Abstract
C27H23Fe2O5PS2, monoclinic, P21/c (no. 14), a = 15.4423(7) Å, b = 9.8615(5) Å, c = 18.0727(8) Å, β = 90.0380(10)°, V = 2752.2(2) Å3, Z = 4, Rgt (F) = 0.0296, wRref (F 2) = 0.0703, T = 296(2) K.
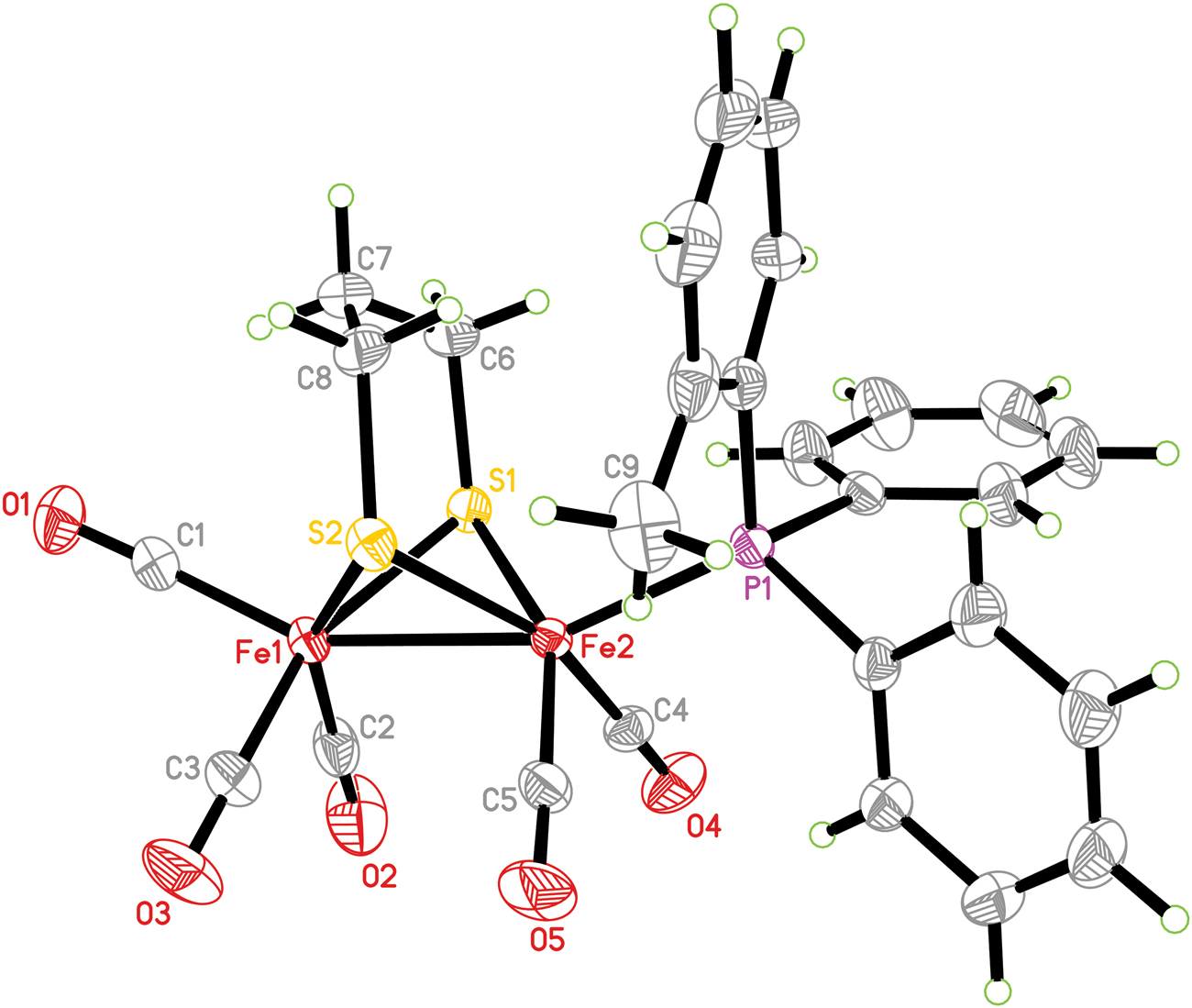
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.38 × 0.36 × 0.32 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.30 mm−1 |
| Diffractometer, scan mode: | BRUKER D8 QUEST, ω |
| θ max, completeness: | 25.1°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 54,372, 4885, 0.073 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4140 |
| N(param)refined: | 335 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Fe1 | 0.43816 (2) | 0.09396 (3) | 0.31662 (2) | 0.03461 (10) |
| Fe2 | 0.28985 (2) | 0.17386 (3) | 0.35269 (2) | 0.02898 (9) |
| S1 | 0.37835 (4) | 0.05643 (6) | 0.42936 (3) | 0.03509 (14) |
| S2 | 0.40624 (4) | 0.31378 (6) | 0.34043 (3) | 0.03888 (15) |
| P1 | 0.19035 (4) | 0.28463 (6) | 0.42070 (3) | 0.03157 (14) |
| O1 | 0.62517 (12) | 0.0545 (2) | 0.33748 (12) | 0.0740 (6) |
| O2 | 0.38477 (16) | −0.1782 (2) | 0.27402 (16) | 0.0949 (9) |
| O3 | 0.44544 (18) | 0.1700 (3) | 0.16077 (11) | 0.0883 (8) |
| O4 | 0.18868 (13) | −0.07373 (19) | 0.34371 (12) | 0.0655 (5) |
| O5 | 0.23554 (15) | 0.2630 (3) | 0.20651 (10) | 0.0838 (7) |
| C1 | 0.55300 (17) | 0.0710 (3) | 0.33058 (14) | 0.0467 (6) |
| C2 | 0.40779 (17) | −0.0733 (3) | 0.29155 (16) | 0.0542 (7) |
| C3 | 0.44286 (18) | 0.1400 (3) | 0.22161 (15) | 0.0516 (7) |
| C4 | 0.22737 (15) | 0.0250 (3) | 0.34688 (13) | 0.0404 (6) |
| C5 | 0.25572 (16) | 0.2310 (3) | 0.26472 (13) | 0.0460 (6) |
| C6 | 0.42621 (17) | 0.1603 (3) | 0.50225 (13) | 0.0496 (6) |
| H6A | 0.3798 | 0.2060 | 0.5284 | 0.060* |
| H6B | 0.4545 | 0.1004 | 0.5373 | 0.060* |
| C7 | 0.48955 (18) | 0.2635 (3) | 0.47824 (15) | 0.0576 (7) |
| H7A | 0.5369 | 0.2179 | 0.4532 | 0.069* |
| H7B | 0.5133 | 0.3072 | 0.5218 | 0.069* |
| C8 | 0.45398 (17) | 0.3711 (3) | 0.42763 (15) | 0.0515 (7) |
| H8A | 0.5004 | 0.4338 | 0.4161 | 0.062* |
| H8B | 0.4102 | 0.4214 | 0.4546 | 0.062* |
| C9 | 0.2452 (2) | 0.5714 (3) | 0.34870 (19) | 0.0713 (9) |
| H9A | 0.2349 | 0.4844 | 0.3265 | 0.107* |
| H9B | 0.2990 | 0.6074 | 0.3308 | 0.107* |
| H9C | 0.1989 | 0.6321 | 0.3360 | 0.107* |
| C10 | 0.22727 (14) | 0.4369 (2) | 0.46970 (14) | 0.0407 (6) |
| C11 | 0.24957 (16) | 0.5562 (3) | 0.43145 (17) | 0.0541 (7) |
| C12 | 0.2779 (2) | 0.6663 (3) | 0.4736 (3) | 0.0761 (11) |
| H12 | 0.2917 | 0.7470 | 0.4498 | 0.091* |
| C13 | 0.2859 (2) | 0.6588 (4) | 0.5486 (3) | 0.0879 (13) |
| H13 | 0.3045 | 0.7344 | 0.5749 | 0.105* |
| C14 | 0.26700 (19) | 0.5411 (4) | 0.5858 (2) | 0.0745 (10) |
| H14 | 0.2742 | 0.5356 | 0.6368 | 0.089* |
| C15 | 0.23715 (16) | 0.4310 (3) | 0.54613 (15) | 0.0522 (7) |
| H15 | 0.2234 | 0.3513 | 0.5710 | 0.063* |
| C16 | 0.09313 (15) | 0.3427 (2) | 0.37106 (13) | 0.0397 (6) |
| C17 | 0.04935 (17) | 0.4597 (3) | 0.39124 (16) | 0.0566 (7) |
| H17 | 0.0724 | 0.5162 | 0.4275 | 0.068* |
| C18 | −0.02828 (19) | 0.4930 (3) | 0.35794 (19) | 0.0701 (9) |
| H18 | −0.0570 | 0.5720 | 0.3715 | 0.084* |
| C19 | −0.06290 (19) | 0.4103 (4) | 0.30515 (18) | 0.0700 (9) |
| H19 | −0.1156 | 0.4326 | 0.2834 | 0.084* |
| C20 | −0.02054 (18) | 0.2948 (3) | 0.28389 (16) | 0.0618 (8) |
| H20 | −0.0441 | 0.2391 | 0.2475 | 0.074* |
| C21 | 0.05768 (16) | 0.2613 (3) | 0.31690 (14) | 0.0464 (6) |
| H21 | 0.0866 | 0.1830 | 0.3023 | 0.056* |
| C22 | 0.13853 (15) | 0.1834 (2) | 0.49418 (12) | 0.0379 (5) |
| C23 | 0.18226 (19) | 0.0808 (3) | 0.52900 (15) | 0.0591 (7) |
| H23 | 0.2382 | 0.0596 | 0.5139 | 0.071* |
| C24 | 0.1452 (2) | 0.0082 (4) | 0.58600 (18) | 0.0765 (10) |
| H24 | 0.1764 | −0.0602 | 0.6094 | 0.092* |
| C25 | 0.0626 (2) | 0.0371 (4) | 0.60806 (18) | 0.0770 (10) |
| H25 | 0.0375 | −0.0113 | 0.6467 | 0.092* |
| C26 | 0.0173 (2) | 0.1365 (4) | 0.57353 (19) | 0.0754 (10) |
| H26 | −0.0391 | 0.1554 | 0.5883 | 0.090* |
| C27 | 0.05411 (18) | 0.2098 (3) | 0.51657 (16) | 0.0578 (7) |
| H27 | 0.0222 | 0.2773 | 0.4931 | 0.069* |
Source of material
To a solution of the complex [Fe2(CO)6(μ-SCH2CH2CH2S)] (1 mmol) and diphenyl(o-tolyl)phosphine (1 mmol) in CH2Cl2 (10 mL) was added a solution of Me3NO ⋅ 2H2O (1 mmol) in CH3CN (10 mL). The mixture was stirred for 1 h and the solvent was reduced by rotary evaporator. The brown residue was subjected to TLC separation to collect the main red band. Slow evaporation of CH2Cl2/isopropanol solution at 4° afforded crystals suitable for X-ray diffraction analysis.
Experimental details
The structure was solved by direct method with the SHELXS program. Hydrogen atoms were positioned geometrically (C–H = 0.93–0.98 Å). Their U iso values were set to 1.2U eq or 1.5U eq of the parent atoms.
Comment
Carbonyl substitution of a diiron propanedithiolate complex [Fe2(CO)6(μ-SCH2CH2CH2S)] with phosphine ligands has received great interest in recent years [5], [6], [7], [8]. A great number of crystal structures of these complexes have been reported.
The asymmetric unit of the title complex is composed of a diiron propanedithiolate moiety ligated by five terminal carbonyls and a phosphine ligand (see the figure). The diiron propanedithiolate fragment contains two six-membered metallocycles, in which the six-membered metallocycle Fe1S1C6C7C8S2 is a boat-conformation and the other six-membered metallocycle Fe2S1C6C7C8S2 is a chair-conformation. The position of the phosphine ligand is apical of the pseudo-octahedral geometry of the Fe2 atom, in accord with other analogues containing monosubstituted ligands [9]. The Fe1–Fe2 bond length [2.5085(4) Å] is slightly shorter than that of the diiron hexacarbonyl complex [Fe2(CO)6(μ-SCH2CH2CH2S)] [2.5103(11) Å] [10], indicating that the phosphine ligand does not affect the Fe–Fe bond length. Moreover, the Fe1–Fe2 bond length is close to those of hexacarbonyl analogues [11], but much shorter than those of diphosphine-containing analogues [12].
Funding source: Zhejiang Provincial Natural Science Foundation of China
Award Identifier / Grant number: LY19B020002
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant LY19B020002.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
5. Sheng, Y. D., Yu, X. Y., Liu, X. F., Li, Y. L. 2-(Diphenylphosphino)benzaldehyde or isopropyldiphenylphosphine substituted diiron dithiolate complexes. Polyhedron 2017, 137, 134–139; https://doi.org/10.1016/j.poly.2017.08.029.Search in Google Scholar
6. Wang, Z., Jiang, W., Liu, J., Jiang, W., Wang, Y., Åkermark, B., Sun, L. Pendant bases as proton transfer relays in diiron dithiolate complexes inspired by [Fe–Fe] hydrogenase active site. J. Organomet. Chem. 2008, 693, 2828–2834, https://doi.org/10.1016/j.jorganchem.2008.06.001.Search in Google Scholar
7. Pandey, I. K., Natarajan, M., Hemlata, Hussain F., Kaur–Ghumaan, S. Diiron complexes [Fe2(CO)5(μ-pdt/Mebdt)(L)] containing a chelating diphosphine ligand L=(oxydi-2,1-phenylene)bis(diphenylphosphine): bioinspired [FeFe] hydrogenase model complexes. ChemistrySelect 2016, 1, 5671–5678; https://doi.org/10.1002/slct.201601216.Search in Google Scholar
8. Li, P., Wang, M., He, C., Li, G., Liu, X., Chen, C., Åkermark, B., Sun, L. Influence of tertiary phosphanes on the coordination configurations and electrochemical properties of iron hydrogenase model complexes: crystal structures of [(μ-S2C3H6)Fe2(CO)6−nLn] (L = PMe2Ph, n = 1, 2; PPh3, P(OEt)3, n = 1). Eur. J. Inorg. Chem. 2005, 2005, 2506–2513; https://doi.org/10.1002/ejic.200400947.Search in Google Scholar
9. Yan, L., Hu, K., Liu, X. F., Li, Y. L., Liu, X. H., Jiang, Z. Q. Diiron ethane-1,2-dithiolate complexes with 1,2,3-thiadiazole moiety: synthesis, X-ray crystal structures, electrochemistry, and fungicidal activity. Appl. Organomet. Chem. 2021, 35, e6084; https://doi.org/10.1002/aoc.6084.Search in Google Scholar
10. Lyon, E. J., Georgakaki, I. P., Reibenspies, J. H., Darensbourg, M. Y. Carbon monoxide and cyanide ligands in a classical organometallic complex model for Fe-only hydrogenase. Angew. Chem. Int. Ed. 1999, 38, 3178–3180; https://doi.org/10.1002/(sici)1521-3773(19991102)38:21<3178::aid-anie3178>3.0.co;2-4.10.1002/(SICI)1521-3773(19991102)38:21<3178::AID-ANIE3178>3.0.CO;2-4Search in Google Scholar
11. Lian, M., He, J., Yu, X. Y., Mu, C., Liu, X. F., Li, Y. L., Jiang, Z. Q. Diiron ethanedithiolate complexes with acetate ester: synthesis, characterization and electrochemical properties. J. Organomet. Chem. 2018, 870, 90–96; https://doi.org/10.1016/j.jorganchem.2018.06.023.Search in Google Scholar
12. Zhao, P. H., Hu, M. Y., Li, J. R., Wang, Y. Z., Lu, B. P., Han, H. F., Liu, X. F. Impacts of coordination modes (chelate versus bridge) of PNP-diphosphine ligands on the redox and electrocatalytic properties of diiron oxadithiolate complexes for proton reduction. Electrochim. Acta 2020, 353, 136615; https://doi.org/10.1016/j.electacta.2020.136615.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-(1-(2-((5-methylthiophen-2-yl)methylene)hydrazinyl)ethylidene)chroman-2,4-dione, C17H14N2O3S
- Crystal structure of chlorido-(η 6-toluene)(5,5′-dimethyl-2,2′-bipyridine-κ2 N,N′)ruthenium(II) hexafluoridophosphate(V) ─ acetone (1/1) C22H26ClN2ORuPF6
- Crystal structure of 4-(((2-(3-(1-(3-(3-cyanophenyl)-6-oxopyridazin-1(6H)-yl)ethyl)phenyl) pyrimidin-5-yl)oxy)methyl)-1-methylpiperidin-1-ium chloride monohydrate, C30H33N6O2Cl
- The crystal structure of 2-chloro-N-((2-chlorophenyl)carbamoyl)nicotinamide, C13H9Cl2N3O2
- Crystal structure of 9-(t-butyl)-3,11-dihydro-6H-pyrazolo [1,5-a]pyrrolo[3′,2′:5,6]pyrido[4,3-d]pyrimidin-6-one hemihydrate, C30H32N10O3
- Crystal structure of di-μ2-hydroxido-tetrakis(6-methylpyridine-2-carboxylato-k2 N,O) diiron(III) trihydrate C28H32Fe2N4O13
- Crystal structure of catena-poly[qua-(μ2-2-aminoisophthalat-κ3 O,O′:O′′)(1,10-phenanthroline-κ2 N,N′)manganese(II)] C20H15MnN3O5
- Crystal structure of poly[(bis(isothiocyano)-bis(μ 2-(E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide))iron(II) – methanol – 1,4-dioxane (1/2/2), C36H44FeN10O8S2
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl)propylidene)-4-hydroxybenzohydrazide, C16H15ClN2O3
- Crystal structure of bis(μ2-benzoato-k2O:O′)-bis(μ2-benzoato-k3O,O′:O′)dinitrato-k2O,O′-bis(phenanthroline-k2 N,N′)dierbium(III), C52H36Er2N6O14
- Crystal structure of 4-ethyl-2-{[(4-nitrophenyl)methyl]sulfanyl}-6-oxo-1,6-dihydropyrimidine-5-carbonitrile, C14H12N4O3S
- Synthesis and crystal structure of 1-((3R,10S,13R,17S)-10,13-dimethyl-3- (phenylamino)hexadecahydro-1H-cyclopenta[α] phenanthren-17-yl)ethan-1-one, C27H39NO
- Crystal structre of 1,4-bis(bromomethyl)-2,3,5,6-tetramethylbenzene, C12H16Br2
- Crystal structure of 2-(adamantan-1-yl)-5-(3,5-dinitrophenyl)-1,3,4-oxadiazole, C18H18N4O5
- Crystal structure of (E)-N′-benzylidene-4-nitrobenzohydrazide – methanol (1/1), C15H15N3O4
- The crystal structure of 3-(2-bromophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23BrO2
- Crystal structure of catena-poly[(μ 2-4,4′-bipyridine-κ2 N:N′)-bis(4-bromobenzoato-κ1 O)zinc(II)], C24H16Br2N2O4Zn
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)zinc(II), C20H14N6ZnS4
- Crystal structure of pentacarbonyl-(μ2-propane-1,3-dithiolato-κ4 S:S,S′:S′)-(diphenyl(o-tolyl)phosphine-κ1 P)diiron (Fe-Fe), C27H23Fe2O5PS2
- The crystal structure of the cocrystal 4-hydroxy-3,5-dimethoxybenzoic acid–pyrazine-2-carboxamide(1/1), C14H15N3O6
- The crystal structure of dichlorido-bis((RS)-2-(4-chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile-κ1 N)zinc(II), C30H34Cl4N8Zn
- Crystal structure of the cocrystal 2,4,6-triamino-1,3,5-triazine – 1H-isoindole-1,3(2H)-dione – methanol (1/1/1), C12H15N7O3
- The crystal structure of methyl 4-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl) benzoate, C23H28O3
- Crystal structure of (poly[µ2-(1H-pyrazol-1-yl)methyl]-1H-benzotriazole-κ 2 N:N)-(nitrato-κ 2 O:O′) silver(I), C9H8AgN7O3
- Crystal structure of tetraaqua-bis[4-(1H-1,2,4-triazol-1-yl)benzoato-k1 N]cadmium(II), C18H20CdN6O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)nickel(II) dihydrate, C14H16N6O8Ni
- Crystal structure of poly[μ2-aqua-aqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2 N:N′)-(μ2-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ2 O:O′)-(μ4-4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ5 O,O′:O″:O′″:O′″)dicobalt(II)] – water – dimethylformamide (1/1/1) C44H43N11O12Co2
- Crystal structure of N-((Z)-amino(((E)-amino(phenylamino)methylene) amino)methylene)benzenaminium chloride – benzo[f]isoquinolino[3,4-b][1,8]naphthyridine – tetrahydrofurane (1/2/2), C60H54ClN11O2
- The crystal structure of Chrysosplenol D, C18H16O8
- Crystal structure of poly[deca aqua-bis(μ 4-2-(triazol-1-yl)-benzene-1,3,5-tricarboxylato)- bis(μ 5-2-(triazol-1-yl)-benzene-1,3-dicarboxylato-5-carboxyl acid) pentamanganese(II)] dihydrate, C44H42Mn5N12O36
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C20H24N2O2
- The crystal structure of 4,4′-dichloro-6,6′-dimethoxy-2,2′,3,3′,5,5′- hexanitroazobenzene, C14H6N8O14Cl2
- Crystal structure of N 2,N 4-dimesitylpentane-2,4-diamine, C23H34N2
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ 6O6)potassium(2-methylphenylamino)ethyl-2-methylphenylamide ammoniate (1/3.5), [K(18-crown-6)](o-CH3C6H4)NH(CH2)2N(o-CH3C6H4) 3.5 NH3, C28H53.5KN5.5O6
- The crystal structure of N′,N″,2-tris((E)-5-chloro-2-hydroxybenzylidene)hydrazine-1-carbohydrazonhydrazide hydrochloride – methanol (1/3), C25H30Cl4N6O6
- Crystal structure of (E)-7-bromo-2-(3,5-dimethoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C19H17BrO3
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-4-hydroxybenzohydrazide, C15H13ClN2O3
- {2-(((2-aminoethyl)imino)methyl)-6-bromophenolato-κ3 N,N′,O}iron(III) nitrate, C18H20Br2FeN5O5
- Crystal structure of 2-(tert-pentyl)anthracene-9,10-dione, C19H18O2
- Crystal structure of 5,5′-(1,4-phenylene)bis(1H-imidazol-3-ium) bis(2-(2-(carboxymethyl)phenyl)acetate), C32H30N4O8
- Crystal structure of N 2,N 6-bis(2-(((E)-naphthalen-1-ylmethylene)amino)phenyl)pyridine-2,6-dicarboxamide, C41H29N5O2
- The crystal structure of 3-amino-1,2,4-triazolium 2,4,5-trinitroimidazolate, C5H5O6N9
- Hydrogen bonded dimers in the crystal structure of 2-chloro-N-(phenylcarbamoyl)nicotinamide, C26H20Cl2N6O4
- The crystal structure of 4,4′-bipyridine-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one-water(1/2/2), C40H32N2O12
- Crystal structure of N,N'-bis(4-fluoro-salicylaldehyde)-3,6-dioxa-1,8-diaminooctane, C20H22F2N2O4
- Crystal structure of 3-(1,3-dinitropropan-2-yl)-4H-chromen-4-one, C12H10N2O6
- The crystal structure of (4-(2-bromoethoxy)-phenyl)(phenyl)methanone, C15H13BrO2
- Crystal structure of (E)-7-bromo-2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C18H15BrO2
- Crystal structure of dichlorido-tetrakis((E)-(RS)-1-(2,4-dichlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pent-1-en-3-ol-κ 1 N)cadmium(II), C60H68O4N12Cl10Cd
- Crystal structure of diaqua-diphenanthroline-κ2 N,N′-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-tetrafluorophthalato-κ3 O,O′:O′)didysprosium(III) – phenanthroline (1/2), C80H38Dy2F16N8O18
- Crystal structure of bis(μ2-2-oxido-2-phenylacetato-κ3 O,O′:O′)-bis(N-oxido-benzamide-κ2 O,O′)-bis(propan-2-olato-κ1 O)dititanium(IV), C36H38N2O12Ti2
- Crystal structure of poly[diaqua-(μ2-1H-benzo[d][1,2,3]triazole-5-carboxylato-κ2 O:O′)(μ2-oxalato-κ4O,O:O″,O′″)europium(III)] monohydrate, C9H10N3O9Eu
- Crystal structure of bis((N-methyl-2-oxyethyl)amine)-bis(μ 2-N,N,N-tris(2-oxoethyl)amine)-bis(isopropoxy)-bis(μ 3-oxo)tetratitanium(IV)– isopropanol (1/2), C34H76N4O16Ti4
- Synthesis and crystal structure of ethyl 4-((4-iodobenzyl)amino)benzoate, C16H16INO2
- Crystal structure of (Z)-2-(tert-butyl)-5-((5-(tert- butyl)-2H-pyrrol-2-ylidene)(mesityl)methyl)-1H-pyrrole, C26H34N2
- Crystal structure of dimethylammonium poly[μ4-1,1′-(1,4- phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylato-κ6 N,O:O′:N′,O″:O‴) manganese(II)], C22H26MnN6O8

