Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
Abstract
C80H38Dy2F16N8O18, triclinic,
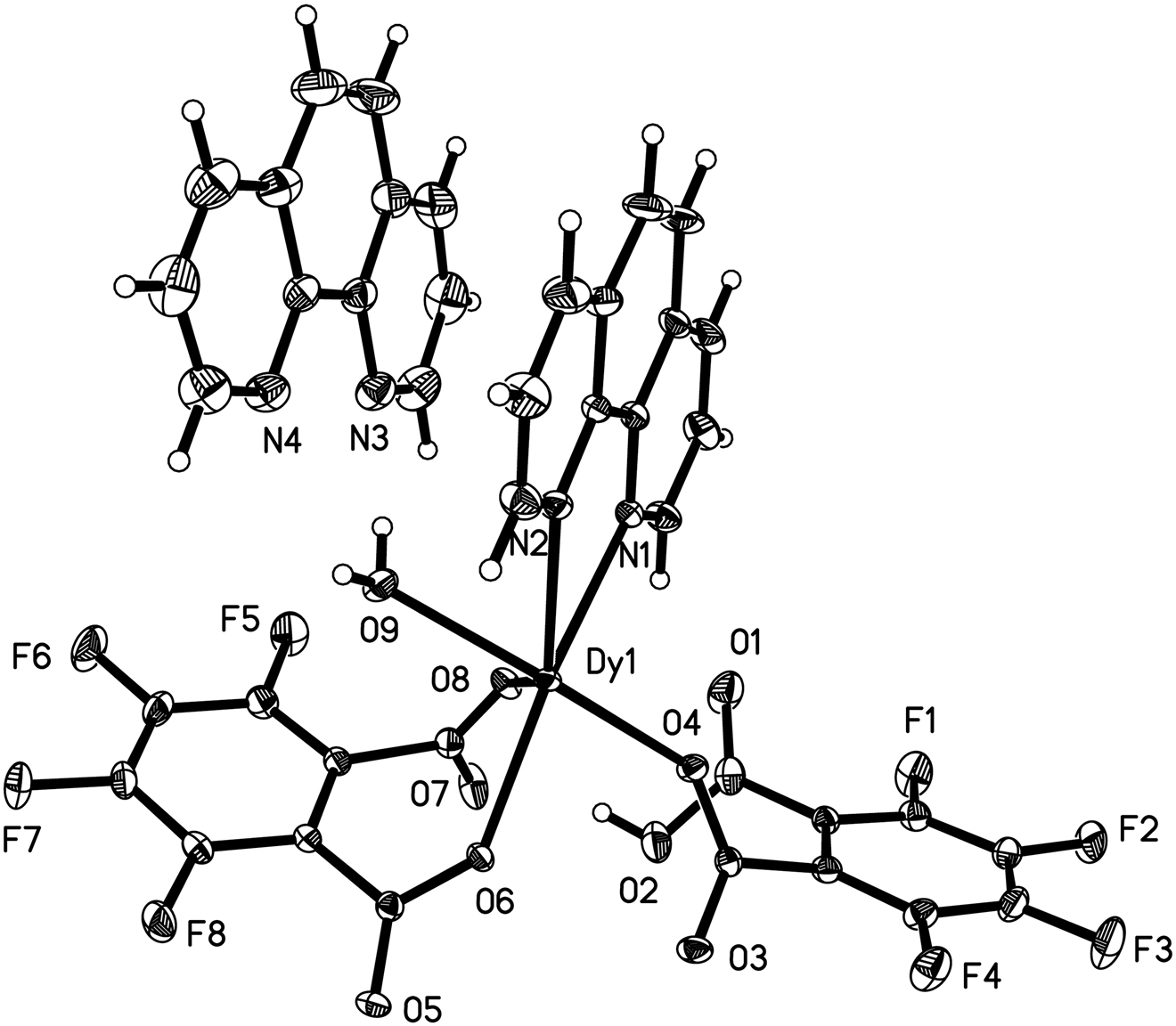
The asymmetric unit of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.19 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.23 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θ max, completeness: | 29.3°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 16224, 8196, 0.028 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 7557 |
| N(param)refined: | 565 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Dy1 | 0.41528 (2) | 0.83634 (2) | 0.44862 (2) | 0.01792 (4) |
| F1 | 0.46272 (19) | 0.80107 (17) | 0.99306 (14) | 0.0465 (5) |
| F2 | 0.70525 (19) | 0.87111 (17) | 1.08137 (14) | 0.0451 (5) |
| F3 | 0.87048 (19) | 1.00941 (19) | 1.00099 (16) | 0.0557 (6) |
| F4 | 0.79487 (18) | 1.07260 (16) | 0.83104 (14) | 0.0428 (5) |
| F5 | −0.06548 (18) | 0.75199 (17) | 0.43227 (16) | 0.0489 (5) |
| F6 | −0.17347 (19) | 0.80968 (18) | 0.26804 (16) | 0.0525 (6) |
| F7 | −0.04658 (19) | 0.99209 (17) | 0.20819 (14) | 0.0434 (5) |
| F8 | 0.17727 (19) | 1.12230 (16) | 0.31852 (15) | 0.0421 (5) |
| O1 | 0.3060 (2) | 0.7478 (2) | 0.78460 (19) | 0.0479 (7) |
| O2 | 0.3239 (2) | 0.9289 (2) | 0.78652 (18) | 0.0401 (6) |
| H2 | 0.2597 | 0.9024 | 0.7407 | 0.060* |
| O3 | 0.5768 (2) | 1.07762 (16) | 0.69644 (15) | 0.0285 (5) |
| O4 | 0.49264 (19) | 0.88821 (16) | 0.63512 (14) | 0.0238 (4) |
| O5 | 0.3519 (2) | 1.17454 (16) | 0.50476 (16) | 0.0317 (5) |
| O6 | 0.38412 (18) | 1.01348 (15) | 0.51152 (14) | 0.0210 (4) |
| O7 | 0.1425 (2) | 0.8920 (2) | 0.63637 (17) | 0.0390 (6) |
| O8 | 0.24177 (19) | 0.80514 (16) | 0.52977 (15) | 0.0258 (5) |
| O9 | 0.2311 (2) | 0.7562 (2) | 0.31571 (17) | 0.0302 (5) |
| H9A | 0.2473 | 0.7649 | 0.2553 | 0.045* |
| H9B | 0.195 (4) | 0.691 (4) | 0.302 (3) | 0.072 (15)* |
| N1 | 0.3704 (2) | 0.63910 (19) | 0.46976 (18) | 0.0228 (5) |
| N2 | 0.4657 (2) | 0.69544 (19) | 0.30468 (18) | 0.0240 (5) |
| C1 | 0.5001 (3) | 0.8921 (2) | 0.8576 (2) | 0.0228 (6) |
| C2 | 0.5856 (3) | 0.9620 (2) | 0.8146 (2) | 0.0233 (6) |
| C3 | 0.7076 (3) | 1.0038 (3) | 0.8668 (2) | 0.0283 (7) |
| C4 | 0.7498 (3) | 0.9734 (3) | 0.9561 (2) | 0.0325 (7) |
| C5 | 0.6662 (3) | 0.9033 (3) | 0.9967 (2) | 0.0300 (7) |
| C6 | 0.5427 (3) | 0.8659 (3) | 0.9483 (2) | 0.0275 (7) |
| C7 | 0.3651 (3) | 0.8467 (3) | 0.8053 (2) | 0.0307 (7) |
| C8 | 0.5470 (3) | 0.9779 (2) | 0.7057 (2) | 0.0214 (6) |
| C9 | 0.1800 (3) | 1.0041 (2) | 0.4349 (2) | 0.0206 (6) |
| C10 | 0.1159 (3) | 0.9065 (2) | 0.4622 (2) | 0.0220 (6) |
| C11 | −0.0025 (3) | 0.8454 (3) | 0.4068 (2) | 0.0295 (7) |
| C12 | −0.0587 (3) | 0.8733 (3) | 0.3226 (2) | 0.0317 (7) |
| C13 | 0.0038 (3) | 0.9665 (3) | 0.2934 (2) | 0.0300 (7) |
| C14 | 0.1197 (3) | 1.0325 (2) | 0.3513 (2) | 0.0262 (7) |
| C15 | 0.3120 (3) | 1.0710 (2) | 0.4878 (2) | 0.0212 (6) |
| C16 | 0.1728 (3) | 0.8648 (2) | 0.5503 (2) | 0.0236 (6) |
| C17 | 0.3245 (3) | 0.6110 (3) | 0.5504 (2) | 0.0308 (7) |
| H17 | 0.3120 | 0.6672 | 0.6017 | 0.037* |
| C18 | 0.2941 (3) | 0.5006 (3) | 0.5618 (3) | 0.0366 (8) |
| H18 | 0.2632 | 0.4849 | 0.6200 | 0.044* |
| C19 | 0.3099 (3) | 0.4170 (3) | 0.4879 (3) | 0.0365 (8) |
| H19 | 0.2883 | 0.3431 | 0.4941 | 0.044* |
| C20 | 0.3815 (4) | 0.3593 (3) | 0.3211 (3) | 0.0435 (9) |
| H20 | 0.3621 | 0.2845 | 0.3247 | 0.052* |
| C21 | 0.4299 (4) | 0.3876 (3) | 0.2411 (3) | 0.0448 (9) |
| H21 | 0.4447 | 0.3323 | 0.1905 | 0.054* |
| C22 | 0.5141 (4) | 0.5346 (3) | 0.1501 (3) | 0.0432 (9) |
| H22 | 0.5305 | 0.4817 | 0.0981 | 0.052* |
| C23 | 0.5430 (4) | 0.6446 (3) | 0.1480 (3) | 0.0434 (9) |
| H23 | 0.5801 | 0.6679 | 0.0951 | 0.052* |
| C24 | 0.5165 (3) | 0.7226 (3) | 0.2259 (2) | 0.0345 (8) |
| H24 | 0.5353 | 0.7974 | 0.2225 | 0.041* |
| C25 | 0.4382 (3) | 0.5855 (2) | 0.3080 (2) | 0.0234 (6) |
| C26 | 0.4593 (3) | 0.5008 (3) | 0.2315 (2) | 0.0323 (7) |
| C27 | 0.3590 (3) | 0.4424 (2) | 0.4016 (2) | 0.0287 (7) |
| C28 | 0.3870 (3) | 0.5553 (2) | 0.3954 (2) | 0.0238 (6) |
| N3 | 0.0923 (3) | 0.5343 (2) | 0.2690 (2) | 0.0396 (7) |
| N4 | 0.1733 (3) | 0.6083 (2) | 0.1020 (2) | 0.0408 (7) |
| C29 | 0.0460 (4) | 0.4962 (4) | 0.3450 (3) | 0.0527 (10) |
| H29 | 0.0407 | 0.5487 | 0.4037 | 0.063* |
| C30 | 0.0049 (4) | 0.3818 (4) | 0.3422 (3) | 0.0594 (12) |
| H30 | −0.0292 | 0.3590 | 0.3967 | 0.071* |
| C31 | 0.0153 (4) | 0.3045 (3) | 0.2589 (3) | 0.0549 (11) |
| H31 | −0.0091 | 0.2279 | 0.2567 | 0.066* |
| C32 | 0.0724 (4) | 0.2629 (3) | 0.0846 (4) | 0.0601 (12) |
| H32 | 0.0524 | 0.1862 | 0.0818 | 0.072* |
| C33 | 0.1095 (4) | 0.2977 (3) | 0.0027 (3) | 0.0611 (12) |
| H33 | 0.1133 | 0.2448 | −0.0564 | 0.073* |
| C34 | 0.1778 (4) | 0.4554 (4) | −0.0808 (3) | 0.0575 (11) |
| H34 | 0.1797 | 0.4047 | −0.1422 | 0.069* |
| C35 | 0.2082 (4) | 0.5668 (4) | −0.0744 (3) | 0.0596 (12) |
| H35 | 0.2307 | 0.5939 | −0.1310 | 0.071* |
| C36 | 0.2052 (4) | 0.6407 (3) | 0.0190 (3) | 0.0520 (10) |
| H36 | 0.2272 | 0.7177 | 0.0230 | 0.062* |
| C37 | 0.1410 (3) | 0.4959 (3) | 0.0952 (3) | 0.0348 (8) |
| C38 | 0.1433 (4) | 0.4154 (3) | 0.0046 (3) | 0.0449 (9) |
| C39 | 0.0630 (3) | 0.3401 (3) | 0.1756 (3) | 0.0428 (9) |
| C40 | 0.0989 (3) | 0.4570 (3) | 0.1827 (3) | 0.0338 (7) |
Source of materials
Dy(NO3)3·6H2O (0.0913 g, 0.2 mmol), 3,4,5,6-tetrafluorophthalate (0.054 g, 0.2 mmol) and phenanthroline (35.7 mg, 0.15 mmol) were mixed with 8 mL of deionized water in a reagent bottle. The mixture was treated by ultrasonic waves for 15 min at room temperature, sealed in a 25 mL Teflon-lined steel autoclave and heated at 110 °C for 72 h. The mixture was cooled to room temperature within 36 h and colorless block crystals were isolated after filtration, washed with distilled water and dried in air.
Experimental details
Using Olex2 [2], the structure was solved with the ShelXT [3] structure solution program and refined with the ShelXL [4] refinement package.
The carbon bound hydrogen atoms were placed in calculated positions and refined using a riding model on attached atoms.
Comment
In the last decades, a large number of Dy(III)-metal organic frameworks (MOFs) have been reported due to their excellent magnetic and optical properties [5], [6], [7]. Dy–MOFs can be constructed from Dy(III) ion and carboxylate and nitrogen-containing ligands [8], [9], [10]. Among various carboxylate ligands, tetrafluorophthalate (TFPA) is a good candidate because it has multiple coordinate modes and can serve as a bridging ligand [11], [12], [13]. In addition, phenanthroline (phen) is one of the commonly used N-containing ligands in combination with carboxylates [14, 15]. In this work, a binuclear Dy–complex was constructed with TFPA and phen as ligands. It is worth noting that, using the same reactants as described in the literature, different framework structures are produced due to different reaction conditions [9, 10]. Thus the dinuclear title complex was obtained unexpectedly.
In the title complex, there are one Dy(III) ion, one phen ligand, two differently deprotonated tetrafluorophthalato ligands (HTFPA− and TFPA2−, respectively), one coordinated water and one cocrystallized phen in the asymmetric unit. The cocrystallized phen molecule is linked to the coordinated water by a hydrogen bond (O9–H9A⃛N3). Dy1 is nine-coordinated by two N atoms (N1, N2) of the phen ligand, one O atom (O9) from coordinated water, two O atoms (O4, O3A) from two mono-deprotonated HTFPA− ligands and four O atoms (O6, O8, O5A and O6A) from two fully-deprotonated TFPA2− ligands. The Dy–N bond lengths are 2.506(2) and 2.561(2) Å and the Dy–O ones are in the range of 2.0560(14)–2.3610(19) Å. Two Dy(III) ions are bridged by two HTFPA− and two TFPA2− ligands to form a dinuclear {Dy2} unit, which is further connected into a 3D structure by π–π interactions and C–H⃛O hydrogen bonds.
Funding source: Training Program for Young Cadre Teachers of Higher Education Institutions in Henan Province
Award Identifier / Grant number: 2018GGJS128
Funding source: Science and Technology Development Project in Henan Province
Award Identifier / Grant number: 172101410037
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Training Program for Young Cadre Teachers of Higher Education Institutions in Henan Province (No. 2018GGJS128) and Science and Technology Development Project in Henan Province (No. 172101410037).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction Ltd. CrysAlisPRO, Version 1.171.39.6a; Rigaku Oxford Diffraction: England, 2018.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Rajak, R., Saraf, M., Verma, S. K., Kumar, R., Mobin, S. M. Dy (III)-based metal–organic framework as a fluorescent probe for highly selective detection of picric acid in aqueous medium. Inorg. Chem. 2019, 58, 16065–16074; https://doi.org/10.1021/acs.inorgchem.9b02611.Search in Google Scholar PubMed
6. Zhang, K., Montigaud, V., Cador, O., Li, G. P., Le Guennic, B., Tang, J. K., Wang, Y. Y. Tuning the magnetic interactions in Dy(III)4 single-molecule magnets. Inorg. Chem. 2018, 57, 8550–8557; https://doi.org/10.1021/acs.inorgchem.8b01269.Search in Google Scholar PubMed
7. Li, R. F., Li, R. H., Liu, X. F., Chang, X. H., Feng, X. Lanthanide complexes based on a conjugated pyridine carboxylate ligand: structures, luminescence and magnetic properties. RSC Adv. 2020, 10, 6192–6199; https://doi.org/10.1039/c9ra10975g.Search in Google Scholar PubMed PubMed Central
8. Wankar, S., Limaye, S. N. Luminescence and electronic spectral studies of some synthesized lanthanide complexes using benzoic acid derivative and o-phenanthroline. J. Fluoresc. 2015, 25, 787–794; https://doi.org/10.1007/s10895-015-1573-6.Search in Google Scholar PubMed
9. Feng, X., Shang, Y., Zhang, H., Li, R., Wang, W., Zhang, D., Wang, L., Li, Z. Correction: enhanced luminescence and tunable magnetic properties of lanthanide coordination polymers based on fluorine substitution and phenanthroline ligand. RSC Adv. 2019, 9, 18182; https://doi.org/10.1039/c9ra90044f.Search in Google Scholar PubMed PubMed Central
10. Li, J. J., Fan, T. T., Qu, X. L., Han, H. L., Li, X. Temperature-induced 1D lanthanide polymeric frameworks based on Ln n (n = 2, 2, 4, 6) cores: synthesis, crystal structures and luminescence properties. Dalton Trans. 2016, 45, 2924–2935; https://doi.org/10.1039/c5dt04262c.Search in Google Scholar PubMed
11. Sakamoto, H., Ito, A., Ohtani, M. Unusual ligand substitution of a metal–organic framework with distorted metal–ligand coordination. CrystEngComm 2022, 24, 1690–1694; https://doi.org/10.1039/d2ce00060a.Search in Google Scholar
12. Blair, L. H., Colakel, A., Vrcelj, R. M., Sinclair, I., Coles, S. J. Metal–organic fireworks: MOFs as integrated structural scaffolds for pyrotechnic materials. Chem. Commun. 2015, 51, 12185–12188; https://doi.org/10.1039/c5cc04174k.Search in Google Scholar PubMed
13. Li, Y., Han, M. Crystal structure of poly[diaqua-(μ2-4,4′-bipyridine -κ2N:N′)-(μ2-3,4,5,6-tetrafluorophthalato-κ2O:O′)nickel(II)], C18H12F4NiN2O6. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 563–564; https://doi.org/10.1515/ncrs-2019-0808.Search in Google Scholar
14. Xin, L. Y., Yang, H. B., Guo, H. Crystal structure of bis (phenanthroline)-zinc(II) 5-(4-carboxy-2-nitrophenoxy) isophthalic hydrate, Zn(C15H9NO9)(C12H8N2)2∙(H2O). Z. Kristallogr. N. Cryst. Struct. 2012, 227, 407–409; https://doi.org/10.1524/ncrs.2012.0166.Search in Google Scholar
15. Zhang, T., Li, R. F., Feng, X., Ng, S. W., Bai, R. F. Synthesis, crystal structure and characterization of a lanthanide complex based on 4-(4-oxypyridinium-1-yl) phthalic acid and 1, 10-phenanthroline. Inorg. Nano-Metal Chem. 2017, 47, 375–379; https://doi.org/10.1080/15533174.2016.1186065.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

