) | 0.58175 (9) | 0.0806 (9) |
1 Source of materials
The title compound was synthesized according to the previous reports [5], [6], [7]. 1,2-O-isopropylidene-α–D-ribose (1.9 g, 10.0 mmol) and triethylamine (6.9 mL, 50.0 mmol) were dissolved in anhydrous dichloromethane (30 mL). After it was cooled to 0 °C, benzoyl chloride (3.5 mL, 30.0 mmol) was added dropwise. After addition, the reaction mixture was stirred at room temperature for 3 h until TLC showed the reaction was finished. Then, the reaction was quenched with ice water. Another dichloromethane (30 mL) was added and the obtained reaction mixture was washed with 5 % aqueous HCl (30 mL × 2), water (30 mL × 2), and brine (30 mL × 2). After dried over anhydrous Na2SO4 and filtration, the solvent was evaporated under vacuum. The residue was purified to afford 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose (3.9 g, 98 %) by column chromatography, which was further recrystallized with ethanol. Crystals were acquired by slow evaporation from the solution at 268–270 K.
2 Experimental details
Fixed U iso at 1.2 times for all C(H) groups, all C(H,H) groups and at 1.5 times for all C(H,H,H) groups; Ternary CH were refined with riding coordinates: C4(H4A), C5(H5A), C6(H6A), C7(H7A); Secondary CH2 were refined with riding coordinates: C15(H15A,H15B); Me were refined with riding coordinates: C1(H1A,H1B,H1C), C2(H2A,H2B,H2C); Aromatic/amide H were refined with riding coordinates: C10(H10A), C11(H11A), C12(H12A), C13(H13A), C14(H14A), C18(H18A), C19(H19A), C20(H20A), C21(H21A), C22(H22A).
3 Comment
Nucleosides, the basic building blocks of nucleic acids such as RNA and DNA, play crucial roles in many biological processes [8]. Modified nucleosides have demonstrated great therapeutic promise for antiviral and anticancer chemotherapies [9, 10]. Among them, sugar-modified nucleosides are a type of modified nucleoside with alterations to the ribose component at the 2′, 3′, or 5′ positions, affecting the conformation, stability, and reactivity of the nucleoside [11]. For instance, 2′-deoxy-2-fluoro-β–D-arabinoadenine (Fludarabine) is an FDA approved drug for the treatment of chronic lymphocytic leukemia (CLL) [12]. During our ongoing work to synthesize novel nucleoside analogues, 3,5-di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose has been identified as a highly desirable key intermediate [13, 14]. Using this intermediate, we can synthesize a series of novel nucleosides and test them for antiviral activity. Recently, our group has developed an improved synthetic approach using tetra-O-acetyl-β–D-ribose as the starting material in three steps, with an overall yield of 86 %. The crystal structure of this intermediate has not been reported previously, which is crucial for analysing its conformation.
The title structure (see Figure) consists of a furanosy D-ribose with four OH groups protected by two benzoyl groups at O4 and O6 and a bridged isopropylidene at O1 and O2, where a large dihedral angle (116.1°) between O3–C7–C4–C5 and O1–C7–C4–O2 was observed. The orientation between C6–C15 bond and C7–O1 bond is a trans-form (that is, α-configuration). Summarily, the bond lengths and angles are in the expected ranges.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This project was supported by the National Natural Science Foundation of China (22177023, 41866005); the Key Science and Technology Program of Hainan Province (No. ZDKJ202008); Hainan Provincial Natural Science Foundation of China (221RC1054); the specific reseach fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202030).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. DIFFRACTION O. CrysAlisPRO; Oxford Diffraction Ltd: Abingdon, Oxfordshire, England, 2006.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Houston, T. A., Koreeda, M. Iodine-promoted ribosylation leads to a facile acetonide-forming reaction. Carbohydr. Res. 2009, 344, 2240–2244; https://doi.org/10.1016/j.carres.2009.08.026.Search in Google Scholar PubMed
6. Li, C., Ding, H., Ruan, Z., Zhou, Y., Xiao, Q. First total synthesis of kipukasin A. Beilstein J. Org. Chem. 2017, 13, 855–862; https://doi.org/10.3762/bjoc.13.86.Search in Google Scholar PubMed PubMed Central
7. Ding, H., Ruan, Z., Kou, P., Dong, X., Bai, J., Xiao, Q. Total synthesis of mycalisine B. Mar. Drugs. 2019, 17, 226; https://doi.org/10.3390/md17040226.Search in Google Scholar PubMed PubMed Central
8. Jordheim, L. P., Durantel, D., Zoulim, F., Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464; https://doi.org/10.1038/nrd4010.Search in Google Scholar PubMed
9. Seley-Radtke, K. L., Yates, M. K. The evolution of nucleoside analogue antivirals: a review for chemists and non-chemists. Part 1: early structural modifications to the nucleoside scaffold. Antiviral Res. 2018, 154, 66–86; https://doi.org/10.1016/j.antiviral.2018.04.004.Search in Google Scholar PubMed PubMed Central
10. Yates, M. K., Seley–Radtke, K. L. The evolution of antiviral nucleoside analogues: a review for chemists and non-chemists. Part II: complex modifications to the nucleoside scaffold. Antiviral Res. 2019, 162, 5–21; https://doi.org/10.1016/j.antiviral.2018.11.016.Search in Google Scholar PubMed PubMed Central
11. Ichikawa, E., Kato, K. Sugar-modified nucleosides in past 10 years, a review. Curr. Med. Chem. 2001, 8, 385–423; https://doi.org/10.2174/0929867013373471.Search in Google Scholar PubMed
12. Thompson, P. A., Tam, C. S., O’Brien, S. M., Wierda, W. G., Stingo, F., Plunkett, W., Smith, S. C., Kantarjian, H. M., Freireich, E. J., Keating, M. J. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 2016, 127, 303–309; https://doi.org/10.1182/blood-2015-09-667675.Search in Google Scholar PubMed PubMed Central
13. Ishido, Y., Sakairi, N., Sekiya, M., Nakazaki, N. Partial protection of carbohydrate derivatives. Part 6. Regioselective O-deacylation of fully acylated glycosides and 1,2-O-isopropylidenealdofuranose derivatives with hydrazine hydrate. Carbohydr. Res. 1981, 97, 51–79; https://doi.org/10.1016/s0008-6215(00)80525-x.Search in Google Scholar
14. Agrofoglio, L. A., Jacquinet, J.-C., Lancelot, G. A multigram, stereoselective synthesis of D-[13C5]ribose from D-[13C6]glucose and its conversion into [13C5]nucleosides. Tetrahedron Lett. 1997, 38, 1411–1412; https://doi.org/10.1016/s0040-4039(97)00080-4.Search in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of a polymorph of potassium picrate, C6H2KN3O7
- The crystal structure of (1E,2E)-1,2-bis(quinolin-2-ylmethylene)hydrazine, C20H14N4
- 5-Amino-2-chloro-4-fluoro-N-(N-isopropyl-N-methylsulfamoyl) benzamide, C11H15O3ClFN3S
- Crystal structure of trans-N 1,N 8-bis(2-cyanoethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H42N6
- The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) – chloroform (1/1), C29H25NO4Si·CHCl3
- Crystal structure of tetracarbonyl-{μ-[N-(diphenylphosphanyl)-N,P,P-triphenylphosphinous amide]}-bis[μ-(phenylmethanethiolato)]diiron (Fe–Fe), C48H39Fe2NO4P2S2
- Crystal structure of baryte from Mine du Pradet (France)
- The crystal structure of [(2,2′-bipyridine-6-carboxylato-κ3 N,N,O)-(6-phenylpyridine-2-carboxylate-κ2 N,O)copper(II)] monohydrate, C23H17N3O5Cu
- Crystal structure of bis(μ-benzeneselenolato)-(μ-[N-benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous amide])-tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2
- The crystal structure of diaqua-methanol-κ1 O- (3-thiophenecarboxylato-κO)-(2,2′-dipyridyl-κ2 N,N′)manganese(II) 3-thiophenecarboxylate, C21H22N2O7S2Mn
- Crystal structure of catena-poly[tetrakis(butyl)-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O:O,O′,N-μ2-2-((oxido(phenyl)methylene)hydrazineylidene)propanoato-κ4 O,N,O′:N′-ditin(IV)], C34H50N6O6Sn2
- Crystal structure of 4-chloro-N′-[(1E)-(2-nitrophenyl)methylidene]benzohydrazide, C14H10ClN3O3
- The crystal structure of 3-(1′-deoxy-3′,5′-O-dibenzy-β-d-ribosyl)adenine dichloromethane solvate, C49H52Cl2N10O6
- The crystal structure of (Z)-4-amino-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H17N3O
- The co-crystal structure of etoricoxib–phthalic acid (1/1), C18H15ClN2O2S·C8H6O4
- Crystal structure of (glycinto-κ 2 O,N ′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ 4 N,N ′,N ″,N ‴]nickel(II) perchlorate monohydrate C18H42ClN5NiO7
- The crystal structure of catena-poly[bis(1-ethylimidazole-k1 N)-(μ 2-benzene-1-carboxyl-3,5-dicarboxylato-κ 2 O, O′)zinc(II)], C19H20N4O6Zn
- Crystal structure of 3-(thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C11H12N2O4S
- Rietveld structure analysis of keatite, a rare, metastable SiO2 polymorph
- Crystal structure of catena-poly[(μ2-isophthalato-k3 O,O′:O″)(4-(4-pyridyl)-2,5-dipyrazylpyridine-k3 N,N′,N″)cobalt(II)] trihydrate C26H22N6O7Co1
- Crystal structure of 3,5–di-O-benzoyl-1,2-O-isopropylidene-α–D-ribose, C22H22O7
- The crystal structure of fac-tricarbonyl(6-bromo-2,2-bipyridine-κ2 N,N)-(nitrato-κO)rhenium(I), C13H7BrN3O6Re
- The crystal structure of (E)-N′-(4-hydroxy-3-methoxybenzylidene)-2-naphthohydrazide monohydrate, C19H18N2O4
- The crystal structure of 5,5′-diselanediyl-bis(2-hydroxybenzaldehyde), C14H10O4Se2
- The crystal structure of catena-poly[diaqua-m2-dicyanido-κ2 C:N-dicyanido-κ1 C-bis(4-(pyridin-4-yl)benzaldehyde-κ1N)iron(II)-platinum(II), C28H22N6O4PtFe
- Redetermination of the crystal structure of 5,14-dihydro-6,17-dimethyl-8,15-diphenyldibenzo(b,i)(1,4,8,11)tetra-azacyclotetradecine, C32H28N4
- Crystal structure of poly[(μ3-2-(3,5-dicarboxyphenyl) benzimidazole-6-carboxylato-κ4O:O:O′:O″)lead(II)] monohydrate, C16H10N2O7Pb
- The crystal structure of fac-tricarbonyl(2-pyridin-2-yl-quinoline-κ2 N,N′)-(pyrazole-κN)rhenium(I)nitrate, C20H14N4O3ReNO3
- Crystal structure of dibromo-dicarbonyl-bis(tricyclohexylphosphine)-osmium(II) dichloromethane solvate, C38H66Br2O2OsP2
- Crystal structure of poly[bis(μ 2-2,6-bis(1-imidazoly)pyridine-κ 2 N:N′)copper(II)] diperchlorate dihydrate, C22H22Cl2CuN10O10
- The crystal structure of fac-tricarbonyl(N-benzoyl-N,N-cyclohexylmethylcarbamimidothioato-κ2 S,O)-(pyridine-κN)rhenium(I), C23H24N3O4ReS
- Crystal structure of (E)-7-fluoro-2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C17H12F2O
- Synthesis and crystal structure of 1-((3R,10S,13S, 17S)-3-((2-methoxyphenyl)amino)-10,13-dimethylhexadecahydro-1H-cyclopenta[α]phenanthren-17-yl)ethan-1-one, C28H41NO2
- The crystal structure of fac-tricarbonyl((pyridin-2-yl)methanamino-κ2 N,N′)-((pyridin-2-yl)methanamino-κN)rhenium(I) nitrate, C15H16O3N4Re
- The crystal structure of (1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-((1-(4-vinylbenzyl)-1H-benzo[d]imidazol-2-yl)methyl)methanamine-κ 4 N,N′,N″,N‴)tris(nitrato-kO,O′)-erbium(III), C29H27ErN8O9
- Crystal structure of tetracene-5,12-dione, C18H10O2
- Crystal structure of (3R,3aS,6R,6aR)-6-hexyl-3-methyltetrahydrofuro[3,4-b]furan-2,4-dione, C13H20O4
- The crystal structure of N′1,N′3-bis((E)-thiophen-2-ylmethylene)isophthalohydrazide monohydrate, C18H16N4O3S2
- Crystal structure of methyl ((4-aminobenzyl)sulfonyl)-L-prolinate, C13H18N2O4S
- Crystal structure of (E)-3-(3-methoxybenzylidene)benzofuran-2(3H)-one, C16H12O3
- Synthesis and crystal structure (E)-1-(4-bromo-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C11H12BrNO2
- Synthesis and crystal structure of (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C23H34O5
- The crystal structure of N,N′-(1,2-phenylene)bis (2-((2-oxopropyl)selanyl)benzamide), C26H24N2O4Se2
- The crystal structure of 1-ethyl-2-nitro-imidazole oxide, C5H7N3O3
- The crystal structure of 2-(2-fluorophenyl)naphtho[2,1-d]thiazole, C17H10FNS
- Crystal structure of (E)-2,4-di-tert-butyl-6-(((2-fluorophenyl)imino) methyl)phenol, C21H26FNO
- Synthesis and crystal structure of 3-methyl-2-(methylthio)-4H-chromen-4-one, C12H12O2S
- Crystal structure of dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-5,10-dione, C14H4O2S4
- The crystal structure of dimethyl 2,2ʹ-((adamantane-1,3-diylbis(4,1-phenylene)) bis(oxy))diacetate, C28H32O6
- The crystal structure of N-(6-chloro-2-methyl-2H-indazol-5-yl)acetamide, C10H10ClN3O
- Crystal structure of triaqua-(5-bromoisophthalato-κ1 O)-(2,2′-bipyridine-κ2 N:N′)nickel(II) hydrate, C18H19BrN2NiO8
- The crystal structure of 2-amino-4-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- The crystal structure of catena-poly[5-aminonicotinic acid-k1 N-m2-bromido-copper(I)], Cu(C6N2H6O2)Br
- The crystal structure of 2,2-bis(3-methoxyphenyl)-1-tosyl-1,2-dihydro- 2λ4,3λ4 -[1,3,2]diazaborolo[4,5,1-ij]quinoline - dichloromethane (1/1)
- The crystal structure of catena-poly[bis(6-phenylpyridine-2-carboxylato-κ2 N,O)-(μ2-4,4′-bipyridne-κ2 N:N)cadmium(II)], C34H24N4O4Cd
- The crystal structure of 5,7-dinitropyrazolo[5,1-b]quinazolin-9(4H)-one, C10H5N5O5
- Crystal structure of rac-1,8-bis(2-carbamoylethyl)-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane, C22H46N6O2
- The crystal structure of (E)-N ′-(2-bromobenzylidene)-2-naphthohydrazide, C36H26Br2N4O2
- The crystal structure of 5-nitronaphthoquinone, C10H5NO4
- The crystal structure of (S, R p )-4–benzhydrylideneamino-12-(4-tert-butyl oxazolin-2-yl)[2.2]paracyclophane, C36H36N2O
- Synthesis and crystal structure of 2-(2-oxo-2-(o-tolyl)ethyl)-4H-chromen-4-one, C18H14O3
- Crystal structure of 2-(thiazol-2-yl)hexahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione, C11H10N2O3S
- Crystal structure of N-(diaminomethylene)-1-(dimethylamino)-1-iminiomethanaminium dichloride, C4H13Cl2N5
- Crystal structure of poly[(μ3-3, 5-dichloro-2-hydroxy-benzoato-κ4 Cl,O:O′:O″) silver(I)], C7H3AgCl2O3
- The crystal structure of tetrakis(1-isopropylimidazole-κ1 N)-[μ2- imidazole-4,5-dicarboxylato-κ4 O,N,O′,N′)]- trioxido-divanadium, C29H41N10O7V2
- Crystal structure of catena-[(μ3-bromido)-(1H-1,2,4-triazol-1-yl)benzoato-κ1 N)copper(I)], C9H7BrCuN3O2
- The crystal structure of (E)-4-fluoro-N′-(1-(2-hydroxyphenyl)propylidene)benzohydrazide, C16H15FN2O2

