Abstract
C36H38N2O12Ti2, monoclinic, P21/n (no. 14), a = 10.3151(7) Å, b = 15.8747(11) Å, c = 11.5020(8) Å, β = 98.471(3)°, V = 1862.9(2) Å3, Z = 2, R gt(F) = 0.0386, wR ref(F 2) = 0.1075, T = 296(2) K.
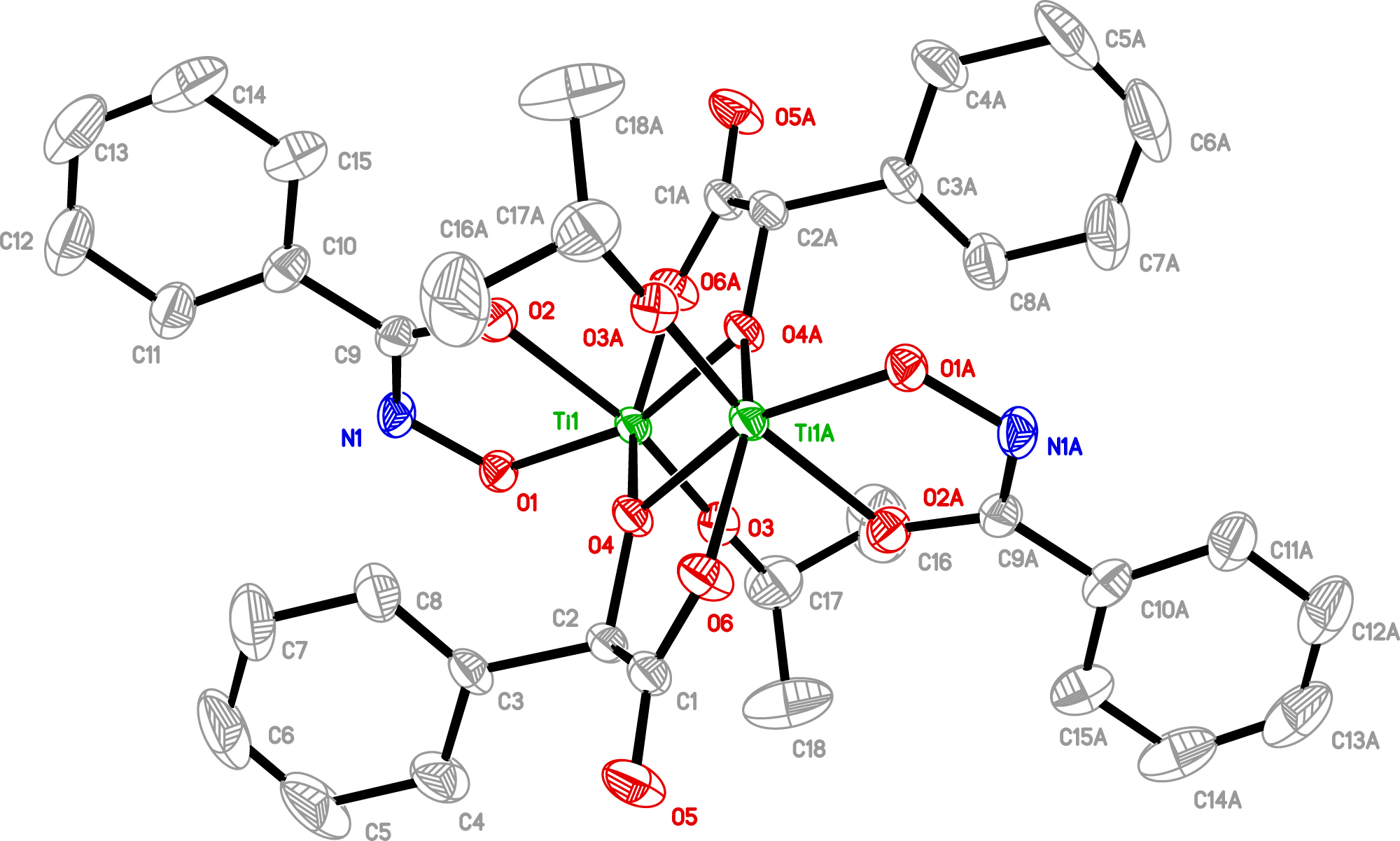
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellowish block |
| Size: | 0.22 × 0.21 × 0.17 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.49 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II |
| θ max, completeness: | 28.4°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 20724, 4653, 0.020 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3841 |
| N(param)refined: | 241 |
| Programs: | Bruker [1, 2], SHELX [3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.45626 (18) | 0.69315 (10) | 0.39588 (14) | 0.0392 (4) |
| C2 | 0.36042 (17) | 0.61868 (10) | 0.39164 (14) | 0.0360 (3) |
| H2 | 0.2960 | 0.6304 | 0.4442 | 0.043* |
| C3 | 0.28918 (18) | 0.60399 (11) | 0.26875 (15) | 0.0416 (4) |
| C4 | 0.1622 (2) | 0.63159 (15) | 0.2392 (2) | 0.0677 (6) |
| H4 | 0.1182 | 0.6562 | 0.2955 | 0.081* |
| C5 | 0.0997 (3) | 0.6221 (2) | 0.1228 (3) | 0.0995 (11) |
| H5 | 0.0150 | 0.6423 | 0.1012 | 0.119* |
| C6 | 0.1626 (4) | 0.5835 (2) | 0.0419 (3) | 0.1034 (12) |
| H6 | 0.1202 | 0.5765 | −0.0346 | 0.124* |
| C7 | 0.2873 (4) | 0.55510 (19) | 0.0719 (2) | 0.0895 (10) |
| H7 | 0.3296 | 0.5285 | 0.0160 | 0.107* |
| C8 | 0.3516 (3) | 0.56565 (15) | 0.18523 (18) | 0.0605 (6) |
| H8 | 0.4373 | 0.5467 | 0.2050 | 0.073* |
| C9 | 0.40037 (17) | 0.35817 (10) | 0.25850 (15) | 0.0381 (4) |
| C10 | 0.4490 (2) | 0.32279 (11) | 0.15463 (17) | 0.0464 (4) |
| C11 | 0.3691 (3) | 0.31484 (14) | 0.04697 (18) | 0.0609 (6) |
| H11 | 0.2827 | 0.3335 | 0.0378 | 0.073* |
| C12 | 0.4191 (4) | 0.27905 (17) | −0.0464 (2) | 0.0811 (8) |
| H12 | 0.3654 | 0.2731 | −0.1183 | 0.097* |
| C13 | 0.5444 (4) | 0.25259 (18) | −0.0349 (3) | 0.0914 (10) |
| H13 | 0.5763 | 0.2285 | −0.0988 | 0.110* |
| C14 | 0.6253 (3) | 0.26097 (17) | 0.0704 (3) | 0.0873 (9) |
| H14 | 0.7119 | 0.2428 | 0.0779 | 0.105* |
| C15 | 0.5772 (2) | 0.29670 (15) | 0.1659 (2) | 0.0640 (6) |
| H15 | 0.6318 | 0.3029 | 0.2373 | 0.077* |
| C16 | 0.2324 (5) | 0.4475 (3) | 0.7661 (3) | 0.1440 (19) |
| H16A | 0.2677 | 0.3915 | 0.7648 | 0.216* |
| H16B | 0.1661 | 0.4487 | 0.8164 | 0.216* |
| H16C | 0.3013 | 0.4861 | 0.7951 | 0.216* |
| C17 | 0.1744 (3) | 0.47213 (19) | 0.6457 (2) | 0.0745 (7) |
| H17 | 0.1064 | 0.4308 | 0.6174 | 0.089* |
| C18 | 0.1134 (4) | 0.5562 (2) | 0.6367 (5) | 0.1316 (16) |
| H18A | 0.1711 | 0.5959 | 0.6808 | 0.197* |
| H18B | 0.0320 | 0.5542 | 0.6677 | 0.197* |
| H18C | 0.0973 | 0.5731 | 0.5557 | 0.197* |
| H1 | 0.217 (2) | 0.3328 (13) | 0.2246 (18) | 0.044(5)* |
| N1 | 0.27581 (15) | 0.35972 (10) | 0.26548 (13) | 0.0411 (3) |
| O1 | 0.24168 (11) | 0.39634 (8) | 0.36442 (10) | 0.0379 (3) |
| O2 | 0.47699 (12) | 0.38863 (8) | 0.34421 (11) | 0.0419 (3) |
| O3 | 0.27218 (13) | 0.46692 (9) | 0.57341 (10) | 0.0463 (3) |
| O4 | 0.43525 (12) | 0.54754 (7) | 0.43393 (9) | 0.0358 (3) |
| O5 | 0.42037 (14) | 0.76294 (8) | 0.36059 (14) | 0.0578 (4) |
| O6 | 0.57385 (12) | 0.67569 (8) | 0.44142 (11) | 0.0452 (3) |
| Ti1 | 0.38148 (3) | 0.43352 (2) | 0.48073 (2) | 0.03263 (10) |
Source of material
All reagents and solvents employed in this work were commercially available and used without further purification.
Firstly, a mixture of mandelic acid (5 mmol, 0.761 g) and benzohydroxamic acid (1 mmol, 0.137 g) were placed in a Teflon-lined stainless vessel (15 mL), in which isopropanol (1 mL) and acetonitrile (5 mL) were added. After stirring for 5 min, Ti(O i Pr)4 (1.63 mmol, 0.5 mL) was added. The resulting mixture was sealed and heated at 373 K for 72 h under autogenous pressure. After cooling to room temperature at a rate of 5 K h−1, yellowish block crystals were obtained and washed with acetonitrile. The yield was 0.106 g (27%, based on mandelic acid).
Experimental details
H atoms were subsequently treated as riding atoms with distances C—H = 0.98 (CH3), 0.99 (CH) and 0.95 (ArH) Å.
Comment
In recent years, titanium oxo clusters (TOCs) have attracted much attention because their atomically precise molecular structures are ideal models for titanium dioxide (TiO2) [5], [6], [7]. Moreover, most reported TOCs exhibit photocatalytic activities, such as photocatalytic degradation, hydrogen production, and water oxidation [8], [9], [10]. The solvothermal synthetic approach using Ti(OR)4 as the titanium source have proven to be effective for construction TOCs, and a lot of TOCs with various nuclearities and diverse structures have been reported [11, 12]. Introduction of dye-functional ligands can enlarge the light-absorption range and reduce band gap values of TOCs, which is of great importance for photocatalytic applications. Generally, the dye-functional ligands for TOCs are characterized with C(sp2)–O or N(sp2)–O atoms as coordinative sites [13]. For example, catechol is a prevalent dye-functional ligand featuring C(sp2)–O as coordinative sites for construction TOCs with narrow band gap values [14]. To be noted, only a few TOCs protected by ligand with type of N(sp2)–O atom as coordinative site have been reported [15]. Herein, benzohydroxamic acid with N(sp2)–O atom was selected as dye-functional ligand to construct the title TOC.
The X-ray crystal diffraction revealed that the title compound crystallizes in the monoclinic system. There is one six-coordinated Ti4+ ion, two one deprotonated benzohydroxamate, one mandelate and one isopropoxide groups in the asymmetric unit. The dinuclear title complex is furnished by an inversion center (see the figure). Thus the two Ti4+ present the same coordination environments featuring the octahedral TiO6 mode. The six coordinated oxygen atoms belong to two mandelate ligands, one deprotonated benzohydroxamate and one isopropoxide groups respectively. Two μ 2–O-type atoms link the two Ti4+ ions together wtith the distance of 3.211 Å which is very close to the reported 3.222 Å of the TOC based on mandelic acid, and the average bond length of Ti–O is 1.956, almost the same as 1.963 Å of the reported structure [16].
Funding source: Anyang Institute of Technology
Award Identifier / Grant number: YPY2019003
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Foundation of Anyang Institute of Technology (YPY2019003).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2; Bruker AXS Inc.: Madison, Wisconsin, USA, 2005.Search in Google Scholar
2. Bruker. SAINT–Plus; Bruker AXS Inc.: Madison, Wisconsin, USA, 2001.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Version 3.2i; Crystal Impact: Bonn, Germany, 2012.Search in Google Scholar
5. Fang, W. H., Zhang, L., Zhang, J. Synthetic strategies, diverse structures and tuneable properties of polyoxo-titanium clusters. Chem. Soc. Rev. 2018, 47, 404–421; https://doi.org/10.1039/c7cs00511c.Search in Google Scholar PubMed
6. Yu, Y. Z., Guo, Y., Zhang, Y. R., Liu, M. M., Feng, Y. R., Geng, C. H., Zhang, X. M. A series of silver doped butterfly-like Ti8Ag2 clusters with two Ag ions panelled on a Ti8 surface. Dalton Trans. 2019, 48, 13423–13429; https://doi.org/10.1039/c9dt02508a.Search in Google Scholar PubMed
7. Zhang, G., Liu, C., Long, D.-L., Cronin, L., Tung, C.-H., Wang, Y. Water-soluble pentagonal-prismatic titanium-oxo clusters. J. Am. Chem. Soc. 2016, 138, 11097–11100; https://doi.org/10.1021/jacs.6b06290.Search in Google Scholar PubMed
8. Fang, W. H., Zhang, L., Zhang, J. A 3.6 nm Ti52 -oxo nanocluster with precise atomic structure. J. Am. Chem. Soc. 2016, 138, 7480–7483; https://doi.org/10.1021/jacs.6b03489.Search in Google Scholar PubMed
9. Wang, C., Wang, S. J., Kong, F. G. Calixarene-protected titanium-oxo clusters and their photocurrent responses and photocatalytic performances. Inorg. Chem. 2021, 60, 5034–5041; https://doi.org/10.1021/acs.inorgchem.1c00063.Search in Google Scholar PubMed
10. Lu, D. F., Kong, X. J., Lu, T. B., Long, L. S., Zheng, L. S. Heterometallic lanthanide-titanium oxo clusters: a new family of water oxidation catalysts. Inorg. Chem. 2017, 56, 1057–1060; https://doi.org/10.1021/acs.inorgchem.6b03072.Search in Google Scholar PubMed
11. Guo, Y.-H., Yu, Y.-Z., Niu, Y.-S., Wang, Z., Shi, W.-Y., Wu, X.-L. Solvothermal synthesis, crystal structure and photocurrent property of a Ti6 -core-based titanium oxo cluster. Chin. J. Struct. Chem. 2021, 40, 357–362.Search in Google Scholar
12. Liu, Y.-J., Fang, W.-H., Zhang, L., Zhang, J. Recent advances in heterometallic polyoxotitanium clusters. Coord. Chem. Rev. 2020, 404, 213099–213106; https://doi.org/10.1016/j.ccr.2019.213099.Search in Google Scholar
13. Yu, Y.-Z., Zhang, Y.-R., Geng, C.-H., Sun, L., Guo, Y., Feng, Y.-R., Wang, Y.-X., Zhang, X.-M. Precise and wide-ranged band-gap tuning of Ti6-core-based titanium oxo clusters by the type and number of chromophore ligands. Inorg. Chem. 2019, 58, 16785–16791; https://doi.org/10.1021/acs.inorgchem.9b02951.Search in Google Scholar PubMed
14. Wang, Y., Liu, C., Hu, J., Zhu, F., Zhan, J., Du, L., Tung, C. H. Functionalization of titanium-oxide cluster Ti17O24(OiC3H7)20 with catechols: structures and ligand-exchange reactivities. Chem. Eur J. 2019, 25, 14843–14849; https://doi.org/10.1002/chem.201902601.Search in Google Scholar PubMed
15. Chen, S., Fang, W.-H., Zhang, L., Zhang, J. Synthesis, structures, and photocurrent responses of polyoxo-titanium clusters with oxime ligands: from Ti4 to Ti18. Inorg. Chem. 2018, 57, 8850–8856; https://doi.org/10.1021/acs.inorgchem.8b00751.Search in Google Scholar PubMed
16. Yu, Y., Guo, Y., Niu, Y., Liu, N., Zhang, H. Crystal structure of bis(μ2-2-oxido-2-phenylacetate-κ3O:O,O′)-bis(1- isopropoxy-2-oxo-2-phenylethan-1-olato-κ2O,O′)-bis(propan-2- olato-κ1O)dititanium(IV), C44H52O14Ti2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 467–469.10.1515/ncrs-2020-0590Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

