Abstract
The recent past witnessed rapid strides in the development of lithium-based rechargeable batteries. Here, some key technological developments in intercalation, conversion, and alloy-type anode and cathode materials are reviewed. Beyond the active electrode materials, we also discuss strategies for improving electrolytes and current collectors. An outlook with remarks on easily misleading battery characteristics reported in the literature, impending challenges, and future directions in lithium-based rechargeable batteries is provided. Lastly, the authors also emphasize the need for lab-based research at the pouch cell level with practical energy densities, in addition to discussing scalability and economic viability of different battery materials and their architectures.
Graphical abstract
1 Introduction
Alternative vehicle technologies, such as battery electric vehicles (BEVs), are being developed to reduce our dependence on oil for transportation and mitigate CO2 emissions [1,2,3,4]. Unlike low-efficiency gasoline or diesel-based internal combustion engines that power most present-day vehicles, a BEV is fully powered by the energy stored in a large onboard battery pack with projected efficiencies up to 70%. The performance of a BEV ultimately hinges on the power and energy capacity of its battery pack. An automotive battery pack typically consists of a large number of cells (few hundreds to thousands) to meet the required energy and power needs of a BEV. The wide deployment of BEVs for maximizing the electrification of the road transportation system demands drastic improvements in the performance of today’s battery packs. Specifically, the U.S. Advanced Battery Consortium developed a set of goals for BEV battery packs, which require that the specific energy be increased beyond 235 W h/kg at the pack level (with a concomitant volumetric energy density of 500 W h/L) at power densities as high as 2,000 W/kg while reducing the cost to <$125 per kW h [5].
The automotive and electronic industries have embraced rechargeable lithium-ion battery (LIB; Figure 1a) as “the component” for battery packs because it provides the highest energy density of all commercially available battery chemistries [1,6,7]. Although presently used 85 kW h LIB-based packs allow up to ∼250 miles driving range on a single charge, their cost is twice the price of a standard economy car. The high cost and limited mileage of present LIB-powered cars are due to the intrinsically limited capacities of the Li-ion insertion cathodes, which are nearing their practical limits. While much progress is being made to improve LIBs, other battery chemistries such as lithium–sulfur batteries (LSBs), Al-ion, Na-ion, and K-ion are also being explored [8,9,10,11,12,13,14]. In this short review, recent progress in improving the electrochemical performance and cycle life of lithium batteries is presented.
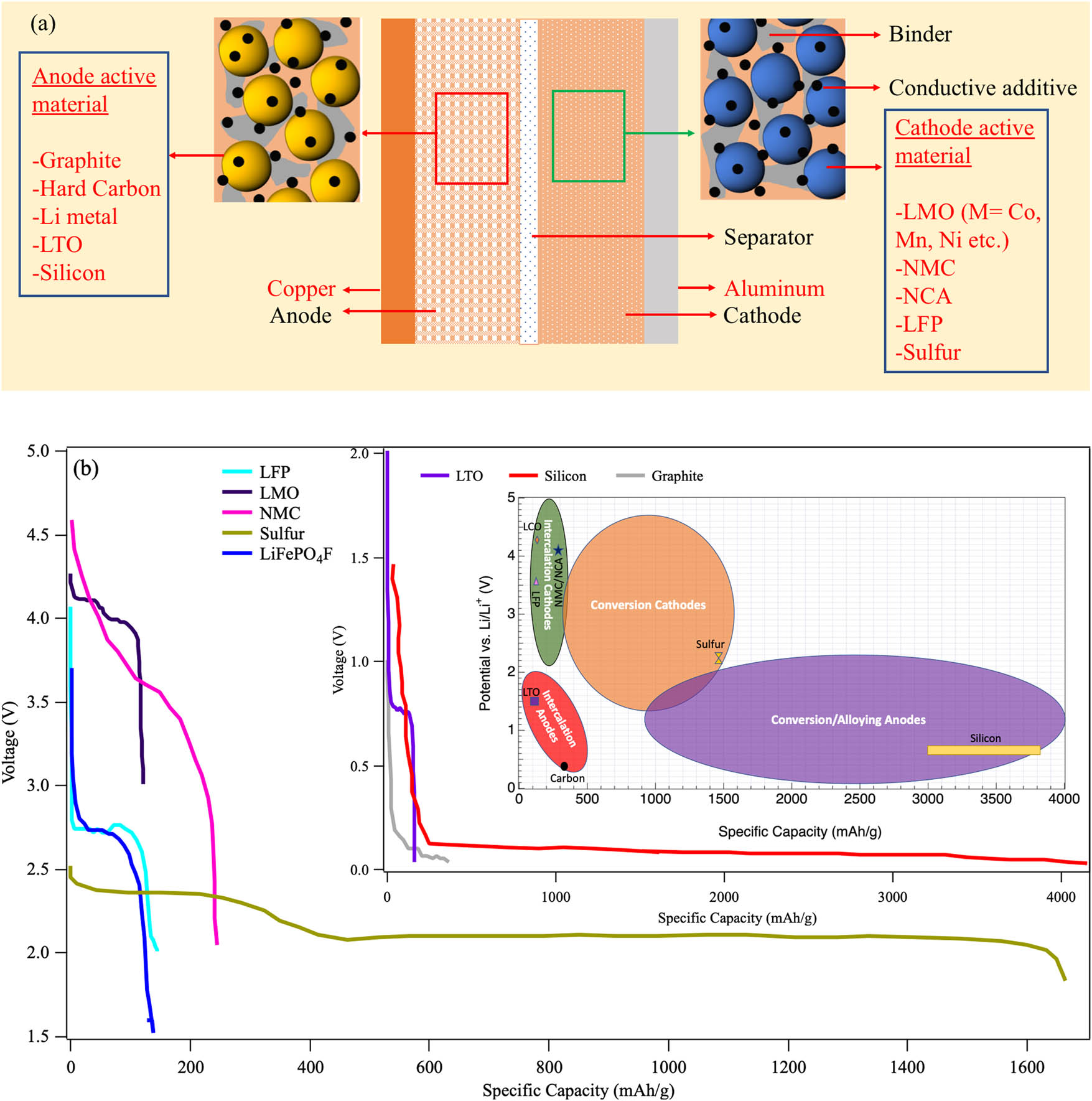
(a) A schematic showing the components of Li-ion batteries. Novel strategies to improve LIB performance by optimizing different components of LIB (red text) will be discussed in this review. (b) Approximate behavior of theoretical discharge curves for selected cathode (inset-anode) materials. The inner inset shows the approximate ranges for average discharge potentials and specific/gravimetric capacities for intercalation and conversion/alloy type electrodes. Some widely used or promising electrode materials that are highlighted in this review are also shown (Li4Ti5O12 (LTO): Li4Ti5O12; LiFePO4 (LFP): LiFePO4; LiCoO2 (LCO): LiCoO2; nickel manganese cobalt oxide (NMC): LiNi0.33Co0.33Mn0.33O2; NCA: LiNi0.8Co0.15Al0.05O2).
Given that lithium batteries can deliver higher energy and power densities, they were the obvious choice for portable electrochemical energy storage. In the recent past, much research has been devoted to realizing LIB electrodes with higher rate capability and charge capacity. For LIB cathodes, achieving sufficiently high voltage can drastically improve their energy and power densities and make them smaller and cheaper. Figure 1b shows the general range of different intercalation and conversion/alloying-type cathodes and anodes for LIBs. This review discusses efforts to improve lithium battery electrodes at various levels via: (1) the identification of the optimal chemical composition of active materials (AMs), (2) tailoring physical properties of AMs such as size and surface, and (3) integrating AMs with binders, conductive additives, and current collectors. More importantly, this review will also discuss how different interfaces such as the solid electrolyte interface (SEI) and current collector active material interface (CCAMI) impact the LIB performance and highlight strategies to overcome adverse interfacial effects.
2 Intercalation cathodes
The LIB intercalation cathodes can be categorized into four different crystal structures, viz., layered, spinel, olivine, and tavorite [15,16]. Although many materials can be used as intercalation cathodes, much attention has been paid to transition metal oxide and polyanion compounds because of their higher operating voltages [17]. These oxide cathodes can deliver specific capacities ∼150–200 mA h/g at the cathode level. To increase the specific capacity and cycling stability, much research has been devoted to identifying the optimal chemical composition for achieving high specific capacity in intercalating AMs.
2.1 Layered intercalation cathodes
The first intercalation cathode (LiTiS2) was developed by Whittingham and Gamble in 1975 [18], but due to a lower voltage (∼2 V), these cathodes were replaced by layered intercalation metal oxide cathodes (LiMO2, M is either bulk transition metal or a mixture of different transition metals) [18]. These cathodes have high operating voltage (3.0–4.8 V) and high theoretical specific capacity (∼250–280 mA h/g). Goodenough, the 2019 Nobel Laureate in Chemistry, introduced LCO as an alternative cathode that was eventually commercialized by SONY [19]. Although it exhibited high capacity (∼275 mA h/g), the cost of Co is high. Also, at higher voltages, LCO suffers from a fast capacity fade due to the structural changes within the material [20,21,22]. Additionally, its thermal instability limits its use in EVs [17,23]. The use of other isostructural oxides has also been explored, for example, LiNiO2 (LNO) is a cheaper alternative to LCO with a comparable theoretical specific capacity. However, LNO is difficult to synthesize [24,25] and is thermally unstable [26]. Another cheaper and environmentally friendly alternative to both LCO and LNO is LiMnO2 (specific capacity ∼285 mA h/g), which too is difficult to synthesize and suffers from high capacity fading due to a change in its crystal structure from layered to spinel (LiMn2O4) with cycling [17,27,28,29,30,31,32,33]. Subsequent studies found that the presence of dopants in LiMnO2 helped reduce the thermal runaway and enhance structural stability and cycling stability at higher voltages [34,35,36,37,38]. Consequently, ternary materials such as LiNi x Co y Mn z O2 (often referred to as NCM or NMC) and LiNi0.8Co0.15Al0.05O2 (NCA) were developed, which proved attractive for commercial use. For example, NMC proved as a cheaper alternative compared to LCO as it contained a minimal amount of Co and exhibited a relatively superior thermal stability and a similar or higher specific capacity compared to LCO at the same operating voltage [39]. LiNi0.33Co0.33Mn0.33O2 (also known as NMC 111) is the common form of NMC that is widely used in today’s battery market. Beyond the NMC 111 composition, higher specific capacities were achieved in Ni-rich NMC cathode materials; however, they suffered from poor thermal stability and cycle life [40,41]. Similar to NMC, NCA is another cathode AM in which Ni is substituted with small amounts of Co and Al, and Al is known to improve the thermal stability of NCA [42]. The theoretical capacity of NCA is ∼280 mA h/g [42], and both Panasonic and Tesla have embraced NCA over LCO as the cathode material for their LIBs. Lastly, a small amount of ion doping (Cl, Mg, Na, F, etc.) has also been shown to improve the thermal stability of NCA [24,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60].
Recently, another family of Li-rich layered oxide composite has been synthesized by structurally incorporating a Li2MnO3 stabilizer into an electrochemically active LiXO2 host (X = Mn, Ni, Co). The excess lithium in such Li-rich oxide composites boosts the specific capacity of the cell up to ∼460 mA h/g (for Li2MnO3) by activating Li2MnO3 at >4.5 V [68]. Such activation releases Li2O initially and facilitates better Li diffusion by acting as a Li reservoir. LiXO2 are cost effective and environmentally friendly [69], and although they exhibit a relatively higher capacity they are considered to be electrochemically inactive (Table 1). Accordingly, much effort has been devoted to increase their electrochemical performance through ion doping (Ni, Mg, Ru, Cr, Mo etc.) [63,64,65,66,67,68,69,70,71,72,73,74,75,17].
Representative layered intercalation cathode materials along with their operating voltage, cycle capacity, and C rate
| Material | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|
| LCO | 2.7–4.3 | 145/140 | 20/1C | 2003 [83] |
| NMC 111 | 3.0–4.3 | 150/150 | 10/0.5C | 2013 [84] |
| NCA | 3.0–4.3 | 184.5/172.3 | 110/1C | 2018 [85] |
| NMC 622 | 3.0–4.2 | 155.4/147.3 | 400/0.1C | 2017 [86] |
| NMC 811 | 3.0–4.2 | 172.5/114.7 | 400/0.1C | 2017 [86] |
| LiNi0.8(1−x)Co0.1Mn0.1Ca0.8x O2 | 2.5–4.5 | 195/130 | 100/0.2C | 2017 [64] |
| Li(Ni0.5Co0.2Mn0.3)1−x Zr x O2 | 3.0–4.6 | 190/166 | 100/1C | 2016 [54] |
| Li2MnO3 | 2.0–4.8 | 180/NA | NA | 2013 [87] |
| Li1.98Mg0.01MnO3 | 2.0–4.6 | 307.5/259.8 | 30/0.1C | 2016 [79] |
| Li1.23Fe0.15Ni0.15Mn0.46O2 | 1.5–4.5 | 255/211 | 50/NA | 2017 [78] |
2.2 Spinel intercalation cathodes
In 1983, Thackeray et al. proposed that LiMn2O4 (LMO) [88] (specific capacity ∼150 mA h/g) could be used as an intercalating cathode. LMO exhibits a cubic “spinel” structure in which Li is present at tetrahedral sites and Mn at the octahedral sites. LMO is relatively cheaper and environmentally friendly; however, its cycling stability is poor because Mn tends to dissolve in electrolytes such as LiPF6 [89,90] and LiAsF6 [89,90]. Surface coating [91,92,93,94,95,96], tuning morphology and size [97,98,99], and doping [100,101,102,103,104] have been found to increase the cycling stability of LMO. Particularly, Ni-substituted (LiMn2−x Ni x O4) and (Li x (MnNi)1−x O4) spinels have emerged as promising cathode materials (Table 2) as they exhibit excellent cycling stability even at a high potential (∼4.8–5.0 V) [105,106,107].
Representative spinel intercalation cathode materials along with their operating voltage, cycle capacity, and C rate
| Material/condition | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|
| LiMn2O4/pure | 3.0–4.5 | 96.00/85.15 | 100/1C | 2019 [97] |
| LiMn2O4/LaF3 coated | 0–1.1 | 109.5/107.9 | 100/10C | 2016 [108] |
| LiMn2O4 nanorods/Li4Ti5O12 coated | 3.2–4.2 | 98.4/80.9 | 500/1C | 2019 [98] |
| LiMn2O4/mesoporous | 3.0–4.5 | 100.51/96.42 | 100/1C | 2019 [97] |
| LiMn2O4/Al doped | 3.2–4.2 | 100.7/94.6 | 400/0.5C | 2020 [104] |
| LiMn1.5Ni0.5O4 | 3.5–5.0 | 111.4/102.3 | 50/5C | 2010 [106] |
| Li1.2Mn0.6Ni0.2O2 | 2.0–4.8 | >200/200 | 50/NA | 2016 [107] |
2.3 Olivine intercalation cathodes
Olivine phosphates (LiMPO4) [109] and olivine silicates (Li2MSiO4) [110], where M = Fe, Mn, Mg, and Co, have also been evaluated as cathode materials [42,111]. Unlike the layered LCO and LNO cathodes, olivine electrodes are significantly more stable and exhibit better power capability [17]. Although LFP has been used in commercial LIBs, it exhibits a relatively low open circuit potential (3.5 V) and low electrical/ionic conductivity [111]. As such, surface coatings and dopants have been used to increase the conductivity of the LFP [112,113,114,115]. Notably, as discussed later in Section 2.6, a new approach involving Al current collector with vertically aligned carbon nanotubes (VACNTs) could resolve the CCAMI issue and improve conductivity across the interface between LFP and the Al current collector [116]. Other olivine-like materials such as LiNiPO4, LiMnPO4, LiMgPO4, and LiCoPO4 also have a higher potential (4.1–4.8 V) but they exhibit a high intrinsic resistance which results in lower electrochemical activity and capacity [117,118,119]. Similar CCAMI approaches as in the case of LFP were used to improve the electrochemical activity and capacity [117,120,121]. Olivine silicates are more thermally stable than phosphates as all four oxygen atoms of tetrahedron SiO4 contribute to connect the structural framework, whereas in phosphate only three oxygen atoms contribute [122]. Also, the theoretical capacity of silicates is almost double (∼330 mA h/g) that of LFP [123,124,125]; however, the practical cycling performance of these silicates is not comparable to that of LFP because of its structural instability/phase change during charging [126,127,128]. Excess lithium was found to improve structural stability [129], specifically Li x MSiO4 (where x = 2), which was modified using approaches similar to those used for LFP to increase the overall electrochemical performance (Table 3) [122,130,131,132].
Representative olivine intercalation cathode materials along with their operating voltage, cycle capacity, and C rate
| Material/condition | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|
| LiFePO4 | 2.0–4.0 | 117/113 | 50/0.1C | 2019 [115] |
| LiFePO4/GdPO4 and C coating | 2.0–4.0 | 158/157 | 50/0.1C | 2019 [115] |
| LiFePO4/Ti doped | 2.3–4.2 | 155/150 | 500/1C | 2020 [112] |
| LiMnPO4/C | 2.5–4.5 | 105/100 | 50/0.05C | 2017 [120] |
| LiMn0.99Y0.01PO4/C | 2.5–4.5 | 153.6/148.1 | 50/0.05C | 2017 [120] |
| Li2FeSiO4/C | 1.5/4.5 | 134/155 | 190/0.2C | 2010 [129] |
| Li2MnSiO4/C | 1.5–4.8 | 280/120 | 16/NA | 2020 [130] |
2.4 Tavorite intercalation cathodes
Considering their exceptional thermal stability and ionic conductivity, tavorite intercalation cathodes (LiMPO4F) are a suitable replacement for olivine cathodes. Tavorite cathodes allow 3D ion transport unlike 1D transport in olivine cathodes [12,133]. Materials with an open structure, such as LiFeSO4F/LiVPO4F/LiVPO4O (specific capacity ∼150 mA h/g) have been considered as promising cathodes as they exhibit an open circuit potential (>4 V) in addition to a stable structure that supports fast Li-ion diffusion (Table 4) [52,133–137]. Although the stability is better, the synthesis of these materials in their pure form remains challenging [135,138].
Representative tavorite intercalation cathode materials along with their operating voltage, cycle capacity, and C rate
| Material/condition | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|
| LiFePO4F | 1.5–4.0 | 110.2/91.5 | 300/0.5C | 2018 [138] |
| LiFePO4F/Ag decorated | 1.5–4.0 | 120.3/115.5 | 300/0.5C | 2018 [138] |
| LiFePO4F nanospheres | 1.5–4.0 | 110/104 | 200/0.5C | 2018 [12] |
| LiVPO4F/C | 2.5–4.6 | 126.6/116.7 | 125/1C | 2018 [139] |
| Li0.99K0.01VPO4F/C | 2.5–4.6 | 131.9/126.8 | 125/1C | 2018 [139] |
2.5 Nanostructured intercalating AMs for cathodes
The recent progress in lithium batteries has largely benefited from the development of nanostructured electrodes in comparison to conventional electrodes because of their unique morphology, significantly enhanced kinetics, and large surface area (Table 5) [3,6,17,140–151]. Nanostructured electrodes shorten the diffusion length and facilitate a higher contact area between the electrolyte and AMs leading to more exposed redox sites, which deliver higher power and energy densities [144,152–154]. Fick’s correlation between the diffusion length L and its diffusion coefficient D can be expressed as τ = L 2/D [144], where τ is the diffusion time.
Representative performance of nanostructured AMs
| Material | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Particle size or morphology | Year/Ref. |
|---|---|---|---|---|---|
| LiFePO4 | 2.5–4.0 | 163/163 | 1/1C | 30 nm | 2006 [194] |
| LiFePO4 | 2.8–4.2 | 130/130 | 1/1C | 150 nm | 2006 [195] |
| LiFePO4 | 2.0–4.5 | 115/115 | 1/1C | 500 nm | 2003 [196] |
| LiFePO4 | 2.1–4.5 | 82/82 | 1/1C | 800 nm | 2006 [197] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | 2.0–4.8 | 153.9/136.7 | 300/3C | Nanotubes | 2016 [177] |
| LiMnPO4/C | 2.5–4.5 | 123/120 | 100/1C | Nanorods | 2018 [163] |
| Li1.2Mn0.54Ni0.13Co0.13O2 | 2.0–4.8 | 138/121 | 100/10C | Nanowires | 2019 [171] |
| LiCoPO4 | 2.5–4.8 | 180/147 | 300/0.1C | Nanoplates | 2016 [179] |
| LiNi0.5Mn1.5O4 | 3.5–4.8 | 136/126 | 100/1C | Nanosheets | 2019 [183] |
| Li1.2Mn0.6Ni0.2O2 | 2.0–4.8 | 197.3/174.6 | 200/1C | 3D micro/nano | 2019 [185] |
| Li1.16Mn0.6Ni0.12Co0.12O2 | 2.0–4.8 | 227/213 | 50/5C | 3D micro/nano | 2017 [187] |
Based on the Li-ion diffusion coefficient D = 2.5 × 10−12 cm2/s, a complete discharge occurs within ∼40 s when the particle size is L = 100 nm while it takes ∼1 h for L = 2 µm. Many physicochemical characteristics such as crystallinity, phase purity, particle morphology, grain size, and surface area depend largely on the AM synthesis methods. Thus, different fabrication methods such as grinding, ball milling, and sol–gel method have been used to synthesize nanostructured intercalating AMs. A detailed review on the synthesis methods can be found in ref. [155].
Okubo et al. [156] demonstrated excellent high-rate capability for nanosized LCO (average particle size ∼17 nm), that is, 65% of the 1C-rate capability retention. However, they also found that extreme reductions in the crystallite size below 15 nm drastically decreased the overall capacity plausibly due to defects. In the case of LFP, the capacity increased linearly with decreasing particle size [152]. In the case of LMO, though the initial capacity was higher, increased capacity fading was observed due to the dissolution of Mn into electrolyte via disproportionation reaction on the larger surface area nanostructured LMO [157–160]. Although nanostructuring can reduce Li+ diffusion length ensuring an improved rate capability, it increases the oxidation of nanoparticles (NPs) leading to a thicker SEI layer on the surface of the electrode and results in capacity fading [153,154]. As discussed in the previous section, both size and structure of AM play a critical role in improving the electrode performance. Recent efforts have focused on 1D morphologies such as nanorods [161–167], nanowires [168–174], and nanotubes [175–178] as they can effectively accommodate volume changes during lithiation/delithiation in addition to promoting diffusion and structural stability [152,174].
Two-dimensional cathode materials with high specific areas are of interest because they provide open 2D channels for Li-ion transport and show better stability than that of the 0D and 1D particles. Although most of the research on 2D electrodes has focused on anode materials, a few reports focused on LMPO4 [117,179–183] 2D cathodes, mostly plausibly due to the unavailability of simple and cost-effective ways of synthesizing 2D cathode materials [181]. Zhao et al. [183] prepared flower-like LiNi0.5Mn1.5O4 nanosheets, which delivered a capacity of 142 mA h/g at 1-C-rate after 100 cycles at 55°C. The LMO microsheets synthesized using a simple carbon gel-combustion synthesis method delivered 91 mA h/g after 300 cycles at 10C [184]. Many challenges such as high preparation cost, low packing density, and inevitable particle aggregation have hampered the use of nanomaterials. Novel 3D hierarchical micro/nanoarchitectures have also been used to improve cell performance [184–193]. Such structures are micron-scale assemblies comprising nanoscale building blocks that enable better electrochemical performance and structural stability [185]. A detailed review of such architectures could be found elsewhere [152].
2.6 Integrating AMs with conductive additives
Most AMs used in LIB electrodes (both anodes and cathodes) are poor electrical conductors and are often mixed with conductive additives (e.g., conductive carbon black often referred to as Super P) [142,198]. In a typical LIB cathode (/anode) manufacturing line, the Al (/Cu) current collector is coated with a slurry containing AM and conductive additives and a binder such as polyvinylidene fluoride or PVDF in N-methyl-2-pyrrolidone (NMP) [199]. Although carbon black (e.g., Super P) is widely used as a conductive additive, other carbon nanomaterials such as graphene, CNTs, and reduced graphene oxide (rGO) have shown great promise in improving the specific capacity (Table 6) [200–216]. When carbon-based additives are used, one should be aware of the fact that such additives could reduce the tap density and affect Li-ion transport. Thus, the use of the minimum amount of additives is suggested [217,218].
Representative electrochemical performance of intercalating AM cathodes made by integrating conductive additives
| Material | Conductive carbon additive | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|---|
| LiFePO4 | Carbon | 2.5–4.2 | 144/138 | 100/0.5C | 2017 [207] |
| LiFePO4 | N-carbon and rGO | 2.0–4.2 | 116/112 | 700/10C | 2017 [219] |
| LiFePO4 | 3D graphene | 2.4–4.2 | 141.1/139.8 | 600/10C | 2018 [220] |
| NCA | Carbon nanotubes | 2.8–4.5 | 187/181 | 60/0.25C | 2017 [200] |
| Li2MnO3 | rGO | 2.0–4.6 | 290/250 | 45/0.1C | 2017 [221] |
| LiMn2O4 | Carbon and carbon nanofibers | 3.0–4.3 | 120/92 | 1,000/1C | 2020 [222] |
| NMC | Graphene | 2.7–4.2 | 140/103 | 500/1C | 2017 [223] |
| LiMn0.8Fe0.2PO4 | Carbon/N-rGO | 2.0–4.6 | 156.9/145.5 | 100/0.2C | 2019 [224] |
2.7 Modifying/replacing traditional Al current collectors for cathodes
Although Al foils are widely used as the cathode current collector, there are several drawbacks such as localized corrosion due to electrolyte, weak adhesion to AMs, and limited contact area [225,226], which adversely affect the electrochemical performance of cells. To improve AM adhesion to Al-foils, the surface is often treated chemically or modified [227–231]. As shown in Figure 2, the total internal resistance of LIBs arises due to the existence of the interfaces between AM, binder, conductive additives, and the current collector. An increase in the internal resistance could decrease the overall electrochemical activity [232]. As described in Sections 2.1–2.5, the present LIB literature is replete with different procedures for improving the interfaces within the active materials using nano/micro-structuring or adding novel conductive additives such as carbon black, graphene, and rGO [2,233]. While conductive carbon additives decrease the resistance of interfaces within the cathode/anode active material, they are ineffective in decreasing the current collector active material interface or CCAMI resistance (see Figure 2) – a key driving factor for increasing energy and power densities that has largely been ignored in LIBs [234].
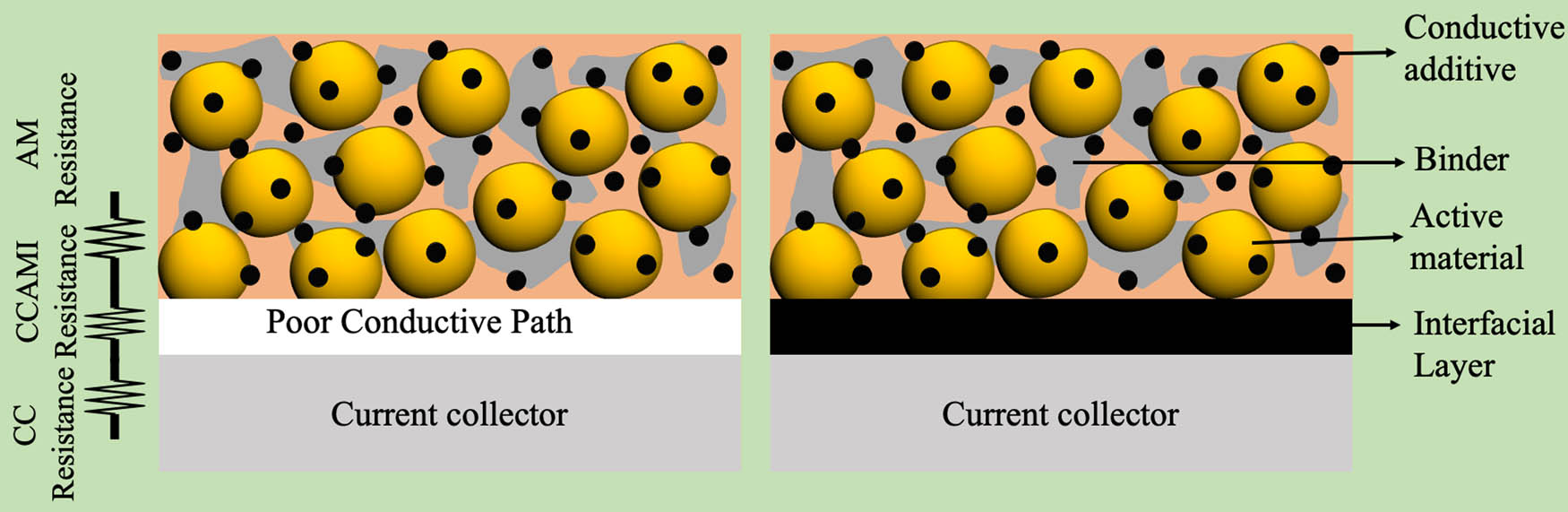
Various resistances in a cathode/anode electrode (left – traditional electrode, right – CCAMI engineered electrode). An interfacial layer of carbon, CNTs, and VACNTs enables better electrical contact between the AM and the current collector (CC), which improves the electrochemical performance of the electrode.
Ventrapragada et al. [116] extensively investigated the role of CCAMI resistance by using VACNTs, either directly grown or spray coated on Al foils. The CCAMI resistance of traditional LFP and NMC electrodes coated on Al/VACNT current collector was reduced by a factor of 3 in comparison to bare Al foil. Moreover, the capillary channels provided by the VACNTs grown on the Al foil promotes the wettability of aqueous slurry due to an increase in surface roughness, thus alleviating the need for toxic organic solvents (e.g., NMP) to prepare the AM slurry. CNT-coated electrodes displayed energy densities as high as follows: ∼500 W h/kg at 170 W/kg for LFP and ∼760 W h/kg at ∼570 W/kg for NMC. The CCAMI engineered LIBs exhibited an initial capacity of ∼103 mA h/g with a capacity retention of ∼80% at 4C after 500 cycles, unlike the commercial electrodes that could not withstand such a high C-rate. By using the carbon black and graphene-modified Al-foil (CG-Al) for the LFP electrode, Wang et al. [228] also demonstrated superior performance over the bare Al-foil (B–Al). The capacity retention at 1C-rate after 500 cycles of CG–Al–LFP battery was ∼94% with initial capacity ∼154 mA h/g, whereas, for B-Al-LFP, the capacity retention was ∼90% with initial capacity ∼120 mA h/g. Interestingly, Loghavi et al. treated the Al surface with a combination of three acids and observed an increase in capacity over the untreated Al foil [229].
There has been a growing interest in displacing relatively heavy metallic current collectors in LIBs with lightweight, low cost, and highly conductive materials that can satisfy the robustness as well as high utility requirement of LIBs (Table 7) [226]. Lightweight and flexible carbon-based current collectors have been researched because of their wide electrochemical window. Using graphene foil as a current collector for NMC523 cathode, Xu et al. [226] reported a ∼45% increase in gravimetric capacity at the electrode level compared to bare Al foil current collector at 0.5C after 200 cycles. The energy density of graphene foil was ∼350 W h/kg after 200 cycles at 0.5C higher than the bare Al foil that showed ∼240 W h/kg. The use of carbon paper [235], carbon fiber [236], and carbon cloth [237] in place of the Al foil have resulted in higher gravimetric and higher energy density. Another alternative for Al-based cathodes is paper/cellulose-based current collectors. Low cost of raw materials, ease of availability, and biodegradability of cellulose-based current collectors have attracted much attention. However, unlike Al foil, these current collectors are not highly conducting. In this regard, many researchers have endeavored to make cellulose fiber-based conducting composites or coat papers with different conducting materials (e.g., activated carbon) using additives, which affects the gravimetric capacity as the amount of inactive material is increased [238–245]. Ventrapragada et al. [246] prepared additive-free paper-CNT current collectors by spray coating CNTs on cellulose paper. Cells assembled using this current collector with LFP coating showed a high energy density of 460 W h/kg at a power density of 250 W/kg. These batteries were able to withstand a high current rate (4C) and showed cycling stability up to 450 cycles at 1C-rate. Wang et al. [237] developed a carbon cloth-based Li1.2Mn0.6Ni0.2O2 electrode, which performed better than commercial Al-foil at a high C-rate (5C).
Representative electrochemical performance of cathodes depending on current collector
| Material | Current collector | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|---|
| LiMnO4 | Bare Al | 3.0–4.4 | 101/76 | 360/0.5C | 2017 [231] |
| LiMn2O4 | Graphene/Al | 3.0–4.4 | 100/91 | 360/0.5C | 2017 [231] |
| LFP | CNT/Al | 2.0–4.2 | 103/79 | 500/4C | 2018 [116] |
| NCA | Acid treated Al | 2.8–4.2 | 187/170 | 80/0.5C | 2019 [229] |
| NMC523 | Graphene foil | 2.8–4.5 | 112.4/42.0 | 200/5C | 2020 [226] |
| Li1.2Mn0.6Ni0.2O2 | Carbon cloth | 2.0–4.8 | 78/78 | 100/5C | 2017 [237] |
| LFP | CNT/printing paper | 2.0–4.2 | 135/90 | 450/1C | 2019 [246] |
3 Conversion-type cathodes
Since SONY’s launch of the first commercial LIBs in 1991, researchers have endeavored to improve the performance of LIBs [6,247–250]. As the gravimetric energy densities of the state-of-the-art LIBs based on intercalating AMs (such as LFP [251], NCA [252], and NMC [86]) reached their practical limits, alternative conversion-type AMs have attracted much attention. Among all the existing conversion-type AMs, sulfur is one of the most promising candidates for the next-generation portable electronic devices owing to its high theoretical gravimetric capacity (1,675 mA h/g) and gravimetric energy density of 2,500 W h/kg (practical energy densities of 400–600 W h/kg at the cell level for lithium-sulfur batteries, or LSBs) in addition to low cost and abundance of sulfur [253]. However, there are many challenges impeding the practical applications of LSBs such as the following: (1) insulating nature of sulfur (S8) and its discharge product (Li2S), (2) polysulfide intermediates resulting in a loss of active material, (3) dendrite formation and unstable SEI leading to the consumption of electrolyte and lithium, (4) low areal capacity of the LSBs due to low sulfur-loadings; and (5) usage of binder, carbon additives and heavy current collector leading to low gravimetric capacity. To overcome these challenges, many efforts have been made as evident from the number of published review articles. From Google scholar search of keyword “lithium-sulfur review” and adjusting the timeline to “since 2019,” we were able to find at least 20 review articles on LSBs, which address challenges presented earlier individually or collectively [254–274].
Although sulfur has a high theoretical capacity, its low electric conductivity ∼5 × 10−30 S/cm (at 25°C) leads to its low utilization as an AM. In the past two decades, carbon-based conductive frameworks have been used for increasing the electrical conductivity of elemental sulfur-based cathodes. These hosts include materials such as carbon black [275–277] carbon fibers [278,279], carbon/graphene sheets [280–282], carbon nanotubes [281,283,284], and hollow porous carbon materials [285–289] The highly soluble polysulfide intermediates (LiS x , 4 ≤ x ≤ 8) generated during the reversible redox reaction between sulfur and its discharge product Li2S (the so-called shuttle effect) lead to the continuous loss of the cathode AMs and degradation of the Li metal anode (Figure 3). Consequently, such elemental sulfur-based cathodes exhibit poor cycling stability and low Coulombic efficiency. These challenges have been addressed in two ways: (i) designing better carbon frameworks and (ii) replacing elemental sulfur with a sulfur-based polymer. Indeed, different carbon framework designs aimed toward suppressing the diffusion of polysulfide intermediates have been developed. Such designs include 1D permeable hollow porous carbon shell cathode [290–296] and 2D conductive polymers or reduced graphene oxide nanosheets wrapped cathode [281,297–301]. Although physically confining sulfur could effectively reduce the outward diffusion of the polysulfides, the weak interaction between the nonpolar carbon and polar polysulfides prohibits the complete immobilization of polysulfides. Zhong et al. [275] prepared the popcorn-inspired macrocelluar carbon interconnected porous network that exhibited high electrical conductivity and was capable of blocking the polysulfide formation. N-doped carbon nanoflakes/carbon nanotubes can also decrease the polysulfide formation by decreasing the active sites for Li2S decomposition [297,302,303]. Also, metal-organic frameworks in combination with carbon can increase the electrical conductivity and enable polysulfide immobilization [292,302,304–308].
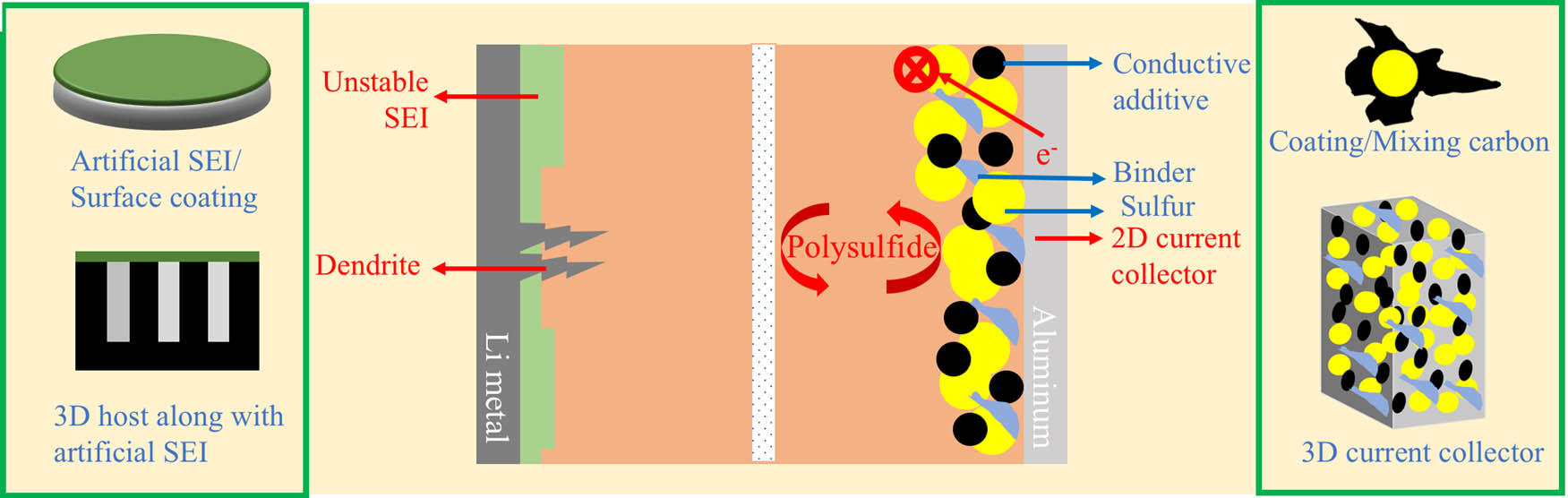
A schematic of a typical LSB battery is shown in middle. The red text corresponds to the challenges that are commonly reported in LSBs. The common solutions are shown in green boxes.
Alternatively, polysulfide formation can be eliminated by using sulfurized polymers. Polyacrylonitrile (PAN) has been widely used as a carbon precursor for synthesizing porous carbon materials mainly due to its ease of dehydrogenation and cyclization into conjugated carbon backbones. The one-pot reaction of PAN with elemental sulfur provides a facile approach for the preparation of sulfurized PAN or SPAN. SPAN is a conductive sulfur-containing compound in which S atoms are covalently bonded to the polymer carbon matrix through a pyrolytic process. During sulfurization, sulfur acts as a mild oxidant to dehydrogenate PAN [309]. The structure and S content in SPAN depend on the pyrolysis temperature and duration. Based on extensive spectroscopic and theoretical studies, a few possible structures have been proposed as shown in Figure 4 [309]. Clearly, all possible SPAN structures do not exhibit the octagonal S8 structure. Indeed, based on thermogravimetric analysis-mass spectrometry data, Zhang [309] proposed that Figure 4a is the most likely structure for SPAN obtained through pyrolysis at relatively lower temperatures (<600°C). In such structures, the lithiation of SPAN is expected to occur without the formation of any higher-order polysulfides, as shown in Figure 4d.
![Figure 4
(a–c) Proposed chemical structures for SPAN based on pyrolysis temperature and duration [310]. (d) Lithiation of SPAN with the chemical structure shown in (a) does not result in the formation of higher-order polysulfides.](/document/doi/10.1515/ntrev-2021-0114/asset/graphic/j_ntrev-2021-0114_fig_004.jpg)
(a–c) Proposed chemical structures for SPAN based on pyrolysis temperature and duration [310]. (d) Lithiation of SPAN with the chemical structure shown in (a) does not result in the formation of higher-order polysulfides.
SPAN-based LSBs were first reported by Wang et al. [310], which was followed by He et al. [311,312] Beyond the one-pot reaction, Lai et al. [313] synthesized SPAN using a two-step technique by first pyrolyzing PAN and then reacting it with elemental sulfur. Their Li/SPAN cell with a 1 M LiPF6 electrolyte showed a single discharge voltage plateau and a stable capacity of 770 mA h/g for 110 cycles at 40 mA/g. Similarly, Guo et al. [314] first pyrolyzed PAN into disordered amorphous carbon nanotubes and subsequently reacted them with sulfur at 500°C for 3 h. Their resulting SPAN tubes contained 40 wt% sulfur and were thermally stable up to 700°C suggesting that sulfur was covalently bonded to carbon as shown in Figure 4a–c. Moreover, their Li/SPAN cell showed stable capacities of nearly 700 mA h/g for 100 cycles at 10 mA/g. Ahn et al. and Ming et al. [315,316] also reported that the polysulfide intermediates could be eliminated by using SPAN as the cathode material. Although SPAN provides a stable conversion-type sulfur cathode, its use is limited by its poor ionic and electronic conductivities, which can be improved using carbon-based additives [317–322].
To increase the cycling stability with Li metal anode in LSBs, an electrolyte that is capable of forming a stable SEI layer is needed. The SEI layer forms on the electrode from the decomposition of electrolytes and solutes. A stable SEI layer passivates the electrode and mitigates dendrite formation, which could short-circuit the battery. Fan et al. [323] developed novel mixed electrolytes for LSB that enable the simultaneous formation of SEIs on both electrodes. Their SPAN-based LSBs with mixed electrolytes showed a high utilization of sulfur (over 80% at 0.15C), superior rate performance, excellent coulombic efficiency (≈100%), and long cycling life (1,000 cycles at 1C and 2,400 cycles at 7.5 C with capacity retention of 86.6 and 82.3%, respectively). Indeed, they were able to achieve high mass loadings and capacities as high as ∼1,250 mA h/g at 0.75 C with no capacity fade up to 200 cycles. Liu et al. developed a gel polymer electrolyte, which showed stable performance even at high temperatures (55°C) compared to traditional liquid electrolytes [324]. A recently published mini-review about electrolyte regulation in lithium metal–SPAN battery can be found in ref. [325].
According to statistical analysis in ref. [253], 57% of >100 publications (in 2015–2016) reported areal sulfur loadings below 2 mg/cm and 49% corresponding areal capacities below 2 mA h/cm. Such electrodes are reasonable to use in reaction mechanism studies but unsuitable for practical application despite their high specific capacities (normalized by the amount of sulfur) because they deliver much lower gravimetric energy densities than commercial lithium-ion batteries [326]. An areal capacity of at least 6 mA h/cm is needed for LSBs to displace commercial LIBs because of the low operating voltage of LSBs (∼2.1 V) [253]. Although there have been advances in the LSBs research, the commercialization is still lagging. This could be due to the fact that there is a vast difference on how research is conducted in academia and industry [327]. For example, usually coin cell is used in academia whereas pouch cell is generally used in industry. The advanced structures of the C/S that are used in academia are hard to produce in larger quantities [327]. Recently published article from Bhargav et al. have outlined some critical metrics, viz., “Five 5s” (S-loading >5 mg/cm2, C-content <5%, E/S ratio <5 µL/mg, N/P ratio <5, E/C ratio <5 µL/(mA h)) to achieve high energy density, which could be helpful to translate to industry [328]. Although “Five 5s” seems attractive, there are several issues that need to be addressed first. While increasing the loading might increase the energy density, it will adversely increase the electrochemical polarization, decrease the utilization and increase thermal instability [327,329,330].
Lastly, most studies report the gravimetric capacities of cathodes calculated based on the mass of sulfur. Unfortunately, although the sulfur content of some reported cathode materials can reach up to 70 wt%, the use of polymer binder (usually 10% of the slurry) and carbon black (usually ∼10–20% of the slurry) in the slurry coating procedure (especially coating on the widely used aluminum foil current collector) results in a low-rate capacity on the electrode level in addition to a low gravimetric energy density [253]. High sulfur loading cathodes with high sulfur utilization and lightweight current collectors are essential for realizing high-energy-density LSBs. Recently, more efforts have been focused on self-supporting cathode electrodes [331–337]. Manthiram et al. [338] developed graphene-wrapped double-shelled hollow carbon sphere cathode with sulfur loading of 3.9 mg/cm2, which exhibited a stable areal capacity of ∼2 mA h/cm2 at a current density of 3.3 mA/cm2. Zheng et al. [339] fabricated a two-dimensional carbon yolk-shell nanosheets cathode with sulfur loading of 10 mg/cm2 that delivered an areal capacity of ∼11.4 mA h/cm2 after 50 cycles at a current density of 1.68 mA/cm2. Lou et al. [300] designed a freestanding pie-like cathode by sulfurizing the electrospun PAN/polystyrene paper and wrapping the carbon–sulfur composite film with a thin layer of functionalized rGO. The pie-like cathode with a sulfur loading of 10.8 mg/cm2 showed an areal capacity of ∼10.7 mA h/cm2 at a current density of 1.2 mA/cm2. Liu et al. [335] developed a continuous core-shell structure of boron-doped carbon–sulfur aerogel (S-loading ∼13.5 mg/cm2) and achieved an areal capacity of 12.3 mA h/cm2. Zhang et al. [332] showed dense monolithic metal-organic framework (MOF) and CNT can be used for sulfur electrode, which exhibited high areal (10.7 mA h/cm2) and volumetric (676 mA h/mL) capacity. Sulfur can be infiltrated inside the VACNTs to get high areal capacity LSBs [331,340]. In this regard, we fabricated LSB cathodes by vacuum filtrating aqueous SPAN slurry through chemical vapor deposition grown freestanding graphene foam. More importantly, porous 3D graphene foams allowed S loadings as high as ∼26 mg/cm2 without any delamination or increased electrical resistance unlike Al foils that could only support a maximum S loading of 0.2–0.4 mg/cm2 (Al–SPAN) without the binders (Figure 5). The graphene foam–SPAN (GF–SPAN) cathodes outperformed conventional Al–SPAN cathodes at the electrode level with areal capacity ∼20 mA h/cm2 at the current density of 3 mA/cm2 (Table 8) [341].
![Figure 5
Top panel: A schematic showing the fabrication steps of a GF/SPAN cathode: GF is grown on a 3D-Ni mesh using the chemical vapor deposition technique. The GF/SPAN cathode electrode was prepared by vacuum filtrating a slurry of SPAN, carboxymethyl cellulose, and carbon black (mass ratio of 70:15:15) onto the GF current collector (with Ni mesh). The as-prepared cathode electrode was air-dried overnight followed by drying in an oven ∼130°C for 12 h. Next, 5 wt% poly(methyl methacrylate) thin layer was coated on the cathode before it was submerged in a 6 M HCl solution for 6 h at 70°C to completely remove the nickel. The resulted cathode electrode was cleaned in KOH solution and washed in DI H2O and dried [341]. Bottom panel: Weight comparison of different components inside the electrode for same S-loadings [341].](/document/doi/10.1515/ntrev-2021-0114/asset/graphic/j_ntrev-2021-0114_fig_005.jpg)
Top panel: A schematic showing the fabrication steps of a GF/SPAN cathode: GF is grown on a 3D-Ni mesh using the chemical vapor deposition technique. The GF/SPAN cathode electrode was prepared by vacuum filtrating a slurry of SPAN, carboxymethyl cellulose, and carbon black (mass ratio of 70:15:15) onto the GF current collector (with Ni mesh). The as-prepared cathode electrode was air-dried overnight followed by drying in an oven ∼130°C for 12 h. Next, 5 wt% poly(methyl methacrylate) thin layer was coated on the cathode before it was submerged in a 6 M HCl solution for 6 h at 70°C to completely remove the nickel. The resulted cathode electrode was cleaned in KOH solution and washed in DI H2O and dried [341]. Bottom panel: Weight comparison of different components inside the electrode for same S-loadings [341].
Representative electrochemical performance of sulfur cathodes
| Material | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|
| Puffed rice derived carbon/Ni/S | 1.7–2.8 | 1,257/821 | 500/0.2C | 2018 [275] |
| Macro porous carbon nanotubes/S | 1.5–2.8 | 1,544/901 | 100/0.5C | 2018 [283] |
| Porous carbon fibers/vanadium nitride/S | 1.7–2.8 | 1310.8/1052.5 | 250/0.1C | 2018 [278] |
| N, O, and P-doped carbon/S | 1.7–3.0 | 921/489 | 300/0.2C | 2019 [287] |
| rGO–carbon composite–S paper | 1.8–2.6 | 997/670 | 400/0.2C | 2019 [298] |
| S/superP@SPAN | 1.0–3.0 | 1,500/1,251 | 100/0.1C | 2019 [322] |
| Porous SPAN fiber | 1.0–3.0 | 903/903 | 150/1C | 2019 [320] |
| Carbon–cotton/S | 1.5–3.0 | 1,173/788 | 100/0.1C | 2016 [301] |
| SPAN–carbon fiber current collector | 1.0–3.5 | 1,640/1,250 | 100/NA | 2017 [319] |
| Boron-doped carbon/S aerogel | 1.8–3.0 | 1,120/836 | 500/1C | 2019 [335] |
| Functionalized super aligned CNT/S | 1.6–2.8 | 1,079/741 | 400/1C | 2019 [334] |
| Dense monolithic MOF and CNT/S | 1.5–3.0 | 580/470 | 300/1C | 2019 [332] |
| Sulfur sandwiched between CeO2–CNT@C membrane | 1.7–2.8 | 1,300/847 | 100/0.2C | 2020 [342] |
| S@LiAlO2 on aluminum foil | 1.8–2.6 | 989/842 | 100/0.2C | 2020 [343] |
| Sulfur infused porous carbon | 1.7–2.8 | 1,143/950 | 200/0.2C | 2020 [336] |
| Graphene foam–SPAN | 1.0–3.0 | 800/728 | 500/1C | 2021 [341] |
4 Anodes
4.1 Li metal
Li metal is the ideal anode for LIBs due to its low density (0.59 g/cm3) and negative electrochemical potential (−3.04 V) and high theoretical gravimetric capacity (3,860 mA h/g) [344,345]. Li-metal anodes are prone to dendrite formation, which can cause short circuits, thermal runaway reactions on the cathode, and could also cause the battery to catch fire. Indeed, in 1976, Exxon tried to commercialize Li metal batteries (anode Li metal, cathode TiS2); however, the task was unsuccessful due to safety concerns that resulted from short circuiting [6,346]. Furthermore, Li-metal anodes also suffer from poor cycle life due to volume changes and subsequent formation of unstable SEI during cycling [345,347–353]. In the past 7 years, many research groups have been trying to revive the Li metal as a stable anode using different strategies such as optimization of solid/liquid electrolytes and additives [354–359], protective surface coatings (/artificial SEI layer) [353,360–363], modified separator [364,365], and the design of 3D conductive Li host [351,366–373]. Although such strategies improve the lifespan of the Li-metal anode, commercial applications of such batteries are still far away as they still have many disadvantages. Although surface coatings and solid electrolyte combination can increase the ionic concentration, it inevitably increases internal interfacial impedance that adversely affects the cycling stability [358,362,374]. Nanostructures such as MOFs [375], carbon materials [376,377], and 3D current collectors [375,378,379], which have pre-stored lithium, along with artificial SEI layer are promising alternatives [375]. This kind of structure can simultaneously address the volume change and unstable SEI formation. In this review, anodes beyond Li are discussed. A detailed review focused on Li-metal batteries can be found in refs [345,358]. Lastly, it should be noted that lithium-air batteries (specific energy ∼5,200 W h/kg) wherein air is used as a cathode, have also been researched extensively in an effort to enhance the specific energy of the battery [380,381]. However, the practical application of such batteries is still far fetched due to the side reaction with electrolytes, poor cycling stability, and elimination of water and CO2 [382]. The recent efforts that are focused on developing the electrolytes and porous cathode materials can be found in the review articles [380–383].
4.2 Intercalating anodes
Graphitic anodes (theoretical capacity ∼372 mA h/g) initially enabled LIBs to become commercially viable and are still used widely due to their low cost and excellent stability [6]. In graphitic anodes, Li ions intercalate between the graphene planes, which offers good 2D mechanical stability, electrical conductivity, and Li+ transport. Different forms of carbon such as graphite, CNTs, carbon fibers, exfoliated graphene, and rGO have been used as anodes [151]. The demand for new anode materials with higher capacity has increased as the theoretical limit of graphitic anodes has already been achieved commercially [384].
Titanium-based oxides (e.g., LTO) also have received much attention despite having low intrinsic conductivity and low theoretical capacity (175 mA h/g) because they show low volume change (2–3%) upon Li insertion/de-insertion. Additionally, they also exhibit excellent cycling life [385–388]. LTO is relatively safer due to its high open-circuit voltage (∼1.55 V), which prevents dendrite formation [384,389–392]. To improve the low intrinsic conductivity, similar approaches as in the case of the spinel layered cathodes have been suggested. For example, many strategies such as doping [393–396], change in size and morphology [397–399], and combining/coating with conducting carbon additives [392,400–403] either individually or in combination have been used (Table 9) [393,404–407].
Representative electrochemical performance of Li metal anode and intercalating anode
| Material | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|
| LiF and Li–Al alloy protected layer–Li metal/LFP | 0.01–4.2 | 120.4/94.2 | 300/3C | 2020 [354] |
| Organophosphate-derived dual-layered Li metal/sulfur | 1.7–2.7 | 800/735 | 100/1C | 2020 [353] |
| Polyacrylic acid-coated Li/Cu matrix//LCO | 3.0–4.2 | 127/103 | 1,000/2C | 2020 [375] |
| Pure LTO | 1.0–2.5 | 55.6/39.5 | 500/10C | 2019 [402] |
| N-doped LTO/carbon | 1.0–2.5 | 121.8/117.1 | 500/10C | 2019 [402] |
| Mesoporous carbon/LTO nanoflakes | 0.01–3.0 | 1,000/300 | 100/1C | 2019 [403] |
| LTO microspheres@C | 1.0–2.5 | 212/201 | 500/1C | 2018 [392] |
| Al3+ and Mn4+ co-doped LTO/CNT | 1.0–3.0 | 286.2/259.5 | 100/0.1C | 2020 [393] |
| Necklace-type LTO/VACNT nanocomposites | 1.0–2.5 | 170/165 | 500/10C | 2016 [401] |
| Nanostructured LTO/crystallite size-7 nm | 1.0–2.5 | 143/129 | 800/10C | 2018 [408] |
5 Alloying-type AMs for anodes
Based on their high specific capacity, low delithiation potential, low cost, and safety, alloying type materials such Sn, Ge, and Si have garnered much attention as promising candidates for anode [409,410]. Although gravimetric capacity (Sn: 994 mA h/g, Ge: 1,625 mA h/g, Si: 4,200 mA h/g) of these materials is different, their volumetric capacity (Sn: 7,216 mA h/cm3, Ge: 8,645 mA h/cm3, Si: 9,786 mA h/cm3) is almost the same. These anode materials suffer from a volume change during cycling and most of the approaches to mitigate volume change are similar to those used for Si. In this review, we will focus on Si as recent reviews on Sn and Ge can be found in refs. [52,411], respectively.
Si has both the highest gravimetric capacity (4,200 mA h/g, Li22Si5) and volumetric capacity (9,786 mA h/cm3) among the anode material candidates (see Figure 1b). The practical application of Si anodes is, however, impeded by its lower conductivity, large volume expansion upon lithiation (∼400%) during first discharge, pulverization during subsequent cycling, delamination from current collector, and unstable SEI layer [52,409,412–414]. Companies such as Sila Nanotechnology, Advano, and NanoGraf are leading the commercialization efforts for Si anodes, but none of them penetrated the market with their Si anode-based batteries yet.
Several strategies have been suggested to overcome the challenges mentioned above (Figure 6). These include change in size/morphology of Si (nanotube, nanorods, nanowires, and porous particles) [52,415–420], coating/mixing with carbon/carbon composite [412,421–426], 3D Si host/conductive framework [427–432], and optimization of electrolyte, binders, and additives [433]. These strategies have also been combined with one another to get better electrochemical performance [422,423,434]. A detailed review of the design/optimization of electrolytes and binders can be found in ref. [433]. Also, limiting the discharge voltage and avoiding the re-crystallization voltage (>80 mV) can mitigate the pulverization issue to some extent [428].
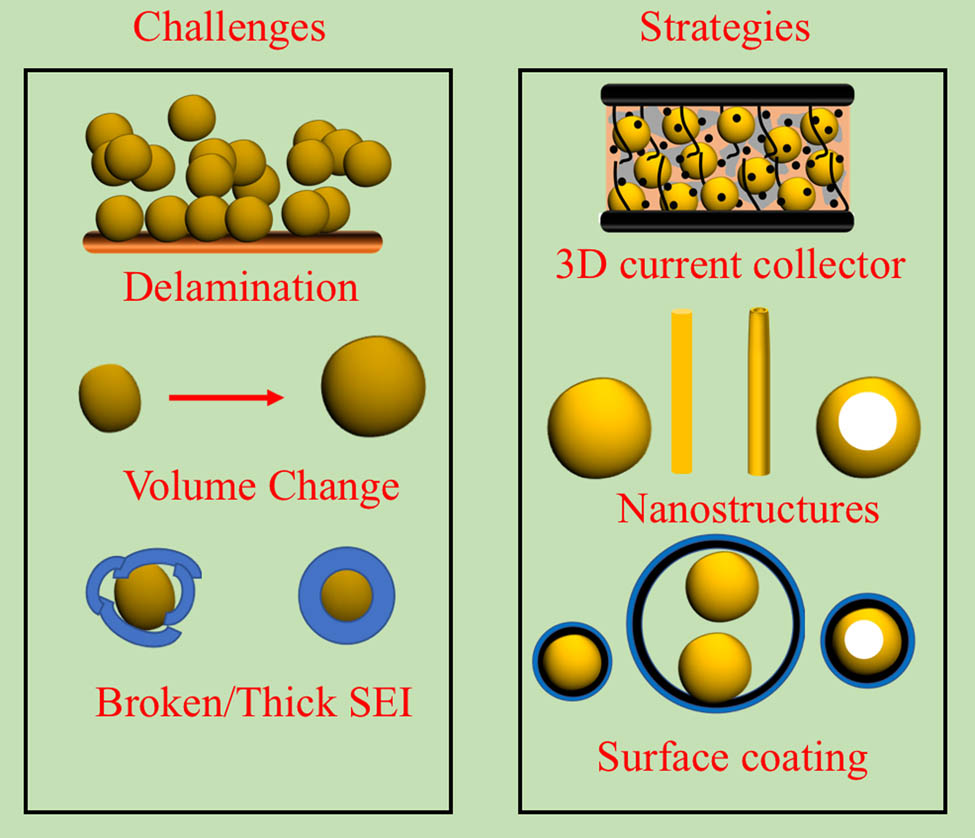
Schematic showing the challenges and strategies commonly found in the literature for Si anodes.
The particle’s ability to resist mechanical degradation increases with tailoring its size from the bulk to the nanometer scale. Many studies have suggested that the stress generation and subsequent cracking/fracture of the particle is size dependent [415–420]. Above 150 nm, Si particles are susceptible to fracture [419]. Although nanostructuring reduces pulverization issues, it also increases side reaction with electrolyte, internal resistance of the electrode, and agglomeration of NPs [433]. Also, the volumetric capacity of the NPs is often lower because of the low density. Nanostructures such as nanorods and nanowires were regarded as better alternatives; however, large capacity fading was observed in such structures due to an unstable SEI and delamination from the current collector [435,436]. Similar problems were observed with thin-film Si anodes with increasing thickness of the films [436]. Porous particles such as porous nanotubes and porous spheres can minimize the mechanical stress by providing the internal space for volumetric expansion and can shorten the Li-diffusion pathways [436,437]. Based on these findings, the development of innovative Si structures has gained momentum in recent years (Table 10) [438]. But, problems such as side reaction at the surface and growth of SEI still exist [437].
Representative electrochemical performance of Si anode (1C = 4.2 A/g)
| Material | Operating voltage (V) | Initial/final capacity (mA h/g) | Cycle/C rate | Year/Ref. |
|---|---|---|---|---|
| Micro-sized Si particle | 0.001–1.5 | 2,100/275 | 20/0.25C | 2019 [458] |
| Micro-sized branched Si particle | 0.001–1.5 | 1,600/1,133 | 100/0.25C | 2019 [458] |
| Si (<100 nm) NPs | 0.01–1.2 | 193.2/68.5 | 100/0.05C | 2019 [459] |
| Si (<100 nm)@mesoporous carbon | 0.01–1.2 | 1,340/1,330 | 100/0.05C | 2019 [459] |
| Si/CNT | 0.001–2.0 | 1,537/246 | 100/0.25C | 2018 [452] |
| Si/CNT/C microsphere | 0.001–2.0 | 1,989/1,392 | 100/0.25C | 2018 [452] |
| Double core-shell porous Si–Ag/C | 0.01–1.5 | 1566.7/1,000 | 100/0.5C | 2018 [450] |
| 3D graphdiyne–Si | 0.01–2.0 | 1,770/1,250 | 100/0.25C | 2018 [430] |
| Si@N-doped carbon | 0.01–2.0 | 2,385/2,003 | 500/0.2C | 2020 [460] |
| Si@graphene | 0.01–1.5 | 3,578/1,909 | 100/0.05C | 2019 [461] |
| Hollow Si@void@C microspheres | 0.01–1.5 | 3,000/1,040 | 500/0.5C | 2019 [447] |
| Si@MOF derived C | 0.005–3.0 | 2,709/1,978 | 350/0.25C | 2020 [462] |
| MXene–Si–CNT | 0.01–1.5 | 1,050/841 | 200/0.5C | 2019 [463] |
| Si@CNT/C-microscroll | 0.01–2.0 | 2,710/2,056 | 300/0.05C | 2020 [464] |
| Amorphous Si@SiO x thin film | 0.01–1.0 | 2,173/2,116 | 100/0.5C | 2019 [465] |
| Si – carbon coaxial nanotube as CC | 0.05–1.0 | 2,750/2,200 | 50/0.2C | 2011 [428] |
| Si – 3D Cu foil/Cu nanowires/CNT as CC | 0.01–2.0 | 2,168/1,845 | 180/0.8C | 2019 [434] |
| Bucky paper/Si/Bucky paper sandwich | 0.01–1.0 | 2,900/1,635 | 100/0.1C | 2020 [457] |
Carbon-based materials such as activated carbon, graphene, CNTs, and graphene oxide have been used to improve the conductivity of the electrode as well as the overall electrochemical activity of Si/C composite [409,439–442]. The electrochemical performance of such composite depends mostly upon the types of carbon matrix used. Pyrolytic carbon-coated Si NPs provide void space for volume change ensuing in excellent performance. Carbon coating can also reduce side reactions of electrolytes with Si and also prevent the growth of SEI [443–448]. Liu et al. [449] first showed the Si/C (yolk/shell) structure can be used. Subsequently, such C shells were replaced by other stable/porous metal/metal oxide as C cannot accommodate the volume change during long cycling [446,450]. 2D carbon forms such as graphene were also used to wrap SiNPs [422–425,439]. Luo et al. [421] showed that crumpled graphene/Si NPs have better cycle performance than bare Si NPs. Si/CNT have also shown promising performance [451,452]. Using CNTs alone may not be enough to mitigate volume change, and therefore CNTs are often used in conjunction with C [437,452]. A detailed review on surface coatings can be found in ref. [453].
Although several efforts have been made to accommodate volume changes by focusing on the AM alone, a key factor for the better electrode performance, that is, the current collector has been overlooked. Mostly, planar current collectors such as Cu-foils have been used in most studies. However, such Cu foil current collectors do not accommodate volume changes in the AM. However, 3D current collectors that can (i) provide better electrical connection between AM and CC reduce the CCAMI resistance, (ii) accommodate the volume change, and (iii) decrease the diffusion time are needed [428,434,454–456]. As an alternative approach to mixing Si NPs with other nanomaterials, we sandwiched SiNPs between freestanding CNT Bucky papers. This method alleviates the need for the traditional Cu current collector due to the high electrically conductive CNTs. Based on exhaustive electrochemical impedance spectroscopy studies, we found that the diffusion time constant for the sandwiched structures is ∼150 times smaller than that of Cu foil [457].
6 CO2 emissions
Although BEVs are touted as cleaner cars based on a comparison of their CO2 emissions with respect to that of gasoline-powered vehicles, there are several factors ranging from mining/manufacturing to driving to disposal or recycling which might have a larger effect on the CO2 emission from BEVs. Despite their low operation (driving) emissions, the CO2 emission from the BEV manufacturing is more because of the added emissions during battery manufacturing, which is negligible during the manufacturing of gasoline-powered vehicles (pink bars in Figure 7a). Overall, present analyses suggest that mid-size BEVs will have a 51% reduction in CO2 emission during operation compared to gasoline-powered vehicles (Figure 7a) [466]. In reality, this percent reduction is highly dependent on how the electricity used during manufacturing was produced. If the electricity grids were not cleaner and used non-renewable sources like coal, then the overall manufacturing CO2 emissions are higher. Therefore, in our analysis of the overall emissions, a key factor that has been overlooked (gray bars in Figure 7a) and must be considered is whether or not clean energy was used during manufacturing. In addition, the battery chemistry also contributes to the CO2 emission (Figure 7b). For mid-size BEV with LCO (hydrothermal process), the CO2 emission is increased by ∼45%, whereas for the LCO (solid-state process) the emission is increased by only ∼9% [466]. If instead LMO is used, then the CO2 emission will be decreased by ∼18% and ∼43% for mid-size and full-size BEVs, respectively (Table 11) [466,467].
Representative battery chemistry and global warming emission change. This table is obtained from ref. [466]
| Battery | Process | Mid-size BEV (%) | Full-size BEV (%) |
|---|---|---|---|
| LCO | Solid state | 9 | −25 |
| LFP | Solid state | −14 | −41 |
| LCO | Hydrothermal | 45 | NA |
| LFP | Hydrothermal | −7 | −36 |
| LMO | NA | −18 | −43 |
In general, the total emissions (T e) from an automobile could be expressed as a sum of emissions from manufacturing (E m), operation (E o), and disposal/recycling (E d).
In equation (1), T e and E o are time dependent as ∼65% emissions of an automobile come from its operation over a period of time [466]. In the case of a BEV, the total manufacturing and disposal emissions contribute up to 30–35% of lifetime global warming emissions [466]. Thus, for BEVs, E m could be decoupled into manufacturing emissions from batteries (E bm) and emissions from other car parts and assembly (E cm).
In a typical mid- or full-size BEVs, E bm contributes ∼15–35% (for present-day LIBs) to E m while E cm contributes ∼65–85%.
The offset in total emissions by using BEVs ΔT e(t) in place of gasoline vehicles would be
In equation (3), (E bm − E gasoline) is the difference between emissions for battery manufacturing and well-to-tank emissions for gasoline or diesel production. The total E bm for LIBs ranges from 123 to 1,090 lbs of CO2 per kW h [468] while E gasoline is 0.16 lbs per kW h of gasoline [469]. Given that well-to-tank emissions of gasoline are at least several magnitudes lower than battery emissions, we approximate E bm − E gasoline ≅ E bm. From the available data on BEV and gasoline car manufacturing [466], ΔE cm could be neglected due to its minimal contribution (i.e., no significant difference in emissions from the car body parts such as tires and metal frame; see Figure 7a). The difference in emissions from battery disposal (ΔE d) is significantly low (<5% of E m for present-day LIBs). Thus, following the earlier approximations, we may express
It is important to note that the term ΔE o(t) is a highly dependent electricity grid mix representative of where BEVs are used. By explicitly including gasoline efficiency (e gas in miles per gallon or mpg), BEV efficiency (e BEV in miles per kW h), and electricity mix (C em, lbs of CO2 emitted per kW h of electricity produced), one obtains
In equation (5), M y stands for average annual miles per car (∼13,500 miles per year in the United States) and t is expressed in years. Factor 20 comes from the fact that one gallon of gasoline results in ∼20 lbs of CO2 emissions. We used lbs of CO2 emission instead of traditional metric tons as ΔT e(t) and ΔE o(t) pertain to a single BEV/gasoline car. Here, we assumed constant automobile efficiency during the lifetime of a car. Using equation (5) and converting all time-dependence into miles driven (M d), the total offset in emissions becomes
In equation (6), E bm depends upon the size of the car. For example, for a mid-size 84-mile-range BEV (/full-size 265-mile-range BEV), manufacturing emissions are approximately 15% (/68%), or 1 ton or 2,000 lbs (/6 tons or 12,000 lbs) of CO2 higher than those of a comparable conventional gasoline vehicle. Accordingly, considering that E bm > 0, ΔT e is offset (i.e., ΔT e = 0) only after BEV is driven for M offset miles, which is given by
Clearly, M
offset directly depends upon emissions from battery manufacturing E
bm in addition to C
em and
Building on the data from Figure 8, Figure 9a shows different ΔT e for different scenarios of E bm and e gas for mid-size vehicles used in the United States. It can be noticed that different lines (corresponding to different E bm and e gas combinations) intersect the x-axis (corresponding to ΔT e = 0) at different points (i.e., each line has a different M offset). In Figure 9b, differences in M offset for various scenarios with different E bm and e gas are shown. It is evident that the increase in M offset is significantly impacted by increases in emissions from battery manufacturing. A 50% increase in battery manufacturing emissions (from the present estimate 2,000 lbs of CO2 to 3,000 lbs) increases M offset from ∼5,000 to 7,500 miles while a 100% increase leads to a higher M offset of 10,000 miles. In other words, as the emission from battery manufacturing increases, it takes longer BEV driving time to offset the emissions (which is the primary goal of BEVs). More importantly, as seen from Figure 8b, e gas increased non-linearly in the last two decades. Further expected increase in e gas to 45 and 55 mpg for passenger vehicles (see Figure 9a and b) increases M offset to ∼11,250 and 20,650 miles, respectively. Lastly, in the developing world, C em is much higher than the United States/Europe. For example, India (C em = 1.63 lbs of CO2 per kW h) and China (C em = 1.35 lbs of CO2 per kW h) have C em, which is ∼100% greater compared to the United States (∼0.8 lbs of CO2 per kW h) with lower average miles per year. In India, the average annual miles for a passenger car is ∼7,500 miles much lower than the US value of ∼13,500 miles. With combined effects of higher C em and lower miles driven per year, a typical mid-size BEVs will take ∼2 years (/3–4 years) to offset emissions that would have come from a gasoline car in the United States (/India). Given that C em is dependent on geographical location, batteries produced in the developing world with higher C em produce more E bm per kW h [468].
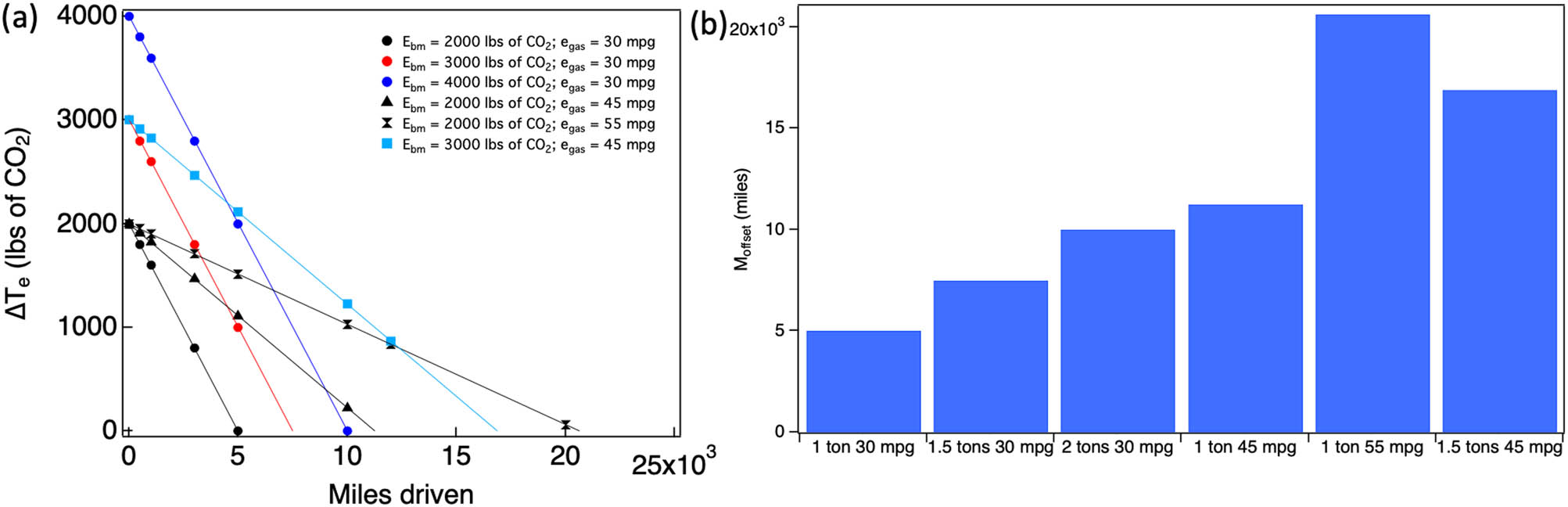
(a) The difference in total emissions (ΔT e) between mid-size BEV and gasoline vehicles for different scenarios of battery manufacturing emissions (E bm) and mileage (e gas). Different scenarios require a different number of miles to be driven (M offset) before total emissions could be offset (ΔT e = 0). (b) The values of M offse for scenarios shown in (a). It should be noted that a conversion factor of 2,000 is used to convert lbs of CO2 into tons.
The primary goal of road electrification is to offset CO2 emissions from gasoline cars. While increasing power and energy density are necessary for ensuring that BEVs provide the same level of comfort as a gasoline car, newer battery chemistries should not significantly increase E bm, E bd, and M offset. Concerted efforts have been focused on discovering new materials, novel architectures, and better chemistries (e.g., Li–S batteries, Na–S batteries) with little or no attention paid to % increase in battery manufacturing emissions for new chemistries. Life cycle analyses (LCA) of present LIB-based BEV batteries suggest that significant emissions result from battery manufacturing. These emissions have been identified to directly depend upon the choice of cathode and anode materials in addition to other components [474–477]. For example, cathode material lithium NMC and anode material graphite together contribute 74% of the total carbon footprint of raw materials needed to make NCM LIBs [477]. Dunn et al. published a series of articles through Argonne National Labs with detailed LCA analysis for five LIB cathode materials [474–476]. Based on their analysis, for at-capacity battery assembly plants, battery materials dominate the carbon footprint of emissions, with cathode materials representing 10–50% of that energy, depending on cathode type. Similarly, based on the LIB LCA analysis from various publications presented in ref. [468], studies indicate that battery production is associated with significant emissions of up to 125 to 1,000 lbs of CO2 per kW h for BEVs. In other words, a 24 kW h battery pack in Nissan Leaf results in E bm = 2,480 lbs of CO2 while a 100 kW h pack in Tesla Model S Long Range Plus leads to E bm = 12,500 lbs of CO2. As described in the mathematical framework earlier (cf. Figure 9), such high emissions imply that a higher M offset (i.e., significantly more miles need to be driven) is needed to offset ΔT e.
Considering that the CO2 emissions are dependent on materials chemistry (Table 11), current collectors, and manufacturing methods, we recommend that researchers use simple LCA based on existing battery chemistries (e.g., GREET™ model from Argonne National Labs) to estimate % change in CO2 emissions for any new active materials, composites, and electrode configurations (including changes in current collector or changes in manufacturing/assembly methods). For example, replacing traditional Al foil current collector with a nano-carbon matrix (e.g., graphene foam, Bucky paper) could possibly lead to a significant increase in CO2 emissions in battery production based on the method used for graphene production (e.g., chemical vapor deposition of graphene from CH4 at 1,000°C produces significantly more emissions than exfoliation). In addition to complete electrochemical characterization, it is important for battery researchers to report % changes in CO2 emissions per kW h that would be caused by their battery chemistry and assembly processes. Ignoring a net increase in CO2 emissions for new materials or manufacturing processes while focusing exclusively on better electrochemical performance will eventually delay our efforts to mitigate the effects of climate change. Furthermore, one should also consider the recyclability of cell components, which ultimately could reduce the net CO2 emissions at the materials production level.
7 Outlook and conclusions
Although AM chemical compositions and physical characteristics have been optimized, many studies are still focused on improving the gravimetric capacity of AMs and largely ignore the need to enhance areal capacity and volumetric capacity beyond existing commercial electrodes. Beyond these incremental studies, future research should also focus on improving the mass loading and tap density of the electrodes while maintaining a high areal capacity. It should be mentioned that there are important issues with how battery parameters are reported or compared in the literature. For example, (i) it is important to report gravimetric capacities at the electrode level and not just the AM level. Inactive binders and additives increase the total weight of the electrode and must be included in normalizing the total capacity to assess the practical usability of the electrodes and (ii) the C rate was developed to compare and evaluate similar electrodes that have similar cross-sectional area [478]. As a result, based on high gravimetric C rates obtained at the coin-cell level (with a small electrode area), it would be incorrect to claim that batteries in BEVs (with relatively higher electrode area) can be charged at a very high C rate within a few minutes. It is easy to maintain a high current density (a high C rate) in coin cells with a very small capacity per unit area for a short time. In larger cells, it may not be possible to maintain such high current densities for longer times because the Li+ ion transport is limited by the diffusivity and the available cross-sectional area of the electrode. Furthermore, gravimetric C rates (calculated per gram of AM) should be converted to appropriate areal C rates (per cm2 of the electrode) while assessing practical applications.
Although a lot of research has been focusing on alternative paper or carbon-based 2D/3D porous or nonporous electrodes, scaling up these techniques using current commercialized manufacturing systems is tricky. For example, to scale up from coin cells level to pouch cells, additional challenges such as gas evolution, placing tabs, or coating the slurry in a cost-effective manner must be addressed. A detailed discussion on gas evolution can be found in ref. [479]. While nano-structuring of AM has shown better results, most of the synthesis approaches reported in the literature are not cost effective because of the low product yield. For example, graphite is still used as the anode even though silicon has been known as an attractive alternative, and commercial silicon batteries remain implausible at this time. Several reports have appeared in the literature in which modified silicon (NPs, porous, core/shell) was used to obtain capacity as close as to its theoretical gravimetric capacity of 4,200 mA h/g. While such studies advance our understanding of battery chemistry, they fall short of commercializing the silicon battery technology. Replacing thermally and structurally stable layered oxides cathodes with sulfur-based cathodes pose a lot of challenges, mainly because of the lower open-circuit voltage (∼2.1 V) in the latter that yields a lower energy density. Therefore, the areal capacity must be increased to 6 mA h/cm2 by overcoming the insulating nature of sulfur and the polysulfide shuttle.
While this review is based on the experimental work reported in the literature, we also acknowledge the fact that extensive modeling of the electrochemical processes has helped advance LIBs [480,481]. Many approaches have been used to understand the electro-chemo mechanics [482], SEI formation [483], development of different components of the battery and its aging [484] as well as the safety [485,486]. Tools such as density functional theory (DFT) and molecular dynamics are used for computation and simulations for Li-ion batteries [323,480,487]. For example, Fan et al. [323] used DFT calculations to investigate the merit of using either ether-based or carbonate-based electrolyte solvents or a mixture of them in their LSBs. Specifically, they demonstrated that 1 M LiTFSI in EC0.5DME0.25DOL0.25 yielded the best battery performance as the mixed electrolyte could promote the formation of a bilateral SEI. In this regard, machine learning is used extensively to identify optimal electrode materials, battery degradation patterns, state of charge, etc. [488–493]. As machine learning requires a significant amount of verified data as input, either experimental or computational data on hand or a combination of both is expected to accelerate new materials discovery for advanced LIBs. Additionally, in-depth knowledge of electrode materials and their electrochemical properties deduced from advanced in situ/operando tools constitutes an important set of inputs for machine learning [494–496]. As such, in situ/operando characterization tools such as imaging (SEM, TEM, AFM), optical (Raman, UV/Vis, FTIR), X-ray, and neutron scattering have been extensively used in recent years. A detailed review on these techniques can be found in ref. [497]. Lastly, more importance must be given to explore environmentally friendly and facile routes that can displace the use of toxic solvents in nanomaterials synthesis and lithium battery manufacturing.
-
Funding information: This work was financially supported by NASA-EPSCoR award under #NNH17ZHA002C and South Carolina EPSCoR/IDeA Program under Award #18-SR03. The authors also thank all the members of Clemson Nanomaterials Institute for their inputs.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Omar N, Daowd M, van den Bossche P, Hegazy O, Smekens J, Coosemans T, et al. Rechargeable energy storage systems for plug-in hybrid electric vehicles – assessment of electrical characteristics. Energies. 2012;5:2952–88.10.3390/en5082952Suche in Google Scholar
[2] Lu J, Chen Z, Ma Z, Pan F, Curtiss LA, Amine K. The role of nanotechnology in the development of battery materials for electric vehicles. Nat Nanotechnol. 2016;11:1031–8.10.1038/nnano.2016.207Suche in Google Scholar PubMed
[3] Zaghib K, Mauger A, Groult H, Goodenough JB, Julien CM. Advanced electrodes for high power Li-ion batteries. Mater (Basel). 2013;6:1028–49.10.3390/ma6031028Suche in Google Scholar PubMed PubMed Central
[4] Brownson DAC, Kampouris DK, Banks CE. An overview of graphene in energy production and storage applications. J Power Sources. 2011;196:4873–85.10.1016/j.jpowsour.2011.02.022Suche in Google Scholar
[5] Auston D, Samuelsen S, Brouwer J, DenBaars S, Glassley W, Jenkins B, et al. Chapter 5. assessing the need for high impact technology research, development & deployment for mitigating climate change. Collabra. 2016;2:1–24.10.1525/collabra.64Suche in Google Scholar
[6] Deng D. Li-ion batteries: basics, progress, and challenges. Energy Sci Eng. 2015;3:385–418.10.1002/ese3.95Suche in Google Scholar
[7] Cairns EJ, Albertus P. Batteries for electric and hybrid-electric vehicles. Annu Rev Chem Biomol Eng. 2010;1:299–320.10.1146/annurev-chembioeng-073009-100942Suche in Google Scholar PubMed
[8] Childress AS, Parajuli P, Zhu J, Podila R, Rao AM. A Raman spectroscopic study of graphene cathodes in high-performance aluminum ion batteries. Nano Energy. 2017;39:69–76.10.1016/j.nanoen.2017.06.038Suche in Google Scholar
[9] Lin MC, Gong M, Lu B, Wu Y, Wang DY, Guan M, et al. An ultrafast rechargeable aluminium-ion battery. Nature. 2015;520:325–8.10.1038/nature14340Suche in Google Scholar PubMed
[10] Bommier C, Ji X. Electrolytes, SEI formation, and binders: a review of nonelectrode factors for sodium-ion battery anodes. Small. 2018;14:1703576.10.1002/smll.201703576Suche in Google Scholar PubMed
[11] Wu C, Tong X, Ai Y, Liu DS, Yu P, Wu J, et al. The surface coating of commercial LiFePO4 by utilizing ZIF-8 for high electrochemical performance lithium ion battery. Nano-Micro Lett. 2018;10:1–18.10.1007/s40820-017-0154-4Suche in Google Scholar
[12] Zhang Y, Liang Q, Huang C, Gao P, Shu H, Zhang X, et al. Nearly monodispersed LiFePO4F nanospheres as cathode material for lithium ion batteries. J Solid State Electrochem. 2018;22:1995–2002.10.1007/s10008-018-3905-3Suche in Google Scholar
[13] Liu Y, Sun Q, Li W, Adair KR, Li J, Sun X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Env. 2017;2:246–77.10.1016/j.gee.2017.06.006Suche in Google Scholar
[14] Zhang W, Mao J, Li S, Chen Z, Guo Z. Phosphorus-based alloy materials for advanced potassium-ion battery anode. J Am Chem Soc. 2017;139:3316–9.10.1021/jacs.6b12185Suche in Google Scholar
[15] Meng YS, Arroyo-De Dompablo ME. Recent advances in first principles computational research of cathode materials for lithium-ion batteries. Acc Chem Res. 2013;46:1171–80.10.1021/ar2002396Suche in Google Scholar
[16] Islam MS, Fisher CAJ. Lithium and sodium battery cathode materials: computational insights into voltage, diffusion and nanostructural properties. Chem Soc Rev. 2014;43:185–204.10.1039/C3CS60199DSuche in Google Scholar
[17] Nitta N, Wu F, Lee JT, Yushin G. Li-ion battery materials: present and future. Mater Today. 2015;18:252–64.10.1016/j.mattod.2014.10.040Suche in Google Scholar
[18] Whittingham MS, Gamble FR. The lithium intercalates of the transition metal dichalcogenides. Mater Res Bull. 1975;10:363–71.10.1016/0025-5408(75)90006-9Suche in Google Scholar
[19] Mizushima K, Jones PC, Wiseman PJ, Goodenough JB. LixCoO2 (0 < x < −1): a new cathode material for batteries of high energy density. Mater Res Bull. 1980;15:783–9.10.1016/0025-5408(80)90012-4Suche in Google Scholar
[20] Jung YS, Cavanagh AS, Dillon AC, Groner MD, George SM, Lee S-H. Enhanced stability of LiCoO2 cathodes in lithium-ion batteries using surface modification by atomic layer deposition. J Electrochem Soc. 2010;157:A75.10.1149/1.3258274Suche in Google Scholar
[21] Chen Z, Dahn JR. Methods to obtain excellent capacity retention in LiCoO2 cycled to 4.5 V. Electrochim Acta. 2004;49:1079–90.10.1016/j.electacta.2003.10.019Suche in Google Scholar
[22] Cho J, Kim YJ, Kim TJ, Park B. Zero-strain intercalation cathode for rechargeable Li-ion cell. Angew Chem - Int Ed. 2001;40:3367–9.10.1002/1521-3773(20010917)40:18<3367::AID-ANIE3367>3.0.CO;2-ASuche in Google Scholar
[23] MacNeil DD, Dahn JR. The reaction of charged cathodes with nonaqueous solvents and electrolytes: II. LiMn2O4 charged to 4.2 V. J Electrochem Soc. 2001;148:A1211.10.1149/1.1407246Suche in Google Scholar
[24] Ohzuku T. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J Electrochem Soc. 1993;140:1862–70.10.1149/1.2220730Suche in Google Scholar
[25] Rougier A, Gravereau P, Delmas C. Optimization of the composition of the Li1 − zNi1+zO2 electrode materials: structural, magnetic, and electrochemical studies. J Electrochem Soc. 1996;143:1168–75.10.1149/1.1836614Suche in Google Scholar
[26] Arai H, Okada S, Sakurai Y, Yamaki JI. Thermal behavior of Li1−yNiO2 and the decomposition mechanism. Solid State Ion. 1998;109:295–302.10.1016/S0167-2738(98)00075-7Suche in Google Scholar
[27] Armstrong AR, Bruce PG. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature. 1996;381:499–500.10.1038/381499a0Suche in Google Scholar
[28] Davidson IJ, McMillan RS, Murray JJ, Greedan JE. Lithium-ion cell based on orthorhombic LiMnO2. J Power Sources. 1995;54:232–5.10.1016/0378-7753(94)02074-DSuche in Google Scholar
[29] Croguennec L, Deniard P, Brec R. Electrochemical cyclability of orthorhombic LiMnO2: characterization of cycled materials. J Electrochem Soc. 1997;144:3323–30.10.1149/1.1838013Suche in Google Scholar
[30] Mishra SK, Ceder G. Structural stability of lithium manganese oxides. Phys Rev B - Condens Matter Mater Phys. 1999;59:6120–30.10.1103/PhysRevB.59.6120Suche in Google Scholar
[31] Fergus JW. Recent developments in cathode materials for lithium ion batteries. J Power Sources. 2010;195:939–54.10.1016/j.jpowsour.2009.08.089Suche in Google Scholar
[32] Ammundsen B, Paulsen J. Novel lithium-ion cathode materials based on layered manganese oxides. Adv Mater. 2001;13:943–56.10.1002/1521-4095(200107)13:12/13<943::AID-ADMA943>3.0.CO;2-JSuche in Google Scholar
[33] Capitaine F, Gravereau P, Delmas C. A new variety of LiMnO2 with a layered structure. Solid State Ion. 1996;89:197–202.10.1016/0167-2738(96)00369-4Suche in Google Scholar
[34] Chen CH, Liu J, Stoll ME, Henriksen G, Vissers DR, Amine K. Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries. J Power Sources. 2004;128:278–85.10.1016/j.jpowsour.2003.10.009Suche in Google Scholar
[35] Armstrong AR, Robertson AD, Bruce PG. Structural transformation on cycling layered Li(Mn1−yCoy)O2 cathode materials. Electrochim Acta. 1999;45:285–94.10.1016/S0013-4686(99)00211-XSuche in Google Scholar
[36] Ceder G, Mishra SK. The stability of orthorhombic and monoclinic-layered LiMnO2. Electrochem Solid-State Lett. 1999;2:550–2.10.1149/1.1390900Suche in Google Scholar
[37] Kalyani P, Kalaiselvi N. Various aspects of LiNiO2 chemistry: a review. Sci Technol Adv Mater. 2005;6:689–703.10.1016/j.stam.2005.06.001Suche in Google Scholar
[38] Armstrong AR, Robertson AD, Gitzendanner R, Bruce PG. The layered intercalation compounds Li(Mn1−yCoy)O2: positive electrode materials for lithium–ion batteries. J Solid State Chem. 1999;145:549–56.10.1006/jssc.1999.8216Suche in Google Scholar
[39] Ding Y, Cano ZP, Yu A, Lu J, Chen Z. Automotive Li-ion batteries: current status and future perspectives. Electrochem Energy Rev. 2019;2:1–28.10.1007/s41918-018-0022-zSuche in Google Scholar
[40] Zhang R, Xia B, Li B, Lai Y, Zheng W, Wang H, et al. Study on the characteristics of a high capacity nickel manganese cobalt oxide (NMC) lithium-ion battery – an experimental investigation. Energies. 2018;11:2275.10.3390/en11092275Suche in Google Scholar
[41] Zeng X, Zhan C, Lu J, Amine K. Stabilization of a high-capacity and high-power nickel-based cathode for Li-ion batteries. Chem. 2018;4:690–704.10.1016/j.chempr.2017.12.027Suche in Google Scholar
[42] Chakraborty A, Kunnikuruvan S, Kumar S, Markovsky B, Aurbach D, Dixit M, et al. Layered cathode materials for lithium-ion batteries: review of computational studies on LiNi1–x–yCoxMnyO2 and LiNi1–x–yCoxAlyO2. Chem Mater. 2020;32:915–52.10.1021/acs.chemmater.9b04066Suche in Google Scholar
[43] Hua W, Zhang J, Zheng Z, Liu W, Peng X, Guo XD, et al. Na-doped Ni-rich LiNi0.5Co0.2Mn0.3O2cathode material with both high rate capability and high tap density for lithium ion batteries. Dalt Trans. 2014;43:14824–32.10.1039/C4DT01611DSuche in Google Scholar PubMed
[44] Dixit M, Markovsky B, Aurbach D, Major DT. Unraveling the effects of al doping on the electrochemical properties of LiNi0.5Co0.2Mn0.3O2 using first principles. J Electrochem Soc. 2017;164:A6359–65.10.1149/2.0561701jesSuche in Google Scholar
[45] Schipper F, Dixit M, Kovacheva D, Talianker M, Haik O, Grinblat J, et al. Stabilizing nickel-rich layered cathode materials by a high-charge cation doping strategy: zirconium-doped LiNi0.6Co0.2Mn0.2O2. J Mater Chem A. 2016;4:16073–84.10.1039/C6TA06740ASuche in Google Scholar
[46] Dixit M, Markovsky B, Schipper F, Aurbach D, Major DT. Origin of structural degradation during cycling and low thermal stability of Ni-rich layered transition metal-based electrode materials. J Phys Chem C. 2017;121:22628–36.10.1021/acs.jpcc.7b06122Suche in Google Scholar
[47] Schipper F, Erickson EM, Erk C, Shin J-Y, Chesneau FF, Aurbach D. Review – recent advances and remaining challenges for lithium ion battery cathodes: I. nickel-rich, LiNixCoyMnzO2. J Electrochem Soc. 2017;164:A6220–8.10.1149/2.0351701jesSuche in Google Scholar
[48] Ates MN, Jia Q, Shah A, Busnaina A, Mukerjee S, Abraham KM. Mitigation of layered to spinel conversion of a Li-rich layered metal oxide cathode material for Li-ion batteries. J Electrochem Soc. 2014;161:A290–301.10.1149/2.040403jesSuche in Google Scholar
[49] Huang B, Li X, Wang Z, Guo H, Xiong X. Synthesis of Mg-doped LiNi0.8Co0.15Al0.05O2 oxide and its electrochemical behavior in high-voltage lithium-ion batteries. Ceram Int. 2014;40:13223–30.10.1016/j.ceramint.2014.05.029Suche in Google Scholar
[50] Yue P, Wang Z, Li X, Xiong X, Wang J, Wu X, et al. The enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials by low temperature fluorine substitution. Electrochim Acta. 2013;95:112–8.10.1016/j.electacta.2013.02.037Suche in Google Scholar
[51] Luo W, Zhou F, Zhao X, Lu Z, Li X, Dahn JR. Synthesis, characterization, and thermal stability of LiNi1/3Mn1/3Co1/3−zMgzO2, LiNi1/3−zMn1/3Co1/3MgzO2, and LiNi1/3Mn1/3−zCo1/3MgzO2. Chem Mater. 2010;22:1164–72.10.1021/cm902593nSuche in Google Scholar
[52] Chen Y, Jiao Q, Wang L, Hu Y, Sun N, Shen Y, et al. Synthesis and characterization of Li1.05Co1/3Ni1/3Mn1/3O1.95X0.05 (X = Cl, Br) cathode materials for lithium-ion battery. Comptes Rendus Chim. 2013;16:845–9.10.1016/j.crci.2013.01.008Suche in Google Scholar
[53] Wan DY, Fan ZY, Dong YX, Baasanjav E, Jun HB, Jin B, et al. Effect of metal (Mn, Ti) doping on NCA cathode materials for lithium ion batteries. J Nanomater. 2018;2018:1–9.10.1155/2018/8082502Suche in Google Scholar
[54] Wang D, Li X, Wang Z, Guo H, Xu Y, Fan Y, et al. Role of zirconium dopant on the structure and high voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Electrochim Acta. 2016;188:48–56.10.1016/j.electacta.2015.11.093Suche in Google Scholar
[55] Krishna Kumar S, Ghosh S, Ghosal P, Martha SK. Synergistic effect of 3D electrode architecture and fluorine doping of Li1.2Ni0.15Mn0.55Co0.1O2 for high energy density lithium-ion batteries. J Power Sources. 2017;356:115–23.10.1016/j.jpowsour.2017.04.077Suche in Google Scholar
[56] Krishna Kumar S, Ghosh S, Martha SK. Synergistic effect of magnesium and fluorine doping on the electrochemical performance of lithium-manganese rich (LMR)-based Ni-Mn-Co-oxide (NMC) cathodes for lithium-ion batteries. Ion (Kiel). 2017;23:1655–62.10.1007/s11581-017-2018-9Suche in Google Scholar
[57] Nisar U, Amin R, Shakoor A, Essehli R, Al-Qaradawi S, Kahraman R, et al. Synthesis and electrochemical characterization of Cr-doped lithium-rich Li1.2Ni0.16Mn0.56Co0.08−xCrxO2 cathodes. Emergent Mater. 2018;1:155–64.10.1007/s42247-018-0014-0Suche in Google Scholar
[58] Liu S, Dang Z, Liu D, Zhang C, Huang T, Yu A. Comparative studies of zirconium doping and coating on LiNi0.6Co0.2Mn0.2O2 cathode material at elevated temperatures. J Power Sources. 2018;396:288–96.10.1016/j.jpowsour.2018.06.052Suche in Google Scholar
[59] Eilers-Rethwisch M, Winter M, Schappacher FM. Synthesis, electrochemical investigation and structural analysis of doped Li[Ni0.6Mn0.2Co0.2-M]O2 (x = 0, 0.05; M = Al, Fe, Sn) cathode materials. J Power Sources. 2018;387:101–7.10.1016/j.jpowsour.2018.02.080Suche in Google Scholar
[60] Pişkin B, Uygur CS, Aydınol MK. Morphology effect on electrochemical properties of doped (W and Mo) 622NMC, 111NMC, and 226NMC cathode materials. Int J Hydrog Energy. 2020;45:7874–80.10.1016/j.ijhydene.2019.07.249Suche in Google Scholar
[61] Liu L, Sun K, Zhang N, Yang T. Improvement of high-voltage cycling behavior of Li(Ni1/3Co1/3Mn1/3)O2 cathodes by Mg, Cr, and Al substitution. J Solid State Electrochem. 2009;13:1381–6.10.1007/s10008-008-0695-zSuche in Google Scholar
[62] Kam KC, Mehta A, Heron JT, Doeff MM. Electrochemical and physical properties of Ti-substituted layered nickel manganese cobalt oxide (NMC) cathode materials. J Electrochem Soc. 2012;159:A1383–92.10.1149/2.060208jesSuche in Google Scholar
[63] Zhang S, Ma J, Hu Z, Cui G, Chen L. Identifying and addressing critical challenges of high-voltage layered ternary oxide cathode materials. Chem Mater. 2019;31:6033–65.10.1021/acs.chemmater.9b01557Suche in Google Scholar
[64] Chen M, Zhao E, Chen D, Wu M, Han S, Huang Q, et al. Decreasing Li/Ni disorder and improving the electrochemical performances of Ni-Rich LiNi0.8Co0.1Mn0.1O2by Ca doping. Inorg Chem. 2017;56:8355–62.10.1021/acs.inorgchem.7b01035Suche in Google Scholar PubMed
[65] Yang L, Ren F, Feng Q, Xu G, Li X, Li Y, et al. Effect of Cu doping on the structural and electrochemical performance of LiNi1/3Co1/3Mn1/3O2 cathode materials. J Electron Mater. 2018;47:3996–4002.10.1007/s11664-018-6284-8Suche in Google Scholar
[66] Kim UH, Jun DW, Park KJ, Zhang Q, Kaghazchi P, Aurbach D, et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries. Energy Env Sci. 2018;11:1271–9.10.1039/C8EE00227DSuche in Google Scholar
[67] Schipper F, Bouzaglo H, Dixit M, Erickson EM, Weigel T, Talianker M, et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries. Adv Energy Mater. 2018;8:1701682.10.1002/aenm.201701682Suche in Google Scholar
[68] Sun Y, Zan L, Zhang Y. Enhanced electrochemical performances of Li2MnO3 cathode materials via adjusting oxygen vacancies content for lithium-ion batteries. Appl Surf Sci. 2019;483:270–7.10.1016/j.apsusc.2019.03.210Suche in Google Scholar
[69] Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney SA. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem. 2007;17:3112–25.10.1039/b702425hSuche in Google Scholar
[70] Zhang X, Xiong Y, Dong M, Hou Z. Pb-Doped lithium-rich cathode material for high energy density lithium-ion full batteries. J Electrochem Soc. 2019;166:A2960–65.10.1149/2.0860913jesSuche in Google Scholar
[71] Xie Q, Li W, Manthiram A. A Mg-doped high-nickel layered oxide cathode enabling safer, high-energy-density Li-ion batteries. Chem Mater. 2019;31:938–46.10.1021/acs.chemmater.8b03900Suche in Google Scholar
[72] Torres-Castro L, Shojan J, Julien CM, Huq A, Dhital C, Paranthaman MP, et al. Synthesis, characterization and electrochemical performance of Al-substituted Li2MnO3. Mater Sci Eng B Solid-State Mater Adv Technol. 2015;201:13–22.10.1016/j.mseb.2015.07.006Suche in Google Scholar
[73] Kim S, Noh JK, Yu S, Chang W, Chung KY, Cho BW. Effects of transition metal doping and surface treatment to improve the electrochemical performance of Li2MnO3. J Electroceram. 2013;30:159–65.10.1007/s10832-012-9778-4Suche in Google Scholar
[74] Mori D, Sakaebe H, Shikano M, Kojitani H, Tatsumi K, Inaguma Y. Synthesis, phase relation and electrical and electrochemical properties of ruthenium-substituted Li2MnO3 as a novel cathode material. J Power Sources. 2011;196:6934–8.10.1016/j.jpowsour.2010.11.150Suche in Google Scholar
[75] Xu Y, Cui Q. Nb-doped Li1.20[Mn0.54Ni0.13Co0.13]O2 cathode material with enhanced electrochemical properties for lithium-ion battery. Int J Electrochem Sci. 2020;15:803–15.10.20964/2020.01.47Suche in Google Scholar
[76] Wu ZL, Xie H, Li Y, Zhang F, Wang Z, Zheng W, et al. Li1.2Ni0.25Mn0.55O2: a high-capacity cathode material with a homogeneous monoclinic Li2MnO3-like superstructure. J Alloy Compd. 2020;827:154202.10.1016/j.jallcom.2020.154202Suche in Google Scholar
[77] Zhao W, Oyama M, Yamada H, Noguchi H. Influence of Ti substitution on the structure and electrochemical properties of lithium-excess layered manganese based oxide for lithium ion batteries. Electrochim Acta. 2015;168:157–66.10.1016/j.electacta.2015.04.017Suche in Google Scholar
[78] Yuge R, Kuroshima S, Toda A, Miyazaki T, Tabuchi M, Doumae K, et al. Structural and electrochemical properties of iron- and nickel-substituted Li 2 MnO 3 cathodes in charged and discharged states. J Power Sources. 2017;365:117–25.10.1016/j.jpowsour.2017.08.049Suche in Google Scholar
[79] Zhao W, Xiong L, Xu Y, Xiao X, Wang J, Ren Z. Magnesium substitution to improve the electrochemical performance of layered Li2MnO3 positive-electrode material. J Power Sources. 2016;330:37–44.10.1016/j.jpowsour.2016.08.135Suche in Google Scholar
[80] Ma J, Zhou YN, Gao Y, Kong Q, Wang Z, Yang XQ, et al. Molybdenum substitution for improving the charge compensation and activity of Li2MnO3. Chem - A Eur J. 2014;20:8723–30.10.1002/chem.201402727Suche in Google Scholar PubMed
[81] Gao Y, Wang X, Ma J, Wang Z, Chen L. Selecting substituent elements for Li-rich Mn-based cathode materials by density functional theory (DFT) calculations. Chem Mater. 2015;27:3456–61.10.1021/acs.chemmater.5b00875Suche in Google Scholar
[82] Tabuchi M, Nabeshima Y, Takeuchi T, Kageyama H, Tatsumi K, Akimoto J, et al. Synthesis and electrochemical characterization of Fe and Ni substituted Li2MnO3 – an effective means to use Fe for constructing “Co-free” Li2MnO3 based positive electrode material. J Power Sources. 2011;196:3611–22.10.1016/j.jpowsour.2010.12.060Suche in Google Scholar
[83] Cho J, Kim YW, Kim B, Lee JG, Park B. A breakthrough in the safety of lithium secondary batteries by coating the cathode material with AlPO4 nanoparticles. Angew Chem - Int Ed. 2003;42:1618–21.10.1002/anie.200250452Suche in Google Scholar
[84] Noh HJ, Youn S, Yoon CS, Sun YK. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J Power Sources. 2013;233:121–30.10.1016/j.jpowsour.2013.01.063Suche in Google Scholar
[85] Song C, Wang W, Peng H, Wang Y, Zhao C, Zhang H, et al. Improving the Electrochemical performance of LiNi0.80Co0.15Al0.05O2 in lithium ion batteries by LiAlO2 surface modification. Appl Sci. 2018;8:378.10.3390/app8030378Suche in Google Scholar
[86] Jung R, Metzger M, Maglia F, Stinner C, Gasteiger HAA. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2(NMC) cathode materials for Li-ion batteries. J Electrochem Soc. 2017;164:A1361–77.10.1149/2.0021707jesSuche in Google Scholar
[87] Wang R, He X, He L, Wang F, Xiao R, Gu L, et al. Atomic structure of Li2MnO3after partial delithiation and Re-lithiation. Adv Energy Mater. 2013;3:1358–67.10.1002/aenm.201200842Suche in Google Scholar
[88] Thackeray MM, David WIF, Bruce PG, Goodenough JB. Lithium insertion into manganese spinels. Mater Res Bull. 1983;18:461–72.10.1016/0025-5408(83)90138-1Suche in Google Scholar
[89] Xia Y, Zhou Y, Yoshio M. Capacity fading on cycling of 4VLi/LiMn2O4 Cells. J Electrochem Soc. 1997;144:2593–600.10.1149/1.1837870Suche in Google Scholar
[90] Aurbach D, Levi MD, Gamulski K, Markovsky B, Salitra G, Levi E, et al. Capacity fading of LixMn2O4 spinel electrodes studied by XRD and electroanalytical techniques. J Power Sources. 1999;81–82:472–9.10.1016/S0378-7753(99)00204-9Suche in Google Scholar
[91] Zhang C, Liu X, Su Q, Wu J, Huang T, Yu A. Enhancing electrochemical performance of LiMn2O4 cathode material at elevated temperature by uniform nanosized TiO2 coating. ACS Sustain Chem Eng. 2017;5:640–7.10.1021/acssuschemeng.6b02011Suche in Google Scholar
[92] Yao Y, Wang Z, Yu X, Zhang Y, Duan J, Zhu C, et al. Interface control strategy of synthesis LiMn2O4@Al2O3 assisted by tert-butanol. Int J Electrochem Sci. 2019;14:6478–87.10.20964/2019.07.01Suche in Google Scholar
[93] Liu Y, Lin XJ, Sun YG, Xu YS, Chang BB, Liu CT, et al. Precise surface engineering of cathode materials for improved stability of lithium-ion batteries. Small. 2019;15:1901019.10.1002/smll.201901019Suche in Google Scholar PubMed
[94] Wang S, Luo C, Feng Y, Fan G, Feng L, Ren M, et al. Electrochemical properties and microstructures of LiMn2O4 cathodes coated with aluminum zirconium coupling agents. Ceram Int. 2020;46:13003–13.10.1016/j.ceramint.2020.02.070Suche in Google Scholar
[95] Feng X, Zhang J, Yin L. Enhanced cycling stability of Co3(PO4)2-coated LiMn2O4 cathode materials for lithium ion batteries. Powder Technol. 2016;287:77–81.10.1016/j.powtec.2015.09.031Suche in Google Scholar
[96] Tron A, Park YD, Mun J. AlF3-coated LiMn2O4 as cathode material for aqueous rechargeable lithium battery with improved cycling stability. J Power Sources. 2016;325:360–4.10.1016/j.jpowsour.2016.06.049Suche in Google Scholar
[97] Hai Y, Zhang Z, Liu H, Liao L, Fan P, Wu Y, et al. Facile controlled synthesis of spinel LiMn2O4 porous microspheres as cathode material for lithium ion batteries. Front Chem. 2019;7:437.10.3389/fchem.2019.00437Suche in Google Scholar PubMed PubMed Central
[98] Zhu C. Novel fabrication of Li4Ti5O12 coated LiMn2O4 nanorods as cathode materials with long-term cyclic stability at high ambient temperature. Int J Electrochem Sci. 2019;14:7673–83.10.20964/2019.08.11Suche in Google Scholar
[99] Li S, Zhu K, Zhao D, Zhao Q, Zhang N. Porous LiMn2O4 with Al2O3 coating as high-performance positive materials. Ion (Kiel). 2019;25:1991–8.10.1007/s11581-018-2643-ySuche in Google Scholar
[100] Liu S, Ni D, Li HF, Hui KN, Ouyang CY, Jun SC. Effect of cation substitution on the pseudocapacitive performance of spinel cobaltite MCo2O4(M = Mn, Ni, Cu, and Co). J Mater Chem A. 2018;6:10674–85.10.1039/C8TA00540KSuche in Google Scholar
[101] Michalska M, Ziółkowska DA, Jasiński JB, Lee PH, Ławniczak P, Andrzejewski B, et al. Improved electrochemical performance of LiMn2O4 cathode material by Ce doping. Electrochim Acta. 2018;276:37–46.10.1016/j.electacta.2018.04.165Suche in Google Scholar
[102] Chen B, Ben L, Yu H, Chen Y, Huang X. Understanding surface structural stabilization of the high-temperature and high-voltage cycling performance of Al3+-modified LiMn2O4 cathode material. ACS Appl Mater Interfaces. 2018;10:550–9.10.1021/acsami.7b14535Suche in Google Scholar PubMed
[103] Yang Z, Wang Y, Chen X, Wu H, Zhang Y. Mg2+and Ti4+Co-doped spinel LiMn2O4 as lithium-ion battery cathode. ChemistrySelect. 2019;4:9583–9.10.1002/slct.201902685Suche in Google Scholar
[104] Cai Z, Ma Y, Huang X, Yan X, Yu Z, Zhang S, et al. High electrochemical stability Al-doped spinel LiMn2O4 cathode material for Li-ion batteries. J Energy Storage. 2020;27:101036.10.1016/j.est.2019.101036Suche in Google Scholar
[105] Talyosef Y, Markovsky B, Lavi R, Salitra G, Aurbach D, Kovacheva D, et al. Comparing the behavior of nano- and microsized particles of LiMn1.5Ni0.5O4 spinel as cathode materials for Li-ion batteries. J Electrochem Soc. 2007;154:A682.10.1149/1.2736657Suche in Google Scholar
[106] Sun Y, Yang Y, Zhan H, Shao H, Zhou Y. Synthesis of high power type LiMn1.5Ni0.5O4 by optimizing its preparation conditions. J Power Sources. 2010;195:4322–6.10.1016/j.jpowsour.2010.01.039Suche in Google Scholar
[107] Rapulenyane N, Ferg E, Luo H. High-performance Li1.2Mn0.6Ni0.2O2 cathode materials prepared through a facile one-pot co-precipitation process for lithium ion batteries. J Alloy Compd. 2018;762:272–81.10.1016/j.jallcom.2018.05.207Suche in Google Scholar
[108] Zhu Q, Zheng S, Lu X, Wan Y, Chen Q, Yang J, et al. Improved cycle performance of LiMn2O4 cathode material for aqueous rechargeable lithium battery by LaF3 coating. J Alloy Compd. 2016;654:384–91.10.1016/j.jallcom.2015.09.085Suche in Google Scholar
[109] Padhi AK, Nanjundaswamy KS, Goodenough JB. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc. 1997;144:1188–94.10.1149/1.1837571Suche in Google Scholar
[110] Nytén A, Abouimrane A, Armand M, Gustafsson T, Thomas JO. Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material. Electrochem commun. 2005;7:156–60.10.1016/j.elecom.2004.11.008Suche in Google Scholar
[111] Zaghib K, Mauger A, Julien CM. Green energy and technology. Green Energy Technol. 2015;172:25–65.10.1007/978-3-319-15458-9_2Suche in Google Scholar
[112] Li Z, Ren X, Zheng Y, Tian W, An L, Sun J, et al. Effect of Ti doping on LiFePO4/C cathode material with enhanced low-temperature electrochemical performance. Ion (Kiel). 2020;26:1599–609.10.1007/s11581-019-03408-4Suche in Google Scholar
[113] Göktepe H, Şahan H, Patat Ş. Effect of silver and carbon double coating on the electrochemical performance of LiFePO4 cathode material for lithium ion batteries. Int J Hydrog Energy. 2016;41:9774–9.10.1016/j.ijhydene.2016.03.074Suche in Google Scholar
[114] Liu Y, Manuel J, Zhao X, Haridas AK, Chauhan GS, Kim JK, et al. Effect of carbon coating and magnesium doping on electrochemical properties of LiFePO4 for lithium ion batteries. Sci Adv Mater. 2017;9:1266–71.10.1166/sam.2017.2891Suche in Google Scholar
[115] Li Y, Wang J, Huang HX, Wang J, Zhang M, Liang MmM. Co-coating effect of GdPO4 and carbon on LiFePO4 cathode surface for lithium ion batteries. Adv Powder Technol. 2019;30:1442–9.10.1016/j.apt.2019.04.017Suche in Google Scholar
[116] Ventrapragada LK, Zhu J, Creager SE, Rao AM, Podila R. A versatile carbon nanotube-based scalable approach for improving interfaces in Li-ion battery electrodes. ACS Omega. 2018;3:4502–8.10.1021/acsomega.8b00027Suche in Google Scholar PubMed PubMed Central
[117] Wang C, Li S, Han Y, Lu Z. Assembly of LiMnPO4 nanoplates into microclusters as a high-performance cathode in lithium-ion batteries. ACS Appl Mater Interfaces. 2017;9:27618–24.10.1021/acsami.7b05868Suche in Google Scholar PubMed
[118] Kim S-W, Kim J, Gwon H, Kang K. Phase stability study of Li1−xMnPO4 (0≤x≤1) cathode for Li rechargeable battery. J Electrochem Soc. 2009;156:A635.10.1149/1.3138705Suche in Google Scholar
[119] Satyavani TVSL, Srinivas Kumar A, Subba Rao PSV. Methods of synthesis and performance improvement of lithium iron phosphate for high rate Li-ion batteries: a review. Eng Sci Technol an Int J. 2016;19:178–88.10.1016/j.jestch.2015.06.002Suche in Google Scholar
[120] Zhang J, Luo S, Wang Q, Wang Z, Zhang Y, Hao A, et al. Yttrium substituting in Mn site to improve electrochemical kinetics activity of sol-gel synthesized LiMnPO4/C as cathode for lithium ion battery. J Solid State Electrochem. 2017;21:3189–94.10.1007/s10008-017-3662-8Suche in Google Scholar
[121] Huang QY, Wu Z, Su J, Long YF, Lv XY, Wen YX. Synthesis and electrochemical performance of Ti–Fe co-doped LiMnPO4/C as cathode material for lithium-ion batteries. Ceram Int. 2016;42:11348–54.10.1016/j.ceramint.2016.04.057Suche in Google Scholar
[122] Sarkar T, Bharadwaj MD, Waghmare UV, Kumar P. Mechanism of charge transfer in olivine-type LiFeSiO4and LiFe0.5M0.5SiO4(M = Mg or Al) cathode materials: first-principles analysis. J Phys Chem C. 2015;119:9125–33.10.1021/acs.jpcc.5b01692Suche in Google Scholar
[123] Islam MS, Dominko R, Masquelier C, Sirisopanaporn C, Armstrong AR, Bruce PG. Silicate cathodes for lithium batteries: alternatives to phosphates. J Mater Chem. 2011;21:9811–8.10.1039/c1jm10312aSuche in Google Scholar
[124] Arroyo-de Dompablo ME, Armand M, Tarascon JM, Amador U. On-demand design of polyoxianionic cathode materials based on electronegativity correlations: an exploration of the Li2MSiO4 system (M = Fe, Mn, Co, Ni). Electrochem commun. 2006;8:1292–8.10.1016/j.elecom.2006.06.003Suche in Google Scholar
[125] Arthur Z, Chiu HC, Lu X, Chen N, Emond V, Zaghib K, et al. Spontaneous reaction between an uncharged lithium iron silicate cathode and a LiPF6-based electrolyte. Chem Commun. 2016;52:190–3.10.1039/C5CC07197FSuche in Google Scholar PubMed
[126] Wang C, Xu Y, Sun X, Zhang B, Chen Y, He S. Enhanced electrochemical properties of F-doped Li2MnSiO4/C for lithium ion batteries. J Power Sources. 2018;378:345–52.10.1016/j.jpowsour.2017.12.004Suche in Google Scholar
[127] Li YX, Gong ZL, Yang Y. Synthesis and characterization of Li2MnSiO4/C nanocomposite cathode material for lithium ion batteries. J Power Sources. 2007;174:528–32.10.1016/j.jpowsour.2007.06.126Suche in Google Scholar
[128] Wang M, Yang M, Ma L, Shen X, Zhang X. Graphene channel liquid container field effect transistor as pH sensor. J Nanomater. 2014;2014:1–6.10.1155/2014/547139Suche in Google Scholar
[129] Fan XY, Li Y, Wang JJ, Gou L, Zhao P, Li DL, et al. Synthesis and electrochemical performance of porous Li2FeSiO4/C cathode material for long-life lithium-ion batteries. J Alloy Compd. 2010;493:77–80.10.1016/j.jallcom.2009.12.179Suche in Google Scholar
[130] Wang M, Ding C, Miao Y, Liu T, Hang K, Zhang J. Improving electrochemical properties and structural stability of lithium manganese silicates as cathode materials for lithium ion batteries via introducing lithium excess. Int J Energy Res. 2020;44:902–12.10.1002/er.4932Suche in Google Scholar
[131] Hou P, Feng J, Wang Y, Wang L, Li S, Yang L, et al. Study on the properties of Li2MnSiO4 as cathode material for lithium-ion batteries by sol-gel method. Ion (Kiel). 2020;26:1611–6.10.1007/s11581-020-03462-3Suche in Google Scholar
[132] Lv X, Zhao X, Wu S, Nguyen MC, Zhu Z, Lin Z, et al. Fe–Si networks and charge/discharge-induced phase transitions in Li2FeSiO4 cathode materials. Phys Chem Chem Phys. 2018;20:14557–63.10.1039/C8CP01962BSuche in Google Scholar
[133] Fedotov SS, Khasanova NR, Samarin AS, Drozhzhin OA, Batuk D, Karakulina OM, et al. AVPO4F (A = Li, K): a 4 V cathode material for high-power rechargeable batteries. Chem Mater. 2016;28:411–5.10.1021/acs.chemmater.5b04065Suche in Google Scholar
[134] Kitajou A, Ishado Y, Inoishi A, Okada S. Amorphous xLiF-FeSO4 (1 ≤ x ≤ 2) composites as a cathode material for lithium ion batteries. Solid State Ion. 2018;326:48–51.10.1016/j.ssi.2018.09.007Suche in Google Scholar
[135] Kim M, Lee S, Kang B. Fast-rate capable electrode material with higher energy density than LiFePO4: 4.2V LiVPO4F synthesized by scalable single-step solid-state reaction. Adv Sci. 2015;3:1500366.10.1002/advs.201500366Suche in Google Scholar PubMed PubMed Central
[136] Harrison KL, Manthiram A. Microwave-assisted solvothermal synthesis and characterization of various polymorphs of LiVOPO4. Chem Mater. 2013;25:1751–60.10.1021/cm400227jSuche in Google Scholar
[137] Kim M, Lee S, Kang B. High energy density polyanion electrode material: LiVPO4O1–xFx(x ≈ 0.25) with tavorite structure. Chem Mater. 2017;29:4690–9.10.1021/acs.chemmater.7b00124Suche in Google Scholar
[138] Zhang Y, Lv T, Gao P, Shu H, Yang X, Liang Q, et al. Ag nanoparticles promoted LiFePO4F nanospheres cathode with superior cycling stability for lithium-ion batteries. J Alloy Compd. 2018;751:12–9.10.1016/j.jallcom.2018.04.075Suche in Google Scholar
[139] Wu J, Xu Y, Sun X, Wang C, Zhang B, Zhao J. The multiple effects of potassium doping on LiVPO4F/C composite cathode material for lithium ion batteries. J Power Sources. 2018;396:155–63.10.1016/j.jpowsour.2018.06.020Suche in Google Scholar
[140] Zhang Y, Huo QY, Du PP, Wang LZ, Zhang AQ, Song YH, et al. Advances in new cathode material LiFePO4 for lithium-ion batteries. Synth Met. 2012;162:1315–26.10.1016/j.synthmet.2012.04.025Suche in Google Scholar
[141] Adib M, Habib N, Ashry A, Fathalla M. On the use of silicon as thermal neutron filter. Ann Nucl Energy. 2003;30:1905–17.10.1016/S0306-4549(03)00129-4Suche in Google Scholar
[142] Yuan LX, Wang ZH, Zhang WX, Hu XL, Chen JT, Huang YH, et al. Development and challenges of LiFePO4cathode material for lithium-ion batteries. Energy Env Sci. 2011;4:269–84.10.1039/C0EE00029ASuche in Google Scholar
[143] Chikkannanavar SB, Bernardi DM, Liu L. A review of blended cathode materials for use in Li-ion batteries. J Power Sources. 2014;248:91–100.10.1016/j.jpowsour.2013.09.052Suche in Google Scholar
[144] Tan H, Xu L, Geng H, Rui X, Li C, Huang S. Nanostructured Li3V2(PO4)3Cathodes. Small. 2018;14:1800567.10.1002/smll.201800567Suche in Google Scholar PubMed
[145] Vu A, Stein A. Lithium iron phosphate spheres as cathode materials for high power lithium ion batteries. J Power Sources. 2014;245:48–58.10.1016/j.jpowsour.2013.06.116Suche in Google Scholar
[146] Qin X, Wang X, Xie J, Wen L. Hierarchically porous and conductive LiFePO4 bulk electrode: binder-free and ultrahigh volumetric capacity Li-ion cathode. J Mater Chem. 2011;21:12444.10.1039/c1jm11642hSuche in Google Scholar
[147] Liu Y, Liu D, Zhang Q, Yu D, Liu J, Cao G. Lithium iron phosphate/carbon nanocomposite film cathodes for high energy lithium ion batteries. Electrochim Acta. 2011;56:2559–65.10.1016/j.electacta.2010.11.050Suche in Google Scholar
[148] Wu XL, Guo YG, Su J, Xiong JW, Zhang YL, Wan LJ. Carbon-nanotube-decorated nano-LiFePO4@C cathode material with superior high-rate and low-temperature performances for lithium-ion batteries. Adv Energy Mater. 2013;3:1155–60.10.1002/aenm.201300159Suche in Google Scholar
[149] Liu C, Li F, Lai-Peng M, Cheng HM. Advanced materials for energy storage. Adv Mater. 2010;22:E28–62.10.1002/adma.200903328Suche in Google Scholar PubMed
[150] Xing Y, He YB, Li B, Chu X, Chen H, Ma J, et al. LiFePO4/C composite with 3D carbon conductive network for rechargeable lithium ion batteries. Electrochim Acta. 2013;109:512–8.10.1016/j.electacta.2013.07.141Suche in Google Scholar
[151] Goriparti S, Miele E, De Angelis F, Di Fabrizio E, Zaccaria RP, Capiglia C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J Power Sources. 2014;257:421–43.10.1016/j.jpowsour.2013.11.103Suche in Google Scholar
[152] Chen R, Zhao T, Zhang X, Li L, Wu F. Advanced cathode materials for lithium-ion batteries using nanoarchitectonics. Nanoscale Horiz. 2016;1:423–44.10.1039/C6NH00016ASuche in Google Scholar
[153] Liu HK, Wang GX, Guo Z, Wang J, Konstantinov K. Nanomaterials for lithium-ion rechargeable batteries. J Nanosci Nanotechnol. 2006;6(1):1–15.10.1166/jnn.2006.103Suche in Google Scholar
[154] Ye SH, Lv JY, Gao XP, Wu F, Song DY. Synthesis and electrochemical properties of LiMn2O4 spinel phase with nanostructure. Electrochim Acta. 2004;49:1623–8.10.1016/S0013-4686(03)00991-5Suche in Google Scholar
[155] Uddin MJ, Alaboina PK, Cho SJ. Nanostructured cathode materials synthesis for lithium-ion batteries. Mater Today Energy. 2017;5:138–57.10.1016/j.mtener.2017.06.008Suche in Google Scholar
[156] Okubo M, Hosono E, Kim J, Enomoto M, Kojima N, Kudo T, et al. Nanosize effect on high-rate Li-ion intercalation in LiCoO2 electrode. J Am Chem Soc. 2007;129:7444–52.10.1021/ja0681927Suche in Google Scholar PubMed
[157] Zhang H, Xu Y, Liu D. Novel nanostructured LiMn2O4 microspheres for high power Li-ion batteries. RSC Adv. 2015;5:11091–95.10.1039/C4RA13041CSuche in Google Scholar
[158] Cai Y, Huang Y, Wang X, Jia D, Tang X. Long cycle life, high rate capability of truncated octahedral LiMn2O4 cathode materials synthesized by a solid-state combustion reaction for lithium ion batteries. Ceram Int. 2014;40:14039–43.10.1016/j.ceramint.2014.05.130Suche in Google Scholar
[159] Li S, Zhu K, Liu J, Zhao D, Cui X. Porous LiMn2O4 microspheres with different pore size: preparation and application as cathode materials for lithium ion batteries. J Electrochem Energy Convers Storage. 2019;16. 10.1115/1.4040567.Suche in Google Scholar
[160] Li B, Wei X, Chang Z, Chen X, Yuan XZ, Wang H. Facile fabrication of LiMn2O4 microspheres from multi-shell MnO2 for high-performance lithium-ion batteries. Mater Lett. 2014;135:75–8.10.1016/j.matlet.2014.07.117Suche in Google Scholar
[161] Yang J, Guo B, He H, Li Y, Song C, Liu G. LiNi0.5Mn0.5O2 hierarchical nanorods as high-capacity cathode materials for Li-ion batteries. J Alloy Compd. 2017;698:714–8.10.1016/j.jallcom.2016.12.264Suche in Google Scholar
[162] Pei Y, Chen Q, Xu CY, Wang HX, Fang HT, Zhou C, et al. Chelate-induced formation of Li2MnSiO4nanorods as a high capacity cathode material for Li-ion batteries. J Mater Chem A. 2016;4:9447–54.10.1039/C6TA01269HSuche in Google Scholar
[163] Li J, Hua Luo S, Wang Q, Yan S, Feng J, Liu H, et al. Facile synthesis of carbon-LiMnPO4 nanorods with hierarchical architecture as a cathode for high-performance Li-ion batteries. Electrochim Acta. 2018;289:415–21.10.1016/j.electacta.2018.09.095Suche in Google Scholar
[164] McNulty D, Buckley DN, O’Dwyer C. V2O3 polycrystalline nanorod cathode materials for Li-ion batteries with long cycle life and high capacity retention. ChemElectroChem. 2017;4:2037–44.10.1002/celc.201700202Suche in Google Scholar
[165] Zhao H, Wang J, Wang G, Liu S, Tan M, Liu X, et al. Facile synthesis of orthorhombic LiMnO2 nanorods by in-situ carbothermal reduction: promising cathode material for Li ion batteries. Ceram Int. 2017;43:10585–89.10.1016/j.ceramint.2017.04.158Suche in Google Scholar
[166] Bao L, Xu G, Sun X, Zeng H, Zhao R, Yang X, et al. Mono-dispersed LiFePO4@C core-shell [001] nanorods for a high power Li-ion battery cathode. J Alloy Compd. 2017;708:685–93.10.1016/j.jallcom.2017.03.052Suche in Google Scholar
[167] Yang F, Zhang Q, Hu X, Peng T, Liu J. Preparation of Li-rich layered-layered type x Li2MnO3(1−x)LiMnO2 nanorods and its electrochemical performance as cathode material for Li-ion battery. J Power Sources. 2017;353:323–32.10.1016/j.jpowsour.2017.04.002Suche in Google Scholar
[168] Wang C, Cao Y, Luo Z, Li G, Xu W, Xiong C, et al. Flexible potassium vanadate nanowires on Ti fabric as a binder-free cathode for high-performance advanced lithium-ion battery. Chem Eng J. 2017;307:382–8.10.1016/j.cej.2016.08.072Suche in Google Scholar
[169] Hua K, Li X, Fang D, Bao R, Yi J, Luo Z, et al. Vanadium trioxide nanowire arrays as a cathode material for lithium-ion battery. Ceram Int. 2018;44:11307–13.10.1016/j.ceramint.2018.03.178Suche in Google Scholar
[170] Su D, Zhao Y, Yan D, Ding C, Ning M, Zhang J, et al. Enhanced composites of V2O5 nanowires decorating on graphene layers as ideal cathode materials for lithium-ion batteries. J Alloy Compd. 2017;695:2974–80.10.1016/j.jallcom.2016.11.363Suche in Google Scholar
[171] Deng B, Chen Y, Wu P, Han J, Li Y, Zheng H, et al. Lithium-rich layered oxide nanowires bearing porous structures and spinel domains as cathode materials for lithium-ion batteries. J Power Sources. 2019;418:122–9.10.1016/j.jpowsour.2019.02.036Suche in Google Scholar
[172] Salvatierra RV, Raji ARO, Lee SK, Ji Y, Li L, Tour JM. Silicon nanowires and lithium cobalt oxide nanowires in graphene nanoribbon papers for full lithium ion battery. Adv Energy Mater. 2016;6:1600918.10.1002/aenm.201600918Suche in Google Scholar
[173] Bai N, Xiang K, Zhou W, Lu H, Zhao X, Chen H. LiFePO4/carbon nanowires with 3D nano-network structure as potential high performance cathode for lithium ion batteries. Electrochim Acta. 2016;191:23–8.10.1016/j.electacta.2016.01.019Suche in Google Scholar
[174] Zhu QP, Wang X, Fan J, Xu Q, Min Y. MnO2 nanowires as precursor synthesis of lithium-rich cathode material with enhanced electrochemical performances. Ion (Kiel). 2019;25:2477–85.10.1007/s11581-018-2747-4Suche in Google Scholar
[175] Zhang LX, Wang YZ, Jiu HF, Wang YL, Sun YX, Li Z. Controllable synthesis of Co-doped spinel LiMn2O4 nanotubes as cathodes for Li-ion batteries. Electron Mater Lett. 2014;10:439–44.10.1007/s13391-013-3188-xSuche in Google Scholar
[176] Ding YL, Xie J, Cao GS, Zhu TJ, Yu HM, Zhao XB. Single-crystalline LiMn2O4 nanotubes synthesized via template-engaged reaction as cathodes for high-power lithium ion batteries. Adv Funct Mater. 2011;21:348–55.10.1002/adfm.201001448Suche in Google Scholar
[177] Ma D, Li Y, Zhang P, Cooper AJ, Abdelkader AM, Ren X, et al. Mesoporous Li1.2Mn0.54Ni0.13Co0.13O2 nanotubes for high-performance cathodes in Li-ion batteries. J Power Sources. 2016;311:35–41.10.1016/j.jpowsour.2016.01.031Suche in Google Scholar
[178] Da F, Que Yu,LF, Wang ZB, Zhang Y, Xue Y, Liu BS, et al. Layered-spinel capped nanotube assembled 3D Li-rich hierarchitectures for high performance Li-ion battery cathodes. J Mater Chem A. 2016;4:18416–25.10.1039/C6TA05676HSuche in Google Scholar
[179] Fang L, Zhang H, Zhang Y, Liu L, Wang Y. Design and synthesis of two-dimensional porous Fe-doped LiCoPO4 nano-plates as improved cathode for lithium ion batteries. J Power Sources. 2016;312:101–8.10.1016/j.jpowsour.2016.02.035Suche in Google Scholar
[180] Sun W, Cao F, Liu Y, Zhao X, Liu X, Yuan J. Nanoporous LiMn2O4 nanosheets with exposed {111} facets as cathodes for highly reversible lithium-ion batteries. J Mater Chem. 2012;22:20952–57.10.1039/c2jm32658bSuche in Google Scholar
[181] Chen KS, Balla I, Luu NS, Hersam MC. Emerging opportunities for two-dimensional materials in lithium-ion batteries. ACS Energy Lett. 2017;2:2026–34.10.1021/acsenergylett.7b00476Suche in Google Scholar
[182] Zhao Y, Peng L, Liu B, Yu G. Single-crystalline LiFePO4 nanosheets for high-rate li-ion batteries. Nano Lett. 2014;14:2849–53.10.1021/nl5008568Suche in Google Scholar PubMed
[183] Zhao Q, Guo Z, Wu Y, Wang L, Han Z, Ma X, et al. Hierarchical flower-like spinel manganese-based oxide nanosheets for high-performance lithium ion battery. Sci China Mater. 2019;62:1385–92.10.1007/s40843-019-9442-xSuche in Google Scholar
[184] Chen J, Zhao N, Zhao J, Li J, Guo FF, Li GD. Facile synthesis of LiMn2O4 microsheets with porous micro-nanostructure as high-rate cathode materials for Li-ion batteries. J Solid State Electrochem. 2018;22:331–8.10.1007/s10008-017-3755-4Suche in Google Scholar
[185] Qiu S, Fang T, Zhu Y, Hua J, Chu H, Zou Y, et al. Li1.2Mn0.6Ni0.2O2 with 3D porous rod-like hierarchical micro/nanostructure for high-performance cathode material. J Alloy Compd. 2019;790:863–70.10.1016/j.jallcom.2019.03.282Suche in Google Scholar
[186] Li Y, Bai Y, Wu C, Qian J, Chen G, Liu L, et al. Three-dimensional fusiform hierarchical micro/nano Li1.2Ni0.2Mn0.6O2 with a preferred orientation (110) plane as a high energy cathode material for lithium-ion batteries. J Mater Chem A. 2016;4:5942–51.10.1039/C6TA00460ASuche in Google Scholar
[187] Deng YP, Yin ZW, Wu ZG, Zhang SJ, Fu F, Zhang T, et al. Layered/spinel heterostructured and hierarchical micro/nanostructured Li-rich cathode materials with enhanced electrochemical properties for Li-ion batteries. ACS Appl Mater Interfaces. 2017;9:21065–70.10.1021/acsami.7b04726Suche in Google Scholar PubMed
[188] Li J, Luo S, Ding X, Wang Q, He P. Three-dimensional honeycomb-structural LiAlO2-modified LiMnPO4 composite with superior high rate capability as Li-ion battery cathodes. ACS Appl Mater Interfaces. 2018;10:10786–95.10.1021/acsami.7b17597Suche in Google Scholar PubMed
[189] Qiu B, Yin C, Xia Y, Liu Z. Synthesis of three-dimensional nanoporous Li-rich layered cathode oxides for high volumetric and power energy density lithium-ion batteries. ACS Appl Mater Interfaces. 2017;9:3661–6.10.1021/acsami.6b14169Suche in Google Scholar PubMed
[190] Deng YP, Fu F, Wu ZG, Yin ZW, Zhang T, Li JT, et al. Layered/spinel heterostructured Li-rich materials synthesized by a one-step solvothermal strategy with enhanced electrochemical performance for Li-ion batteries. J Mater Chem A. 2015;4:257–63.10.1039/C5TA06945ASuche in Google Scholar
[191] Di Zhang Y, Li Y, Niu XQ, Wang DH, Zhou D, Wang CD, et al. A peanut-like hierarchical micro/nano-Li1.2Mn0.54Ni0.18Co0.08O2 cathode material for lithium-ion batteries with enhanced electrochemical performance. J Mater Chem A. 2015;3:14291–97.10.1039/C5TA02915ESuche in Google Scholar
[192] Li W, Zhang H, Mu Y, Liu L, Wang Y. Unique synthesis of novel octahedral micro/nano-hierarchical LiFePO4cages as an enhanced cathode material for lithium-ion batteries. J Mater Chem A. 2015;3:15661–7.10.1039/C5TA04146ESuche in Google Scholar
[193] Wu Y, Cao C, Zhu Y, Li J, Wang L. Cube-shaped hierarchical LiNi1/3Co1/3Mn1/3O2with enhanced growth of nanocrystal planes as high-performance cathode materials for lithium-ion batteries. J Mater Chem A. 2015;3:15523–8.10.1039/C5TA03225CSuche in Google Scholar
[194] Kim DH, Kim J. Synthesis of LiFePO4 nanoparticles in polyol medium and their electrochemical properties. Electrochem Solid-State Lett. 2006;9:A439.10.1149/1.2218308Suche in Google Scholar
[195] Shin HC, Cho WI, Jang H. Electrochemical properties of carbon-coated LiFePO4 cathode using graphite, carbon black, and acetylene black. Electrochim Acta. 2006;52:1472–6.10.1016/j.electacta.2006.01.078Suche in Google Scholar
[196] Dominko R, Gaberscek M, Drofenik J, Bele M, Pejovnik S, Jamnik J. The role of carbon black distribution in cathodes for Li ion batteries. J Power Sources. 2003;119:770–3.10.1016/S0378-7753(03)00250-7Suche in Google Scholar
[197] Liu H, Cao Q, Fu LJ, Li C, Wu YP, Wu HQ. Doping effects of zinc on LiFePO4 cathode material for lithium ion batteries. Electrochem commun. 2006;8:1553–7.10.1016/j.elecom.2006.07.014Suche in Google Scholar
[198] Zhang WJ. Structure and performance of LiFePO4 cathode materials: a review. J Power Sources. 2011;196:2962–70.10.1016/j.jpowsour.2010.11.113Suche in Google Scholar
[199] Olivetti EA, Ceder G, Gaustad GG, Fu X, Chung D, Elgqvist E, et al. Lithium-ion battery supply chain considerations: analysis of potential bottlenecks in critical metals. Joule. 2016;1:229–43.10.1016/j.joule.2017.08.019Suche in Google Scholar
[200] Zhang L, Fu J, Zhang C. Mechanical composite of LiNi0.8Co0.15Al0.05O2/carbon nanotubes with enhanced electrochemical performance for lithium-ion batteries. Nanoscale Res Lett. 2017;12:376.10.1186/s11671-017-2143-4Suche in Google Scholar PubMed PubMed Central
[201] Mollazadeh M, Habibi B. LiFePO4/carbon/reduced graphene oxide nanostructured composite as a high capacity and fast rate cathode material for rechargeable lithium ion battery. Catal Lett. 2019;149:7–18.10.1007/s10562-018-2589-8Suche in Google Scholar
[202] Wang Z, He W, Zhang X, Yue Y, Liu J, Zhang C, et al. Multilevel structures of Li3V2(PO4)3/phosphorus-doped carbon nanocomposites derived from hybrid V-MOFs for long-life and cheap lithium ion battery cathodes. J Power Sources. 2017;366:9–17.10.1016/j.jpowsour.2017.08.078Suche in Google Scholar
[203] Qiao YQ, Feng WL, Li J, De Shen T. Ultralong cycling stability of carbon-nanotube/LiFePO4 nanocomposites as electrode materials for lithium-ion batteries. Electrochim Acta. 2017;232:323–31.10.1016/j.electacta.2017.02.161Suche in Google Scholar
[204] Meng X, IOP Conference Series: Earth and Environmental Science; 2019. Vol. 300, p. 42039.10.1088/1755-1315/300/4/042039Suche in Google Scholar
[205] Chen Y, Tian Y, Qiu Y, Liu Z, He H, Li B, et al. Synthesis and superior cathode performance of sandwiched LiMn2O4@rGO nanocomposites for lithium-ion batteries. Mater Today Adv. 2019;1:100001.10.1016/j.mtadv.2018.12.001Suche in Google Scholar
[206] Dong J, Lin Y, Zong H, Yang H, Wang L, Dai Z. Three-dimensional architecture reduced graphene oxide–LiFePO4 composite: preparation and excellent microwave absorption performance. Inorg Chem. 2019;58:2031–41.10.1021/acs.inorgchem.8b03043Suche in Google Scholar PubMed
[207] Dong B, Huang X, Yang X, Li G, Xia L, Chen G. Rapid preparation of high electrochemical performance LiFePO4/C composite cathode material with an ultrasonic-intensified micro-impinging jetting reactor. Ultrason Sonochem. 2017;39:816–26.10.1016/j.ultsonch.2017.06.010Suche in Google Scholar PubMed
[208] Khan S, Raj RP, Mohan TVR, Bhuvaneswari S, Varadaraju UV, Selvam P. Electrochemical performance of nano-LiFePO4 embedded ordered mesoporous nitrogenous carbon composite as cathode material for Li-ion battery applications. J Electroanal Chem. 2019;848:113242.10.1016/j.jelechem.2019.113242Suche in Google Scholar
[209] Liu J-YJ-H, Li X-X, Huang J-R, Li J-J, Zhou P, Liu J-YJ-H, et al. Three-dimensional graphene-based nanocomposites for high energy density Li-ion batteries. J Mater Chem A. 2017;5:5977–94.10.1039/C7TA00448FSuche in Google Scholar
[210] Ding YH, Ren HM, Huang YY, Chang FH, He X, Fen JQ, et al. Co-precipitation synthesis and electrochemical properties of graphene supported LiMn1/3Ni1/3Co1/3O2cathode materials for lithium-ion batteries. Nanotechnology. 2013;24:375401.10.1088/0957-4484/24/37/375401Suche in Google Scholar PubMed
[211] Jiang R, Cui C, Ma H. Using graphene nanosheets as a conductive additive to enhance the rate performance of spinel LiMn2O4 cathode material. Phys Chem Chem Phys. 2013;15:6406–15.10.1039/c3cp44516jSuche in Google Scholar PubMed
[212] Xin Y, Qi L, Zhang Y, Zuo Z, Zhou H, Zhang X. Organic solvent-assisted free-standing Li2MnO3LiNi1/3Co1/3Mn1/3O2on 3D graphene as a high energy density cathode. Chem Commun. 2015;51:16381–4.10.1039/C5CC06798GSuche in Google Scholar
[213] Zhou Y, Lu J, Deng C, Zhu H, Chen GZ, Zhang S, et al. Nitrogen-doped graphene guided formation of monodisperse microspheres of LiFePO4 nanoplates as the positive electrode material of lithium-ion batteries. J Mater Chem A. 2016;4:12065–72.10.1039/C6TA03440CSuche in Google Scholar
[214] Tao S, Huang WF, Wu GX, Zhu XB, Wang XB, Zhang M, et al. Performance enhancement of lithium-ion battery with LiFePO4@C/RGO hybrid electrode. Electrochim Acta. 2014;144:406–11.10.1016/j.electacta.2014.08.092Suche in Google Scholar
[215] Dhindsa KS, Mandal BP, Bazzi K, Lin MW, Nazri M, Nazri GA, et al. Enhanced electrochemical performance of graphene modified LiFePO4 cathode material for lithium ion batteries. Solid State Ion. 2013;253:94–100.10.1016/j.ssi.2013.08.030Suche in Google Scholar
[216] Venkateswara Rao C, Leela Mohana Reddy A, Ishikawa Y, Ajayan PM. LiNi1/3Co1/3Mn1/3O2–graphene composite as a promising cathode for lithium-ion batteries. ACS Appl Mater Interfaces. 2011;3:2966–72.10.1021/am200421hSuche in Google Scholar PubMed
[217] Zhao N, Zhi X, Wang L, Liu Y, Liang G. Effect of microstructure on low temperature electrochemical properties of LiFePO4/C cathode material. J Alloy Compd. 2015;645:301–8.10.1016/j.jallcom.2015.05.097Suche in Google Scholar
[218] Eftekhari A. LiFePO4/C nanocomposites for lithium-ion batteries. J Power Sources. 2017;343:395–411.10.1016/j.jpowsour.2017.01.080Suche in Google Scholar
[219] Oh J, Lee J, Hwang T, Kim JM, Dong Seoung K, Piao Y. Dual layer coating strategy utilizing N-doped carbon and reduced graphene oxide for high-performance LiFePO4 cathode material. Electrochim Acta. 2017;231:85–93.10.1016/j.electacta.2017.01.185Suche in Google Scholar
[220] Yi X, Zhang F, Zhang B, Yu WJ, Dai Q, Hu S, et al. (010) Facets dominated LiFePO4 nano-flakes confined in 3D porous graphene network as a high-performance Li-ion battery cathode. Ceram Int. 2018;44:18181–8.10.1016/j.ceramint.2018.07.026Suche in Google Scholar
[221] Zhao W, Xiong L, Xu Y, Li H, Ren Z. High performance Li2MnO3/rGO composite cathode for lithium ion batteries. J Power Sources. 2017;349:11–7.10.1016/j.jpowsour.2017.03.022Suche in Google Scholar
[222] Yu X, Deng J, Yang X, Li J, Huang ZH, Li B, et al. A dual-carbon-anchoring strategy to fabricate flexible LiMn2O4 cathode for advanced lithium-ion batteries with high areal capacity. Nano Energy. 2020;67:104256.10.1016/j.nanoen.2019.104256Suche in Google Scholar
[223] Wu F, Yan Y, Wang R, Cai H, Tong W, Tang H. Synthesis of LiNi1/3Mn1/3Co1/3O2@graphene for lithium-ion batteries via self-assembled polyelectrolyte layers. Ceram Int. 2017;43:7668–73.10.1016/j.ceramint.2017.03.066Suche in Google Scholar
[224] Guo L, Ren L, Wan L, Li J. Heterogeneous carbon/N-doped reduced graphene oxide wrapping LiMn0.8Fe0.2PO4 composite for higher performance of lithium ion batteries. Appl Surf Sci. 2019;476:513–20.10.1016/j.apsusc.2018.12.227Suche in Google Scholar
[225] Kim YS, Lee SH, Son MY, Jung YM, Song HK, Lee H. Succinonitrile as a corrosion inhibitor of copper current collectors for overdischarge protection of lithium ion batteries. ACS Appl Mater Interfaces. 2014;6:2039–43.10.1021/am405092ySuche in Google Scholar PubMed
[226] Xu H, Jin H, Qi Z, Guo Y, Wang J, Zhu Y, et al. Graphene foil as a current collector for NCM material-based cathodes. Nanotechnology. 2020;31:205710.10.1088/1361-6528/ab72baSuche in Google Scholar PubMed
[227] Wu HCHC, Wu HCHC, Lee E, Wu NL. High-temperature carbon-coated aluminum current collector for enhanced power performance of LiFePO4 electrode of Li-ion batteries. Electrochem commun. 2010;12:488–91.10.1016/j.elecom.2010.01.028Suche in Google Scholar
[228] Wang R, Li W, Liu L, Qian Y, Liu F, Chen M, et al. Carbon black/graphene-modified aluminum foil cathode current collectors for lithium ion batteries with enhanced electrochemical performances. J Electroanal Chem. 2019;833:63–9.10.1016/j.jelechem.2018.11.007Suche in Google Scholar
[229] Loghavi MM, Askari M, Babaiee M, Ghasemi A. Improvement of the cyclability of Li-ion battery cathode using a chemical-modified current collector. J Electroanal Chem. 2019;841:107–10.10.1016/j.jelechem.2019.04.037Suche in Google Scholar
[230] Fritsch M, Standke G, Heubner C, Langklotz U, Michaelis A. 3D-cathode design with foam-like aluminum current collector for high energy density lithium-ion batteries. J Energy Storage. 2018;16:125–32.10.1016/j.est.2018.01.006Suche in Google Scholar
[231] Wang M, Tang M, Chen S, Ci H, Wang K, Shi L, et al. Graphene-armored aluminum foil with enhanced anticorrosion performance as current collectors for lithium-ion battery. Adv Mater. 2017;29:1703882.10.1002/adma.201703882Suche in Google Scholar PubMed
[232] Cao WJ, Greenleaf M, Li YX, Adams D, Hagen M, Doung T, et al. The effect of lithium loadings on anode to the voltage drop during charge and discharge of Li-ion capacitors. J Power Sources. 2015;280:600–5.10.1016/j.jpowsour.2015.01.102Suche in Google Scholar
[233] Liu T, Feng Y, Duan Y, Cui S, Lin L, Hu J, et al. Formation of mono/bi-layer iron phosphate and nucleation of LiFePO4 nano-crystals from amorphous 2D sheets in charge/discharge process for cathode in high-performance Li-ion batteries. Nano Energy. 2015;18:187–95.10.1016/j.nanoen.2015.10.016Suche in Google Scholar
[234] Nara H, Mukoyama D, Shimizu R, Momma T, Osaka T. Systematic analysis of interfacial resistance between the cathode layer and the current collector in lithium-ion batteries by electrochemical impedance spectroscopy. J Power Sources. 2019;409:139–47.10.1016/j.jpowsour.2018.09.014Suche in Google Scholar
[235] Kretschmer K, Sun B, Xie X, Chen S, Wang G. A free-standing LiFePO4–carbon paper hybrid cathode for flexible lithium-ion batteries. Green Chem. 2016;18:2691–8.10.1039/C5GC02602DSuche in Google Scholar
[236] Liu YH, Lin HH, Tai YJ. Binder-free carbon fiber-based lithium-nickel-manganese-oxide composite cathode with improved electrochemical stability against high voltage: effects of composition on electrode performance. J Alloy Compd. 2018;735:580–7.10.1016/j.jallcom.2017.11.056Suche in Google Scholar
[237] Wang CC, Lin YC, Chiu KF, Leu HJ, Ko TH. Advanced carbon cloth as current collector for enhanced electrochemical performance of lithium-rich layered oxide cathodes. ChemistrySelect. 2017;2:4419–27.10.1002/slct.201700420Suche in Google Scholar
[238] Leijonmarck S, Cornell A, Lindbergh G, Wågberg L. Flexible nano-paper-based positive electrodes for Li-ion batteries – preparation process and properties. Nano Energy. 2013;2:794–800.10.1016/j.nanoen.2013.02.002Suche in Google Scholar
[239] Wang C, Li D, Too CO, Wallace GG. Electrochemical properties of graphene paper electrodes used in lithium batteries. Chem Mater. 2009;21:2604–6.10.1021/cm900764nSuche in Google Scholar
[240] Lu H, Behm M, Leijonmarck S, Lindbergh G, Cornell A. Flexible paper electrodes for li-ion batteries using low amount of TEMPO-oxidized cellulose nanofibrils as binder. ACS Appl Mater Interfaces. 2016;8:18097–106.10.1021/acsami.6b05016Suche in Google Scholar PubMed
[241] Hu L, Choi JW, Yang Y, Jeong S, La Mantia F, Cui LF, et al. Highly conductive paper for energy-storage devices. Proc Natl Acad Sci U S A. 2009;106:21490–4.10.1073/pnas.0908858106Suche in Google Scholar PubMed PubMed Central
[242] Kang YR, Li YL, Hou F, Wen YY, Su D. Fabrication of electric papers of graphene nanosheet shelled cellulose fibres by dispersion and infiltration as flexible electrodes for energy storage. Nanoscale. 2012;4:3248–53.10.1039/c2nr30318cSuche in Google Scholar PubMed
[243] Wang J, Li L, Wong CL, Madhavi S. Flexible single-walled carbon nanotube/polycellulose papers for lithium-ion batteries. Nanotechnology. 2012;23:495401.10.1088/0957-4484/23/49/495401Suche in Google Scholar PubMed
[244] Pang Z, Sun X, Wu X, Nie Y, Liu Z, Yue L. Fabrication and application of carbon nanotubes/cellulose composite paper. Vacuum. 2015;122:135–42.10.1016/j.vacuum.2015.09.020Suche in Google Scholar
[245] Sharifi F, Ghobadian S, Cavalcanti FR, Hashemi N. Paper-based devices for energy applications. Renew Sustain Energy Rev. 2015;52:1453–72.10.1016/j.rser.2015.08.027Suche in Google Scholar
[246] Ventrapragada LK, Creager SE, Rao AM, Podila R. Carbon nanotubes coated paper as current collectors for secondary Li-ion batteries. Nanotechnol Rev. 2019;8:18–23.10.1515/ntrev-2019-0002Suche in Google Scholar
[247] Li M, Lu J, Chen Z, Amine K. Macroscopic self-assembly: versatile hydrogel ensembles with macroscopic multidimensions (Adv. Mater. 52/2018). Adv Mater. 2018;30:1–24.10.1002/adma.201870400Suche in Google Scholar
[248] Goodenough JB, Park KS. The Li-ion rechargeable battery: a perspective. J Am Chem Soc. 2013;135:1167–76.10.1021/ja3091438Suche in Google Scholar PubMed
[249] Xu K. Electrolytes and interphases in Li-ion batteries and beyond. Chem Rev. 2014;114:11503–618.10.1021/cr500003wSuche in Google Scholar PubMed
[250] Manthiram A. An outlook on lithium ion battery technology. ACS Cent Sci. 2017;3:1063–9.10.1021/acscentsci.7b00288Suche in Google Scholar PubMed PubMed Central
[251] Zhou X, Wang F, Zhu Y, Liu Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J Mater Chem. 2011;21:3353–8.10.1039/c0jm03287eSuche in Google Scholar
[252] Purwanto A, Yudha CS, Ubaidillah U, Widiyandari H, Ogi T, Haerudin H. NCA cathode material: synthesis methods and performance enhancement efforts. Mater Res Express. 2018;5(12):122001.10.1088/2053-1591/aae167Suche in Google Scholar
[253] Fang R, Zhao S, Sun Z, Wang DW, Cheng HM, Li F. More reliable lithium-sulfur batteries: status, solutions and prospects. Adv Mater. 2017;29:1–25.10.1002/adma.201606823Suche in Google Scholar PubMed
[254] Zheng Y, Zheng S, Xue H, Pang H. Metal–organic frameworks for lithium–sulfur batteries. J Mater Chem A. 2019;7:3469–91.10.1039/C8TA11075ASuche in Google Scholar
[255] Fu A, Wang C, Pei F, Cui J, Fang X, Zheng N. Recent advances in hollow porous carbon materials for lithium–sulfur batteries. Small. 2019;15:1804786.10.1002/smll.201804786Suche in Google Scholar PubMed
[256] Yuan H, Liu T, Liu Y, Nai J, Wang Y, Zhang W, et al. A review of biomass materials for advanced lithium–sulfur batteries. Chem Sci. 2019;10:7484–95.10.1039/C9SC02743BSuche in Google Scholar
[257] Zhu J, Zhu P, Yan C, Dong X, Zhang X. Recent progress in polymer materials for advanced lithium-sulfur batteries. Prog Polym Sci. 2019;90:118–63.10.1016/j.progpolymsci.2018.12.002Suche in Google Scholar
[258] Liu M, Deng N, Ju J, Fan L, Wang L, Li Z, et al. A review: electrospun nanofiber materials for lithium-sulfur batteries. Adv Funct Mater. 2019;29:1905467.10.1002/adfm.201905467Suche in Google Scholar
[259] Rana M, Ahad SA, Li M, Luo B, Wang L, Gentle I, et al. Review on areal capacities and long-term cycling performances of lithium sulfur battery at high sulfur loading. Energy Storage Mater. 2019;18:289–310.10.1016/j.ensm.2018.12.024Suche in Google Scholar
[260] He J, Manthiram A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019;20:55–70.10.1016/j.ensm.2019.04.038Suche in Google Scholar
[261] Li T, Bai X, Gulzar U, Bai YJ, Capiglia C, Deng W, et al. A comprehensive understanding of lithium–sulfur battery technology. Adv Funct Mater. 2019;29:1901730.10.1002/adfm.201901730Suche in Google Scholar
[262] Guo J, Liu J. A binder-free electrode architecture design for lithium–sulfur batteries: a review. Nanoscale Adv. 2019;1:2104–22.10.1039/C9NA00040BSuche in Google Scholar
[263] Jana M, Xu R, Cheng XB, Yeon JS, Park JM, Huang JQ, et al. Rational design of two-dimensional nanomaterials for lithium–sulfur batteries. Energy Env Sci. 2020;13:1049–75.10.1039/C9EE02049GSuche in Google Scholar
[264] Mukkabla R, Buchmeiser MR. Cathode materials for lithium–sulfur batteries based on sulfur covalently bound to a polymeric backbone. J Mater Chem A. 2020;8:5379–94.10.1039/C9TA12619HSuche in Google Scholar
[265] Huang L, Li J, Liu B, Li Y, Shen S, Deng S, et al. Electrode design for lithium–sulfur batteries: problems and solutions. Adv Funct Mater. 2020;30:1910375.10.1002/adfm.201910375Suche in Google Scholar
[266] Zhang M, Chen W, Xue L, Jiao Y, Lei T, Chu J, et al. Adsorption-catalysis design in the lithium-sulfur battery. Adv Energy Mater. 2020;10:1903008.10.1002/aenm.201903008Suche in Google Scholar
[267] Fan L, Li M, Li X, Xiao W, Chen Z, Lu J. Interlayer material selection for lithium-sulfur batteries. Joule. 2019;3:361–86.10.1016/j.joule.2019.01.003Suche in Google Scholar
[268] Chen X, Hou T, Persson KA, Zhang Q. Combining theory and experiment in lithium–sulfur batteries: current progress and future perspectives. Mater Today. 2019;22:142–58.10.1016/j.mattod.2018.04.007Suche in Google Scholar
[269] Ren W, Ma W, Zhang S, Tang B. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019;23:707–32.10.1016/j.ensm.2019.02.022Suche in Google Scholar
[270] Lim WG, Kim S, Jo C, Lee J. A comprehensive review of materials with catalytic effects in Li–S batteries: enhanced redox kinetics. Angew Chem - Int Ed. 2019;58:18746–57.10.1002/anie.201902413Suche in Google Scholar PubMed
[271] Knoop JE, Ahn S. Recent advances in nanomaterials for high-performance Li–S batteries. J Energy Chem. 2020;47:86–106.10.1016/j.jechem.2019.11.018Suche in Google Scholar
[272] Qi Q, Lv X, Lv W, Yang QH. Multifunctional binder designs for lithium-sulfur batteries. J Energy Chem. 2019;39:88–100.10.1016/j.jechem.2019.02.001Suche in Google Scholar
[273] Li B, Xu H, Ma Y, Yang S. Harnessing the unique properties of 2D materials for advanced lithium–sulfur batteries. Nanoscale Horiz. 2019;4:77–98.10.1039/C8NH00170GSuche in Google Scholar
[274] Zhang L, Wang Y, Niu Z, Chen J. Advanced nanostructured carbon-based materials for rechargeable lithium-sulfur batteries. Carbon N Y. 2019;141:400–16.10.1016/j.carbon.2018.09.067Suche in Google Scholar
[275] Zhong Y, Xia X, Deng S, Zhan J, Fang R, Xia Y, et al. Popcorn inspired porous macrocellular carbon: rapid puffing fabrication from rice and its applications in lithium-sulfur batteries. Adv Energy Mater. 2018;8:1701110.10.1002/aenm.201701110Suche in Google Scholar
[276] Jian Z, Li H, Cao R, Zhou H, Xu H, Zhao G, et al. Polydopamine-coated hierarchical tower-shaped carbon for high-performance lithium-sulfur batteries. Electrochim Acta. 2019;319:359–65.10.1016/j.electacta.2019.06.145Suche in Google Scholar
[277] Li GC, Hu JJ, Li GR, Ye SH, Gao XP. Sulfur/activated-conductive carbon black composites as cathode materials for lithium/sulfur battery. J Power Sources. 2013;240:598–605.10.1016/j.jpowsour.2013.02.095Suche in Google Scholar
[278] Zhong Y, Chao D, Deng S, Zhan J, Fang RY, Xia Y, et al. Confining sulfur in integrated composite scaffold with highly porous carbon fibers/vanadium nitride arrays for high-performance lithium-sulfur batteries. Adv Funct Mater. 2018;28:1706391.10.1002/adfm.201706391Suche in Google Scholar
[279] Ji X, Lee KT, Nazar LF. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat Mater. 2009;8:500–6.10.1038/nmat2460Suche in Google Scholar PubMed
[280] Perez Beltran S, Balbuena PB. Formation of multilayer graphene domains with strong sulfur–carbon interaction and enhanced sulfur reduction zones for lithium–sulfur battery cathodes. ChemSusChem. 2018;11:1970–80.10.1002/cssc.201702446Suche in Google Scholar PubMed
[281] Yang W, Yang W, Song A, Sun G, Shao G. 3D interconnected porous carbon nanosheets/carbon nanotubes as a polysulfide reservoir for high performance lithium–sulfur batteries. Nanoscale. 2018;10:816–24.10.1039/C7NR06805KSuche in Google Scholar
[282] Wang H, Yang Y, Liang Y, Robinson JT, Li Y, Jackson A, et al. Graphene-wrapped sulfur particles as a rechargeable lithium–sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011;11:2644–7.10.1021/nl200658aSuche in Google Scholar PubMed
[283] Gueon D, Hwang JT, Yang SB, Cho E, Sohn K, Yang DK, et al. Spherical macroporous carbon nanotube particles with ultrahigh sulfur loading for lithium–sulfur battery cathodes. ACS Nano. 2018;12:226–33.10.1021/acsnano.7b05869Suche in Google Scholar PubMed
[284] Abdul Razzaq A, Yao Y, Shah R, Qi P, Miao L, Chen M, et al. High-performance lithium sulfur batteries enabled by a synergy between sulfur and carbon nanotubes. Energy Storage Mater. 2019;16:194–202.10.1016/j.ensm.2018.05.006Suche in Google Scholar
[285] Moon S, Jung YH, Jung WK, Jung DS, Choi JW, Kim DK. Encapsulated monoclinic sulfur for stable cycling of Li-S rechargeable batteries. Adv Mater. 2013;25:6547–53.10.1002/adma.201303166Suche in Google Scholar PubMed
[286] Zheng G, Lee SW, Liang Z, Lee HW, Yan K, Yao H, et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat Nanotechnol. 2014;9:618–23.10.1038/nnano.2014.152Suche in Google Scholar PubMed
[287] Ren J, Zhou Y, Wu H, Xie F, Xu C, Lin D. Sulfur-encapsulated in heteroatom-doped hierarchical porous carbon derived from goat hair for high performance lithium–sulfur batteries. J Energy Chem. 2019;30:121–31.10.1016/j.jechem.2018.01.015Suche in Google Scholar
[288] Zhou S, Hu J, Liu S, Lin JX, Cheng J, Mei T, et al. Biomimetic micro cell cathode for high performance lithium–sulfur batteries. Nano Energy. 2020;72:104680.10.1016/j.nanoen.2020.104680Suche in Google Scholar
[289] Fawaz W, Mosavati N, Abdelhamid E, Simon Ng KY. Synthesis of activated carbons derived from avocado shells as cathode materials for lithium–sulfur batteries. SN Appl Sci. 2019;1:289.10.1007/s42452-019-0300-3Suche in Google Scholar
[290] Qin X, Wu J, Xu ZL, Chong WG, Huang JQ, Liang G, et al. Electrosprayed multiscale porous carbon microspheres as sulfur hosts for long-life lithium-sulfur batteries. Carbon N Y. 2019;141:16–24.10.1016/j.carbon.2018.09.048Suche in Google Scholar
[291] Diéz N, Ferrero GA, Sevilla M, Fuertes AB. A simple and general approach for in situ synthesis of sulfur–porous carbon composites for lithium–sulfur batteries. Sustain Energy Fuels. 2019;3:3498–509.10.1039/C9SE00722ASuche in Google Scholar
[292] Wang Y, Zhang R, Chao Pang Y, Chen X, Lang J, Xu J, et al. Carbon@titanium nitride dual shell nanospheres as multi-functional hosts for lithium sulfur batteries. Energy Storage Mater. 2019;16:228–35.10.1016/j.ensm.2018.05.019Suche in Google Scholar
[293] Hu L, Dai C, Liu H, Li Y, Shen B, Chen Y, et al. Double-shelled NiO-NiCo2O4 heterostructure@carbon hollow nanocages as an efficient sulfur host for advanced lithium-sulfur batteries. Adv Energy Mater. 2018;8:1800709.10.1002/aenm.201800709Suche in Google Scholar
[294] Li G, Lei W, Luo D, Deng YP, Wang D, Chen Z. 3D porous carbon sheets with multidirectional ion pathways for fast and durable lithium-sulfur batteries. Adv Energy Mater. 2018;8:1702381.10.1002/aenm.201702381Suche in Google Scholar
[295] Chen S, Huang X, Sun B, Zhang J, Liu H, Wang G. Multi-shelled hollow carbon nanospheres for lithium–sulfur batteries with superior performances. J Mater Chem A. 2014;2:16199–207.10.1039/C4TA03877KSuche in Google Scholar
[296] Sun Q, He B, Zhang XQ, Lu AH. Engineering of hollow core–shell interlinked carbon spheres for highly stable lithium–sulfur batteries. ACS Nano. 2015;9:8504–13.10.1021/acsnano.5b03488Suche in Google Scholar PubMed
[297] Yuan H, Zhang W, Guo Wang J, Zhou G, Zhuang Z, Luo J, et al. Facilitation of sulfur evolution reaction by pyridinic nitrogen doped carbon nanoflakes for highly-stable lithium-sulfur batteries. Energy Storage Mater. 2018;10:1–9.10.1016/j.ensm.2017.07.015Suche in Google Scholar
[298] Kim J, Kang Y, Song SW, Suk J. Freestanding sulfur-graphene oxide/carbon composite paper as a stable cathode for high performance lithium-sulfur batteries. Electrochim Acta. 2019;299:27–33.10.1016/j.electacta.2018.12.165Suche in Google Scholar
[299] Li W, Liang Z, Lu Z, Yao H, Seh ZW, Yan K, et al. A sulfur cathode with pomegranate-like cluster structure. Adv Energy Mater. 2015;5:1500211.10.1002/aenm.201500211Suche in Google Scholar
[300] Li Z, Zhang JT, Chen YM, Li J, Lou XW. Correlated compositional and mineralogical investigations at the Chang′e-3 landing site. Nat Commun. 2015;6:1–8.10.1038/ncomms9880Suche in Google Scholar PubMed PubMed Central
[301] Chung SH, Chang CH, Manthiram A. A carbon-cotton cathode with ultrahigh-loading capability for statically and dynamically stable lithium–sulfur batteries. ACS Nano. 2016;10:10462–70.10.1021/acsnano.6b06369Suche in Google Scholar
[302] Lin C, Qu L, Li J, Cai Z, Liu H, He P, et al. Porous nitrogen-doped carbon/MnO coaxial nanotubes as an efficient sulfur host for lithium sulfur batteries. Nano Res. 2019;12:205–10.10.1007/s12274-018-2203-9Suche in Google Scholar
[303] Kensy C, Härtel P, Maschita J, Dörfler S, Schumm B, Abendroth T, et al. Scalable production of nitrogen-doped carbons for multilayer lithium-sulfur battery cells. Carbon N Y. 2020;161:190–7.10.1016/j.carbon.2020.01.037Suche in Google Scholar
[304] Zhou Y, Shu H, Zhou Y, Sun T, Han M, Chen Y, et al. Flower-like Bi4Ti3O12/Carbon nanotubes as reservoir and promoter of polysulfide for lithium sulfur battery. J Power Sources. 2020;453:227896.10.1016/j.jpowsour.2020.227896Suche in Google Scholar
[305] Abualela S, Lv X, Hu Y, Abd-Alla MD. NiO nanosheets grown on carbon cloth as mesoporous cathode for High-performance lithium-sulfur battery. Mater Lett. 2020;268:127622.10.1016/j.matlet.2020.127622Suche in Google Scholar
[306] Radhika G, Subadevi R, Sivakumar M. Sulfur nested with mixture of MnO2/AB composite as efficient host for high-performance Li–S batteries. J Chem Sci. 2020;132:53.10.1007/s12039-020-1755-xSuche in Google Scholar
[307] Liu S, Li J, Yan X, Su Q, Lu Y, Qiu J, et al. Superhierarchical cobalt-embedded nitrogen-doped porous carbon nanosheets as two-in-one hosts for high-performance lithium-sulfur batteries. Adv Mater. 2018;30:1706895.10.1002/adma.201706895Suche in Google Scholar
[308] Chen K, Sun Z, Fang R, Shi Y, Cheng HM, Li F. Metal-organic frameworks (MOFs)-derived nitrogen-doped porous carbon anchored on graphene with multifunctional effects for lithium-sulfur batteries. Adv Funct Mater. 2018;28:1707592.10.1002/adfm.201707592Suche in Google Scholar
[309] Zhang SS. Understanding of sulfurized polyacrylonitrile for superior performance lithium/sulfur battery. Energies. 2014;7:4588–600.10.3390/en7074588Suche in Google Scholar
[310] Wang J, Yang J, Xie J, Xu N. A novel conductive polymer-sulfur composite cathode material for rechargeable lithium batteries. Adv Mater. 2002;14:963–5.10.1002/1521-4095(20020705)14:13/14<963::AID-ADMA963>3.0.CO;2-PSuche in Google Scholar
[311] He X, Shi Q, Zhou X, Wan C, Jiang C. In situ composite of nano SiO2–P(VDF-HFP) porous polymer electrolytes for Li-ion batteries. Electrochim Acta. 2005;51:1069–75.10.1016/j.electacta.2005.05.048Suche in Google Scholar
[312] He X, Ren J, Wang L, Pu W, Wan C, Jiang C. Electrochemical characteristics of sulfur composite cathode for reversible lithium storage. Ion (Kiel). 2009;15:477–81.10.1007/s11581-008-0267-3Suche in Google Scholar
[313] Lai C, Gao XP, Zhang B, Yan TY, Zhou Z. Synthesis and electrochemical performance of sulfur/highly porous carbon composites. J Phys Chem C. 2009;113:4712–6.10.1021/jp809473eSuche in Google Scholar
[314] Guo J, Xu Y, Wang C. Sulfur-impregnated disordered carbon nanotubes cathode for lithium–sulfur batteries. Nano Lett. 2011;11:4288–94.10.1021/nl202297pSuche in Google Scholar PubMed
[315] Liu Y, Haridas AK, Cho KK, Lee Y, Ahn JH. Highly ordered mesoporous sulfurized polyacrylonitrile cathode material for high-rate lithium sulfur batteries. J Phys Chem C. 2017;121:26172–9.10.1021/acs.jpcc.7b06625Suche in Google Scholar
[316] Wang W, Cao Z, Elia GA, Wu Y, Wahyudi W, Abou-Hamad E, et al. Recognizing the mechanism of sulfurized polyacrylonitrile cathode materials for Li–S batteries and beyond in Al–S batteries. ACS Energy Lett. 2018;3:2899–907.10.1021/acsenergylett.8b01945Suche in Google Scholar
[317] Wang X, Qian Y, Wang L, Yang H, Li H, Zhao Y, et al. Sulfurized polyacrylonitrile cathodes with high compatibility in both ether and carbonate electrolytes for ultrastable lithium–sulfur batteries. Adv Funct Mater. 2019;29:1902929.10.1002/adfm.201902929Suche in Google Scholar
[318] Xiang J, Guo Z, Yi Z, Zhang Y, Yuan L, Cheng Z, et al. Facile synthesis of sulfurized polyacrylonitrile composite as cathode for high-rate lithium-sulfur batteries. J Energy Chem. 2020;49:161–5.10.1016/j.jechem.2020.01.037Suche in Google Scholar
[319] Bong Cho G, Seung Jeong J, Rang Chae M, Pil Noh J, Koo Cho K, Kwang Kim J, et al. Electrochemical properties of sulfurized poly-acrylonitrile (SPAN) cathode containing carbon fiber current collectors. Surf Coat Technol. 2017;326:443–9.10.1016/j.surfcoat.2016.11.098Suche in Google Scholar
[320] Liu Y, Haridas AK, Lee Y, Cho KK, Ahn JH. Freestanding porous sulfurized polyacrylonitrile fiber as a cathode material for advanced lithium sulfur batteries. Appl Surf Sci. 2019;472:135–42.10.1016/j.apsusc.2018.03.062Suche in Google Scholar
[321] Choudhury S, Fischer D, Formanek P, Simon F, Stamm M, Ionov L. Porous carbon prepared from polyacrylonitrile for lithium-sulfur battery cathodes using phase inversion technique. Polym (Guildf). 2018;151:171–8.10.1016/j.polymer.2018.07.026Suche in Google Scholar
[322] Kuo CJ, Weret MA, Hung HY, Tsai MC, Huang CJ, Su WN, et al. Sulfurized−poly(acrylonitrile) wrapped carbon sulfur composite cathode material for high performance rechargeable lithium sulfur batteries. J Power Sources. 2019;412:670–6.10.1016/j.jpowsour.2018.11.094Suche in Google Scholar
[323] Fan L, Chen S, Zhu J, Ma R, Li S, Podila R, et al. Simultaneous suppression of the dendrite formation and shuttle effect in a lithium–sulfur battery by bilateral solid electrolyte interface. Adv Sci. 2018;5:1700934.10.1002/advs.201700934Suche in Google Scholar PubMed PubMed Central
[324] Liu Y, Yang D, Yan W, Huang Q, Zhu Y, Fu L, et al. Synergy of sulfur/polyacrylonitrile composite and gel polymer electrolyte promises heat-resistant lithium-sulfur batteries. iScience. 2019;19:316–25.10.1016/j.isci.2019.07.027Suche in Google Scholar PubMed PubMed Central
[325] Chen W-J, Li B-Q, Zhao C, Zhao M, Yuan T-Q, Sun R-C, et al. Electrolyte regulation towards stable lithium-metal anodes in lithium–sulfur batteries with sulfurized polyacrylonitrile cathodes. Angew Chem. 2020;132:10821–34.10.1002/ange.201912701Suche in Google Scholar
[326] Peng HJ, Huang JQ, Cheng XB, Zhang Q. Lithium-sulfur batteries: review on high-loading and high-energy lithium-sulfur batteries (Adv. Energy Mater. 24/2017). Adv Energy Mater. 2017;7:1–54.10.1002/aenm.201770141Suche in Google Scholar
[327] Zhu K, Wang C, Chi Z, Ke F, Yang Y, Wang A, et al. How far away are lithium-sulfur batteries from commercialization. Front Energy Res. 2019;7:123.10.3389/fenrg.2019.00123Suche in Google Scholar
[328] Bhargav A, He J, Gupta A, Manthiram A. Lithium-sulfur batteries: attaining the critical metrics. Joule. 2020;4:285–91.10.1016/j.joule.2020.01.001Suche in Google Scholar
[329] Zhao R, Liu J, Gu J. The effects of electrode thickness on the electrochemical and thermal characteristics of lithium ion battery. Appl Energy. 2015;139:220–9.10.1016/j.apenergy.2014.11.051Suche in Google Scholar
[330] Du Z, Wood DL, Daniel C, Kalnaus S, Li J. Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries. J Appl Electrochem. 2017;47:405–15.10.1007/s10800-017-1047-4Suche in Google Scholar
[331] Li M, Carter R, Douglas A, Oakes L, Pint CL. Sulfur vapor-infiltrated 3D carbon nanotube foam for binder-free high areal capacity lithium–sulfur battery composite cathodes. ACS Nano. 2017;11:4877–84.10.1021/acsnano.7b01437Suche in Google Scholar PubMed
[332] Zhang H, Zhao W, Wu Y, Wang Y, Zou M, Cao A. Dense monolithic MOF and carbon nanotube hybrid with enhanced volumetric and areal capacities for lithium–sulfur battery. J Mater Chem A. 2019;7:9195–201.10.1039/C9TA00485HSuche in Google Scholar
[333] Rana M, Luo B, Kaiser MR, Gentle I, Knibbe R. The role of functional materials to produce high areal capacity lithium sulfur battery. J Energy Chem. 2020;42:195–209.10.1016/j.jechem.2019.06.015Suche in Google Scholar
[334] Jia L, Wang J, Chen Z, Su Y, Zhao W, Wang D, et al. High areal capacity flexible sulfur cathode based on multi-functionalized super-aligned carbon nanotubes. Nano Res. 2019;12:1105–13.10.1007/s12274-019-2356-1Suche in Google Scholar
[335] Liu Y, Yan Y, Li K, Yu Y, Wang Q, Liu M. A high-areal-capacity lithium–sulfur cathode achieved by a boron-doped carbon–sulfur aerogel with consecutive core–shell structures. Chem Commun. 2019;55:1084–7.10.1039/C8CC07594HSuche in Google Scholar
[336] Cho CS, Chang JY, Li CC. Highly symmetric gigaporous carbon microsphere as conductive host for sulfur to achieve high areal capacity for lithium–sulfur batteries. J Power Sources. 2020;451:227818.10.1016/j.jpowsour.2020.227818Suche in Google Scholar
[337] Abdul Razzaq A, Yuan X, Chen Y, Hu J, Mu Q, Ma Y, et al. Anchoring MOF-derived CoS2 on sulfurized polyacrylonitrile nanofibers for high areal capacity lithium–sulfur batteries. J Mater Chem A. 2020;8:1298–306.10.1039/C9TA11390HSuche in Google Scholar
[338] Zhou G, Zhao Y, Manthiram A. Dual-confined flexible sulfur cathodes encapsulated in nitrogen-doped double-shelled hollow carbon spheres and wrapped with graphene for Li–S batteries. Adv Energy Mater. 2015;5(9):1402263.10.1002/aenm.201402263Suche in Google Scholar
[339] Pei F, Lin L, Ou D, Zheng Z, Mo S, Fang X, et al. In situ click chemistry generation of cyclooxygenase-2 inhibitors. Nat Commun. 2017;8:1–10.10.1038/s41467-016-0009-6Suche in Google Scholar PubMed PubMed Central
[340] Carter R, Davis B, Oakes L, Maschmann MR, Pint CL. A high areal capacity lithium–sulfur battery cathode prepared by site-selective vapor infiltration of hierarchical carbon nanotube arrays. Nanoscale. 2017;9:15018–26.10.1039/C7NR02368ESuche in Google Scholar PubMed
[341] Liu F, Chiluwal S, Childress AS, Etteh C, Miller K, Washington M, et al. Conjugated polymers for photon-to-electron and photon-to-fuel conversions. ACS Appl Nano Mater. 2021;2021:60–92.10.1021/acsapm.0c00791Suche in Google Scholar
[342] Li X, Zhang Y, Wang S, Liu Y, Ding Y, He G, et al. Scalable high-areal-capacity Li–S batteries enabled by sandwich-structured hierarchically porous membranes with intrinsic polysulfide adsorption. Nano Lett. 2020;20:6922–9.10.1021/acs.nanolett.0c03088Suche in Google Scholar PubMed
[343] Ghosh A, Kumar A, Roy A, Nguyen C, Ahuja A, Adil M, et al. Ultrathin lithium aluminate nanoflake-inlaid sulfur as a cathode material for lithium–sulfur batteries with high areal capacity. ACS Appl Energy Mater. 2020;3:5637–45.10.1021/acsaem.0c00597Suche in Google Scholar
[344] Bruce PG, Freunberger SA, Hardwick LJ, Tarascon JM. Li–O2 and Li–S batteries with high energy storage. Nat Mater. 2012;11:19–29.10.1038/nmat3191Suche in Google Scholar PubMed
[345] Guo Y, Li H, Zhai T. Reviving lithium-metal anodes for next-generation high-energy batteries. Adv Mater. 2017;29:1700007.10.1002/adma.201700007Suche in Google Scholar PubMed
[346] Whittingham MS. Electrical energy storage and intercalation chemistry. Science. 1976;192:1126–7.10.1126/science.192.4244.1126Suche in Google Scholar PubMed
[347] Tikekar MD, Choudhury S, Tu Z, Archer LA. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat Energy. 2016;1:1–7.10.1038/nenergy.2016.114Suche in Google Scholar
[348] Liu J, Bao Z, Cui Y, Dufek EJ, Goodenough JB, Khalifah P, et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat Energy. 2019;4:180–6.10.1038/s41560-019-0338-xSuche in Google Scholar
[349] Cheng XB, Zhang R, Zhao CZ, Zhang Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev. 2017;117:10403–73.10.1021/acs.chemrev.7b00115Suche in Google Scholar PubMed
[350] Xu W, Wang J, Ding F, Chen X, Nasybulin E, Zhang Y, et al. Lithium metal anodes for rechargeable batteries. Energy Env Sci. 2014;7:513–37.10.1039/C3EE40795KSuche in Google Scholar
[351] Kim J, Lee J, Yun J, Choi SH, Han SA, Moon J, et al. Functionality of dual-phase lithium storage in a porous carbon host for lithium-metal anode. Adv Funct Mater. 2020;30:1910538.10.1002/adfm.201910538Suche in Google Scholar
[352] Cheng XB, Hou TZ, Zhang R, Peng HJ, Zhao CZ, Huang JQ, et al. Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv Mater. 2016;28:2888–95.10.1002/adma.201506124Suche in Google Scholar PubMed
[353] Liu X, Liu J, Qian T, Chen H, Yan C. Novel organophosphate-derived dual-layered interface enabling air-stable and dendrite-free lithium metal anode. Adv Mater. 2020;32:1902724.10.1002/adma.201902724Suche in Google Scholar PubMed
[354] Wang L, Fu S, Zhao T, Qian J, Chen N, Li L, et al. In situ formation of a LiF and Li–Al alloy anode protected layer on a Li metal anode with enhanced cycle life. J Mater Chem A. 2020;8:1247–53.10.1039/C9TA10965JSuche in Google Scholar
[355] McGrogan FP, Swamy T, Bishop SR, Eggleton E, Porz L, Chen X, et al. Compliant yet brittle mechanical behavior of Li2S–P2S5 lithium-ion-conducting solid electrolyte. Adv Energy Mater. 2017;7:1602011.10.1002/aenm.201602011Suche in Google Scholar
[356] Zheng J, Engelhard MH, Mei D, Jiao S, Polzin BJ, Zhang JG, et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat Energy. 2017;2(3):1–8.10.1038/nenergy.2017.12Suche in Google Scholar
[357] Li NW, Yin YX, Yang CP, Guo YG. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv Mater. 2016;28:1853–8.10.1002/adma.201504526Suche in Google Scholar PubMed
[358] Zhang XQ, Cheng XB, Zhang Q. Advances in interfaces between li metal anode and electrolyte. Adv Mater Interfaces. 2018;5:1701097.10.1002/admi.201701097Suche in Google Scholar
[359] Wu S, Yi J, Zhu K, Bai S, Liu Y, Qiao Y, et al. A super-hydrophobic quasi-solid electrolyte for Li-O2 battery with improved safety and cycle life in humid atmosphere. Adv Energy Mater. 2017;7:1601759.10.1002/aenm.201601759Suche in Google Scholar
[360] Huang Z, Ren J, Zhang W, Xie M, Li Y, Sun D, et al. Protecting the Li-metal anode in a Li-O2 battery by using boric acid as an SEI-forming additive. Adv Mater. 2018;30:1803270.10.1002/adma.201803270Suche in Google Scholar PubMed
[361] Lang J, Long Y, Qu J, Luo X, Wei H, Huang K, et al. One-pot solution coating of high quality LiF layer to stabilize Li metal anode. Energy Storage Mater. 2019;16:85–90.10.1016/j.ensm.2018.04.024Suche in Google Scholar
[362] Luo W, Gong Y, Zhu Y, Li Y, Yao Y, Zhang Y, et al. Reducing interfacial resistance between garnet-structured solid-state electrolyte and Li-metal anode by a germanium layer. Adv Mater. 2017;29:1606042.10.1002/adma.201606042Suche in Google Scholar PubMed
[363] Zhou B, Guo L, Zhang Y, Wang J, Ma L, Zhang WH, et al. A high-performance Li-O2 battery with a strongly solvating hexamethylphosphoramide electrolyte and a LiPON-protected lithium anode. Adv Mater. 2017;29:1701568.10.1002/adma.201701568Suche in Google Scholar PubMed
[364] Ryou MH, Lee DJ, Lee JN, Lee YM, Park JK, Choi JW. Excellent cycle life of lithium-metal anodes in lithium-ion batteries with mussel-inspired polydopamine-coated separators. Adv Energy Mater. 2012;2:645–50.10.1002/aenm.201100687Suche in Google Scholar
[365] Luo W, Zhou L, Fu K, Yang Z, Wan J, Manno M, et al. A thermally conductive separator for stable Li metal anodes. Nano Lett. 2015;15:6149–54.10.1021/acs.nanolett.5b02432Suche in Google Scholar PubMed
[366] Zhang R, Chen X, Shen X, Zhang XQ, Chen XR, Cheng XB, et al. Coralloid carbon fiber-based composite lithium anode for robust lithium metal batteries. Joule. 2018;2:764–77.10.1016/j.joule.2018.02.001Suche in Google Scholar
[367] Luo Z, Liu C, Tian Y, Zhang Y, Jiang Y, Hu J, et al. Dendrite-free lithium metal anode with lithiophilic interphase from hierarchical frameworks by tuned nucleation. Energy Storage Mater. 2020;27:124–32.10.1016/j.ensm.2020.01.025Suche in Google Scholar
[368] Yang T, Sun Y, Qian T, Liu J, Liu X, Rosei F, et al. Lithium dendrite inhibition via 3D porous lithium metal anode accompanied by inherent SEI layer. Energy Storage Mater. 2020;26:385–90.10.1016/j.ensm.2019.11.009Suche in Google Scholar
[369] Li J, Zou P, Chiang SW, Yao W, Wang Y, Liu P, et al. A conductive-dielectric gradient framework for stable lithium metal anode. Energy Storage Mater. 2020;24:700–6.10.1016/j.ensm.2019.06.019Suche in Google Scholar
[370] Yuan Y, Wu F, Bai Y, Li Y, Chen G, Wang Z, et al. Regulating Li deposition by constructing LiF-rich host for dendrite-free lithium metal anode. Energy Storage Mater. 2019;16:411–8.10.1016/j.ensm.2018.06.022Suche in Google Scholar
[371] Zhang Y, Liu B, Hitz E, Luo W, Yao Y, Li Y, et al. A carbon-based 3D current collector with surface protection for Li metal anode. Nano Res. 2017;10:1356–65.10.1007/s12274-017-1461-2Suche in Google Scholar
[372] Zhang Y, Luo W, Wang C, Li Y, Chen C, Song J, et al. High-capacity, low-tortuosity, and channel-guided lithium metal anode. Proc Natl Acad Sci U S A. 2017;114:3584–9.10.1073/pnas.1618871114Suche in Google Scholar PubMed PubMed Central
[373] Shi P, Li T, Zhang R, Shen X, Cheng XB, Xu R, et al. Lithiophilic LiC6 layers on carbon hosts enabling stable Li metal anode in working batteries. Adv Mater. 2019;31:1807131.10.1002/adma.201807131Suche in Google Scholar PubMed
[374] Tsai CL, Roddatis V, Chandran CV, Ma Q, Uhlenbruck S, Bram M, et al. Li7La3Zr2O12 interface modification for Li dendrite prevention. ACS Appl Mater Interfaces. 2016;8:10617–26.10.1021/acsami.6b00831Suche in Google Scholar PubMed
[375] Feng Y, Zhang C, Jiao X, Zhou Z, Song J. Highly stable lithium metal anode with near-zero volume change enabled by capped 3D lithophilic framework. Energy Storage Mater. 2020;25:172–9.10.1016/j.ensm.2019.10.017Suche in Google Scholar
[376] Ye H, Xin S, Yin YX, Li JY, Guo YG, Wan LJ. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons. J Am Chem Soc. 2017;139:5916–22.10.1021/jacs.7b01763Suche in Google Scholar PubMed
[377] Liu L, Yin YX, Li JY, Wang SH, Guo YG, Wan LJ. Uniform lithium nucleation/growth induced by lightweight nitrogen-doped graphitic carbon foams for high-performance lithium metal anodes. Adv Mater. 2018;30:1706216.10.1002/adma.201706216Suche in Google Scholar PubMed
[378] Jin C, Sheng O, Luo J, Yuan H, Fang C, Zhang W, et al. 3D lithium metal embedded within lithiophilic porous matrix for stable lithium metal batteries. Nano Energy. 2017;37:177–86.10.1016/j.nanoen.2017.05.015Suche in Google Scholar
[379] Sen Chi S, Liu Y, Song WL, Fan LZ, Zhang Q. Prestoring lithium into stable 3D nickel foam host as dendrite-free lithium metal anode. Adv Funct Mater. 2017;27:1700348.10.1002/adfm.201700348Suche in Google Scholar
[380] Jung JW, Cho SH, Nam JS, Kim ID. Current and future cathode materials for non-aqueous Li-air (O2) battery technology – A focused review. Energy Storage Mater. 2020;24:512–28.10.1016/j.ensm.2019.07.006Suche in Google Scholar
[381] Rahman MA, Wang X, Wen C. A review of high energy density lithium–air battery technology. J Appl Electrochem. 2014;44:5–22.10.1007/s10800-013-0620-8Suche in Google Scholar
[382] Imanishi N, Yamamoto O. Perspectives and challenges of rechargeable lithium–air batteries. Mater Today Adv. 2019;4:100031.10.1016/j.mtadv.2019.100031Suche in Google Scholar
[383] Lai J, Xing Y, Chen N, Li L, Wu F, Chen R. Electrolytes for rechargeable lithium–air batteries. Angew Chem - Int Ed. 2020;59:2974–97.10.1002/anie.201903459Suche in Google Scholar PubMed
[384] Eftekhari A. Low voltage anode materials for lithium-ion batteries. Energy Storage Mater. 2017;7:157–80.10.1016/j.ensm.2017.01.009Suche in Google Scholar
[385] Wagemaker M, Mulder FM. Properties and promises of nanosized insertion materials for Li-ion batteries. Acc Chem Res. 2013;46:1206–15.10.1021/ar2001793Suche in Google Scholar PubMed
[386] Moretti A, Kim GT, Bresser D, Renger K, Paillard E, Marassi R, et al. Investigation of different binding agents for nanocrystalline anatase TiO2 anodes and its application in a novel, green lithium-ion battery. J Power Sources. 2013;221:419–26.10.1016/j.jpowsour.2012.07.142Suche in Google Scholar
[387] Hong Z, Wei M. Layered titanate nanostructures and their derivatives as negative electrode materials for lithium-ion batteries. J Mater Chem A. 2013;1:4403–14.10.1039/c2ta01312fSuche in Google Scholar
[388] Chen Z, Belharouak I, Sun YK, Amine K. Titanium-based anode materials for safe lithium-ion batteries. Adv Funct Mater. 2013;23:959–69.10.1002/adfm.201200698Suche in Google Scholar
[389] Wang L, Tang C, Takeuchi KJ, Takeuchi ES, Marschilok AC. Synthesis and characterization of Li4Ti5O12 anode materials with enhanced high-rate performance in lithium-ion batteries. MRS Adv. 2018;3:575–80. Materials Research Society10.1557/adv.2018.247Suche in Google Scholar
[390] Tojo T, Kawashiri S, Tsuda T, Kadowaki M, Inada R, Sakurai Y. Electrochemical performance of single Li4Ti5O12 particle for lithium ion battery anode. J Electroanal Chem. 2019;836:24–9.10.1016/j.jelechem.2019.01.061Suche in Google Scholar
[391] Yi TF, Yang SY, Xie Y. Recent advances of Li4Ti5O12as a promising next generation anode material for high power lithium-ion batteries. J Mater Chem A. 2015;3:5750–77.10.1039/C4TA06882CSuche in Google Scholar
[392] Li K, Zhang Y, Sun Y, Xu Y, Zhang H, Ye P, et al. Template-free synthesis of biomass-derived carbon coated Li4Ti5O12 microspheres as high performance anodes for lithium-ion batteries. Appl Surf Sci. 2018;459:572–82.10.1016/j.apsusc.2018.08.047Suche in Google Scholar
[393] Zou S, Wang G, Zhang Y, Xue C, Chen H, Yang G, et al. Nano-structure and characterization of carbon composite with Al3+ and Mn4+ co-doped Li4Ti5O12 as anodes for Li-ion batteries. J Alloy Compd. 2020;816:152609.10.1016/j.jallcom.2019.152609Suche in Google Scholar
[394] Meng WW, Yan BL, Xu YJ. Scalable synthesis of Ti3+ self-doped Li4Ti5O12 microparticles as an improved performance anode material for Li-ion batteries. J Alloy Compd. 2019;788:21–9.10.1016/j.jallcom.2019.01.362Suche in Google Scholar
[395] Sun L, Liu Z, Wang Z, Yang W, Yang J, Sun K, et al. The synergic effects of Ca and Sm co-doping on the crystal structure and electrochemical performances of Li4−xCaxTi5−xSmxO12 anode material. Solid State Sci. 2019;87:110–7.10.1016/j.solidstatesciences.2018.11.010Suche in Google Scholar
[396] Wang Z, Sun L, Yang W, Yang J, Sun K, Chen D, et al. Unveiling the synergic roles of Mg/Zr co-doping on rate capability and cycling stability of Li4Ti5O12. J Electrochem Soc. 2019;166:A658–66.10.1149/2.0791904jesSuche in Google Scholar
[397] Meng WW, Xu YJ, Yan BL. In situ nano-sized spinel Li4Ti5O12 powder fabricated by a one-step roasting process in molten salts. J Alloy Compd. 2018;732:784–91.10.1016/j.jallcom.2017.10.261Suche in Google Scholar
[398] Luo S, Zhang P, Yuan T, Ruan J, Peng C, Pang Y, et al. Molecular self-assembly of a nanorod N-Li4Ti5O12/TiO2/C anode for superior lithium ion storage. J Mater Chem A. 2018;6:15755–61.10.1039/C8TA05860ASuche in Google Scholar
[399] Odziomek M, Chaput F, Lerouge F, Rutkowska A, Świerczek K, Carlier D, et al. Impact of the synthesis parameters on the microstructure of nano-structured LTO prepared by glycothermal routes and 7Li NMR structural investigations. J Sol-Gel Sci Technol. 2019;89:225–33.10.1007/s10971-018-4844-2Suche in Google Scholar
[400] Zhu X, Zhou S, Jiang X, Yao X, Xu X, Peng A, et al. High-performances of Li4Ti5O12 anodes for lithium-ion batteries via modifying the Cu current collector through magnetron sputtering amorphous carbon. J Alloy Compd. 2020;830:154682.10.1016/j.jallcom.2020.154682Suche in Google Scholar
[401] Pawlitzek F, Pampel J, Schmuck M, Althues H, Schumm B, Kaskel S. High-power lithium ion batteries based on preorganized necklace type Li4Ti5O12/VACNT nano-composites. J Power Sources. 2016;325:1–6.10.1016/j.jpowsour.2016.06.016Suche in Google Scholar
[402] Ji X, Liu H, Wu X, Lu Q, Li Z, Pang Y. Toward rational design of N-doped Li4Ti5O12@carbon anode materials for high-performance lithium-ion batteries. Ion (Kiel). 2020;26:1211–20.10.1007/s11581-019-03324-7Suche in Google Scholar
[403] Bon CY, Isheunesu P, Mwemezi M, Kim S, Afrifah VA, Hamenu L, et al. Mesoporous carbon/Li4Ti5O12 nanoflakes composite anode material lithiated to 0.01 V. J Ind Eng Chem. 2019;80:551–7.10.1016/j.jiec.2019.08.026Suche in Google Scholar
[404] Li D, Liu Y, Zhao W, Gao Y, Cao L, Liu Y, et al. Synthesis of Ce modified Li4Ti5O12 using biomass as carbon source. J Electroanal Chem. 2019;851:113441.10.1016/j.jelechem.2019.113441Suche in Google Scholar
[405] Li Y, Chen Q, Meng Q, Lei S, Li C, Li X, et al. One-step synthesis of a nanosized cubic Li2TiO3-coated Br, C, and N Co-doped Li4Ti5O12 anode material for stable high-rate lithium-ion batteries. ACS Appl Mater Interfaces. 2019;11:25804–16.10.1021/acsami.9b04041Suche in Google Scholar PubMed
[406] Meng T, Zeng R, Sun Z, Yi F, Shu D, Li K, et al. Chitosan-confined synthesis of N-doped and carbon-coated Li4Ti5O12 nanoparticles with enhanced lithium storage for lithium-ion batteries. J Electrochem Soc. 2018;165:A1046–53.10.1149/2.0901805jesSuche in Google Scholar
[407] Liu Y, Zhao M, Xu H, Chen J. Fabrication of continuous conductive network for Li4Ti5O12 anode by Cu-doping and graphene wrapping to boost lithium storage. J Alloy Compd. 2019;780:1–7.10.1016/j.jallcom.2018.11.355Suche in Google Scholar
[408] Yue J, Badaczewski FM, Voepel P, Leichtweiß T, Mollenhauer D, Zeier WG, et al. Critical role of the crystallite size in nanostructured Li4Ti5O12 anodes for lithium-ion batteries. ACS Appl Mater Interfaces. 2018;10:22580–90.10.1021/acsami.8b05057Suche in Google Scholar PubMed
[409] Zuo X, Zhu J, Müller-Buschbaum P, Cheng YJ. Silicon based lithium-ion battery anodes: a chronicle perspective review. Nano Energy. 2017;31:113–43.10.1016/j.nanoen.2016.11.013Suche in Google Scholar
[410] Li W, Sun X, Yu Y. Si-, Ge-, Sn-Based anode materials for lithium-ion batteries: from structure design to electrochemical performance. Small Methods. 2017;1:1600037.10.1002/smtd.201600037Suche in Google Scholar
[411] Ying H, Han WQ. Metallic Sn-based anode materials: application in high-performance lithium-ion and sodium-ion batteries. Adv Sci. 2017;4:1700298.10.1002/advs.201700298Suche in Google Scholar PubMed PubMed Central
[412] Wu H, Cui Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today. 2012;7:414–29.10.1016/j.nantod.2012.08.004Suche in Google Scholar
[413] Szczech JR, Jin S. Nanostructured silicon for high capacity lithium battery anodes. Energy Env Sci. 2011;4:56–72.10.1039/C0EE00281JSuche in Google Scholar
[414] Ma D, Cao Z, Hu A. Si-Based anode materials for Li-ion batteries: a mini review. Nano-Micro Lett. 2014;6:347–58.10.1007/s40820-014-0008-2Suche in Google Scholar PubMed PubMed Central
[415] Sun Y, Liu N, Cui Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat Energy. 2016;1:16071.10.1038/nenergy.2016.71Suche in Google Scholar
[416] Kalnaus S, Rhodes K, Daniel C. A study of lithium ion intercalation induced fracture of silicon particles used as anode material in Li-ion battery. J Power Sources. 2011;196:8116–24.10.1016/j.jpowsour.2011.05.049Suche in Google Scholar
[417] Lee SW, McDowell MT, Berla LA, Nix WD, Cui Y. Fracture of crystalline silicon nanopillars during electrochemical lithium insertion. Proc Natl Acad Sci U S A. 2012;109:4080–5.10.1073/pnas.1201088109Suche in Google Scholar PubMed PubMed Central
[418] McDowell MT, Ryu I, Lee SW, Wang C, Nix WD, Cui Y. Studying the kinetics of crystalline silicon nanoparticle lithiation with in situ transmission electron microscopy. Adv Mater. 2012;24:6034–41.10.1002/adma.201202744Suche in Google Scholar PubMed
[419] Liu XH, Zhong L, Huang S, Mao SX, Zhu T, Huang JY. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano. 2012;6:1522–31.10.1021/nn204476hSuche in Google Scholar PubMed
[420] Aghajamali M, Xie H, Javadi M, Kalisvaart WP, Buriak JM, Veinot JGC. Size and surface effects of silicon nanocrystals in graphene aerogel composite anodes for lithium ion batteries. Chem Mater. 2018;30:7782–92.10.1021/acs.chemmater.8b03198Suche in Google Scholar
[421] Luo J, Zhao X, Wu J, Jang HD, Kung HH, Huang J. Crumpled graphene-encapsulated Si nanoparticles for lithium ion battery anodes. J Phys Chem Lett. 2012;3:1824–9.10.1021/jz3006892Suche in Google Scholar PubMed
[422] Lee JK, Smith KB, Hayner CM, Kung HH. Silicon nanoparticles–graphene paper composites for Li ion battery anodes. Chem Commun. 2010;46:2025–7.10.1039/b919738aSuche in Google Scholar PubMed
[423] Zhou X, Yin Y-X, Wan L-J, Guo Y-G. Facile synthesis of silicon nanoparticles inserted into graphene sheets as improved anode materials for lithium-ion batteries. Chem Commun. 2012;48:2198–200.10.1039/c2cc17061bSuche in Google Scholar PubMed
[424] Zhou X, Yin Y-X, Wan L-J, Guo Y-G. Self-assembled nanocomposite of silicon nanoparticles encapsulated in graphene through electrostatic attraction for lithium-ion batteries. Adv Energy Mater. 2012;2:1086–90.10.1002/aenm.201200158Suche in Google Scholar
[425] Chang J, Huang X, Zhou G, Cui S, Hallac PB, Jiang J, et al. Multilayered Si nanoparticle/reduced graphene oxide hybrid as a high-performance lithium-ion battery anode. Adv Mater. 2014;26:758–64.10.1002/adma.201302757Suche in Google Scholar PubMed
[426] Wu H, Yu G, Pan L, Liu N, McDowell MT, Bao Z, et al. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat Commun. 2013;4:1943.10.1038/ncomms2941Suche in Google Scholar PubMed
[427] Bai X, Yu Y, Kung HH, Wang B, Jiang J. Si@SiOx/graphene hydrogel composite anode for lithium-ion battery. J Power Sources. 2016;306:42–8.10.1016/j.jpowsour.2015.11.102Suche in Google Scholar
[428] Hu L, Wu H, Gao Y, Cao A, Li H, McDough J, et al. Silicon-carbon nanotube coaxial sponge as Li-ion anodes with high areal capacity. Adv Energy Mater. 2011;1:523–7.10.1002/aenm.201100056Suche in Google Scholar
[429] Mu T, Zuo P, Lou S, Pan Q, Zhang H, Du C, et al. A three-dimensional silicon/nitrogen-doped graphitized carbon composite as high-performance anode material for lithium ion batteries. J Alloy Compd. 2019;777:190–7.10.1016/j.jallcom.2018.10.177Suche in Google Scholar
[430] Li L, Zuo Z, Shang H, Wang F, Li Y. In-situ constructing 3D graphdiyne as all-carbon binder for high-performance silicon anode. Nano Energy. 2018;53:135–43.10.1016/j.nanoen.2018.08.039Suche in Google Scholar
[431] Batmaz R, Hassan FM, Higgins D, Cano ZP, Xiao X, Chen Z. Highly durable 3D conductive matrixed silicon anode for lithium-ion batteries. J Power Sources. 2018;407:84–91.10.1016/j.jpowsour.2018.08.035Suche in Google Scholar
[432] Wang F, Hu Z, Mao L, Mao J. Nano-silicon@soft carbon embedded in graphene scaffold: high-performance 3D free-standing anode for lithium-ion batteries. J Power Sources. 2020;450:227692.10.1016/j.jpowsour.2019.227692Suche in Google Scholar
[433] Eshetu GG, Figgemeier E. Confronting the challenges of next-generation silicon anode-based lithium-ion batteries: role of designer electrolyte additives and polymeric binders. ChemSusChem. 2019;12:2515–39.10.1002/cssc.201900209Suche in Google Scholar PubMed
[434] Zhao Y, Liu C, Sun Y, Yi R, Cai Y, Li Y, et al. 3D-structured multi-walled carbon nanotubes/copper nanowires composite as a porous current collector for the enhanced silicon-based anode. J Alloy Compd. 2019;803:505–13.10.1016/j.jallcom.2019.06.302Suche in Google Scholar
[435] Chakrapani V, Rusli F, Filler MA, Kohl PA. Silicon nanowire anode: improved battery life with capacity-limited cycling. J Power Sources. 2012;205:433–8.10.1016/j.jpowsour.2012.01.061Suche in Google Scholar
[436] Franco Gonzalez A, Yang NH, Liu RS. Silicon anode design for lithium-ion batteries: progress and perspectives. J Phys Chem C. 2017;121:27775–87.10.1021/acs.jpcc.7b07793Suche in Google Scholar
[437] Luo W, Chen X, Xia Y, Chen M, Wang L, Wang Q, et al. Surface and interface engineering of silicon-based anode materials for lithium-ion batteries. Adv Energy Mater. 2017;7:1701083.10.1002/aenm.201701083Suche in Google Scholar
[438] Liu L, Lyu J, Li T, Zhao T. Well-constructed silicon-based materials as high-performance lithium-ion battery anodes. Nanoscale. 2016;8:701–22.10.1039/C5NR06278KSuche in Google Scholar PubMed
[439] Agyeman DA, Song K, Lee GH, Park M, Kang YM. Carbon-coated Si nanoparticles anchored between reduced graphene oxides as an extremely reversible anode material for high energy-density Li-ion battery. Adv Energy Mater. 2016;6:1600904.10.1002/aenm.201600904Suche in Google Scholar
[440] Wu L, Yang J, Zhou X, Zhang M, Ren Y, Nie Y. Silicon nanoparticles embedded in a porous carbon matrix as a high-performance anode for lithium-ion batteries. J Mater Chem A. 2016;4:11381–7.10.1039/C6TA04398DSuche in Google Scholar
[441] Bie Y, Yu J, Yang J, Lu W, Nuli Y, Wang J. Porous microspherical silicon composite anode material for lithium ion battery. Electrochim Acta. 2015;178:65–73.10.1016/j.electacta.2015.07.173Suche in Google Scholar
[442] Jung CH, Choi J, Kim WS, Hong SH. A nanopore-embedded graphitic carbon shell on silicon anode for high performance lithium ion batteries. J Mater Chem A. 2018;6:8013–20.10.1039/C8TA01471JSuche in Google Scholar
[443] Cho H, Kim K, Park CM, Jeong G. In situ fabrication of nanohybrid carbon/polyamide film providing robust binding and conductive network in silicon anode for lithium-ion battery. J Power Sources. 2019;410–411:25–30.10.1016/j.jpowsour.2018.11.005Suche in Google Scholar
[444] Zhang L, Guo H, Rajagopalan R, Hu X, Huang Y, Dou SX, et al. One-step synthesis of a silicon/hematite@carbon hybrid nanosheet/silicon sandwich-like composite as an anode material for Li-ion batteries. J Mater Chem A. 2016;4:4056–61.10.1039/C5TA09123CSuche in Google Scholar
[445] Zhang L, Rajagopalan R, Guo H, Hu X, Dou S, Liu H. A green and facile way to prepare granadilla-like silicon-based anode materials for Li-ion batteries. Adv Funct Mater. 2016;26:440–6.10.1002/adfm.201503777Suche in Google Scholar
[446] Zhang L, Wang C, Dou Y, Cheng N, Cui D, Du Y, et al. A yolk–shell structured silicon anode with superior conductivity and high tap density for full lithium-ion batteries. Angew Chem Int Ed. 2019;58:8824–8.10.1002/anie.201903709Suche in Google Scholar PubMed
[447] Zhou Z, Pan L, Liu Y, Zhu X, Xie X. From sand to fast and stable silicon anode: synthesis of hollow Si@void@C yolk–shell microspheres by aluminothermic reduction for lithium storage. Chin Chem Lett. 2019;30:610–7.10.1016/j.cclet.2018.08.018Suche in Google Scholar
[448] Zhu L, Chen Y, Wu C, Chu R, Zhang J, Jiang H, et al. Double-carbon protected silicon anode for high performance lithium-ion batteries. J Alloy Compd. 2020;812:151848.10.1016/j.jallcom.2019.151848Suche in Google Scholar
[449] Liu N, Wu H, McDowell MT, Yao Y, Wang C, Cui Y. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 2012;12:3315–21.10.1021/nl3014814Suche in Google Scholar PubMed
[450] Xing Y, Shen T, Guo T, Wang X, Xia X, Gu C, et al. A novel durable double-conductive core-shell structure applying to the synthesis of silicon anode for lithium ion batteries. J Power Sources. 2018;384:207–13.10.1016/j.jpowsour.2018.02.051Suche in Google Scholar
[451] Feng X, Yang J, Bie Y, Wang J, Nuli Y, Lu W. Nano/micro-structured Si/CNT/C composite from nano-SiO2for high power lithium ion batteries. Nanoscale. 2014;6:12532–9.10.1039/C4NR03948CSuche in Google Scholar PubMed
[452] Park BH, Jeong JH, Lee GW, Kim YH, Roh KC, Kim KB. Highly conductive carbon nanotube micro-spherical network for high-rate silicon anode. J Power Sources. 2018;394:94–101.10.1016/j.jpowsour.2018.04.112Suche in Google Scholar
[453] Wang F, Chen G, Zhang N, Liu X, Ma R. Engineering of carbon and other protective coating layers for stabilizing silicon anode materials. Carbon Energy. 2019;1:219–45.10.1002/cey2.24Suche in Google Scholar
[454] Shelke MV, Gullapalli H, Kalaga K, Rodrigues MTF, Devarapalli RR, Vajtai R, et al. Facile synthesis of 3D anode assembly with Si nanoparticles sealed in highly pure few layer graphene deposited on porous current collector for long life Li-ion battery. Adv Mater Interfaces. 2017;4:1601043.10.1002/admi.201601043Suche in Google Scholar
[455] Ababtain K, Babu G, Susarla S, Gullapalli H, Masurkar N, Ajayan PM, et al. Porous graphene current collectors filled with silicon as high-performance lithium battery anode. Mater Res Express. 2018;5:014004.10.1088/2053-1591/aaa0f9Suche in Google Scholar
[456] Breitung B, Aguiló-Aguayo N, Bechtold T, Hahn H, Janek J, Brezesinski T. Embroidered copper microwire current collector for improved cycling performance of silicon anodes in lithium-ion batteries. Sci Rep. 2017;7:13010.10.1038/s41598-017-13261-ySuche in Google Scholar PubMed PubMed Central
[457] Chiluwal S, Sapkota N, Rao AM, Podila R. Three-dimensional Si anodes with fast diffusion, high capacity, high rate capability, and long cycle life. ACS Appl Mater Interfaces. 2020;12:34763–70.10.1021/acsami.0c05888Suche in Google Scholar PubMed
[458] Cen Y, Fan Y, Qin Q, Sisson RD, Apelian D, Liang J. Synthesis of Si anode with a microsized-branched structure from recovered Al scrap for use in Li-Ion batteries. J Power Sources. 2019;410–411:31–7.10.1016/j.jpowsour.2018.10.097Suche in Google Scholar
[459] Prakash S, Zhang C, Park JD, Razmjooei F, Yu JS. Silicon core-mesoporous shell carbon spheres as high stability lithium-ion battery anode. J Colloid Interface Sci. 2019;534:47–54.10.1016/j.jcis.2018.09.004Suche in Google Scholar PubMed
[460] Zhou Y, Yang Y, Hou G, Yi D, Zhou B, Chen S, et al. Stress-relieving defects enable ultra-stable silicon anode for Li-ion storage. Nano Energy. 2020;70:104568.10.1016/j.nanoen.2020.104568Suche in Google Scholar
[461] Wang MS, Wang GL, Wang S, Zhang J, Wang J, Zhong W, et al. In situ catalytic growth 3D multi-layers graphene sheets coated nano-silicon anode for high performance lithium-ion batteries. Chem Eng J. 2019;356:895–903.10.1016/j.cej.2018.09.110Suche in Google Scholar
[462] Gao R, Tang J, Yu X, Tang S, Ozawa K, Sasaki T, et al. In situ synthesis of MOF-derived carbon shells for silicon anode with improved lithium-ion storage. Nano Energy. 2020;70:104444.10.1016/j.nanoen.2019.104444Suche in Google Scholar
[463] Liu S, Zhang X, Yan P, Cheng R, Tang Y, Cui M, et al. Dual bond enhanced multidimensional constructed composite silicon anode for high-performance lithium ion batteries. ACS Nano. 2019;13:8854–64.10.1021/acsnano.9b02129Suche in Google Scholar PubMed
[464] Wang H, Fu J, Wang C, Wang J, Yang A, Li C, et al. A binder-free high silicon content flexible anode for Li-ion batteries. Energy Env Sci. 2020;13:848–58.10.1039/C9EE02615KSuche in Google Scholar
[465] Sitinamaluwa HS, Li H, Wasalathilake KC, Wolff A, Tesfamichael T, Zhang S, et al. Nanoporous SiO coated amorphous silicon anode material with robust mechanical behavior for high-performance rechargeable Li-ion batteries. Nano Mater Sci. 2019;1:70–6.10.1016/j.nanoms.2019.02.005Suche in Google Scholar
[466] Nealer R, Reichmuth D, Anair D. Cleaner cars from cradle to grave. J Union Concerned Sci. 2015;1–54.Suche in Google Scholar
[467] Emilsson E, Dahllöf L. Lithium-ion vehicle battery production. Stockholm, Sweden: IVL Swedish Environmental Research Institute; 2019.Suche in Google Scholar
[468] Hall D, Lutsey N. Effects of battery manufacturing on electric vehicle life-cycle greenhouse gas emissions. ICCT Brief. 2018;12.Suche in Google Scholar
[469] Masnadi MS, El-Houjeiri HM, Schunack D, Li Y, Englander JG, Badahdah A, et al. Global carbon intensity of crude oil production. Science. 2018;361:851–3.10.1126/science.aar6859Suche in Google Scholar PubMed
[470] Fuel economy improvements are projected to reduce future gasoline use - Today in Energy - U.S. Energy Information Administration (EIA). https://www.eia.gov/todayinenergy/detail.php?id=31332#, (accessed 6 March 2021).Suche in Google Scholar
[471] Electricity generation, capacity, and sales in the United States - U.S. Energy Information Administration (EIA). https://www.eia.gov/energyexplained/electricity/electricity-in-the-us-generation-capacity-and-sales.php, (accessed 6 March 2021).Suche in Google Scholar
[472] Environment - U.S. Energy Information Administration (EIA) - U.S. Energy Information Administration (EIA). https://www.eia.gov/environment/emissions/co2_vol_mass.php, (accessed 6 March 2021).Suche in Google Scholar
[473] Peters JF, Baumann M, Zimmermann B, Braun J, Weil M. The environmental impact of Li-ion batteries and the role of key parameters – a review. Renew Sustain Energy Rev. 2017;67:491–506.10.1016/j.rser.2016.08.039Suche in Google Scholar
[474] Dunn JB, James C, Gaines L, Gallagher K, Dai Q, Kelly JC. Material and energy flows in the production of cathode and anode materials for lithium ion batteries. Argonne, IL (United States); 2015.10.2172/1224963Suche in Google Scholar
[475] Dunn JB, Gaines L, Kelly JC, James C, Gallagher KG. The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling's role in its reduction. Energy Env Sci. 2015;8:158–68.10.1039/C4EE03029JSuche in Google Scholar
[476] Dunn JB, Gaines L, Kelly JC, Gallagher KG. REWAS 2016. Cham: Springer International Publishing; 2016. p. 73–7910.1002/9781119275039.ch11Suche in Google Scholar
[477] Wang Y, Yu Y, Huang K, Tang B. From the perspective of battery production: energy–environment–economy (3E) analysis of lithium-ion batteries in China. Sustainability. 2019;11:6941.10.3390/su11246941Suche in Google Scholar
[478] Jacob J. The chalkboard: C rating of batteries: a misleading concept, C flux rather than C rate. Electrochem Soc Interface. 2018;27:42–3.10.1149/2.F01182ifSuche in Google Scholar
[479] Rowden B, Garcia-Araez N. A review of gas evolution in lithium ion batteries. Energy Rep. 2020;6:10–8.10.1016/j.egyr.2020.02.022Suche in Google Scholar
[480] Leiva EPM. Modeling of lithium-ion batteries is becoming viral: where to go. J Solid State Electrochem. 2020;24:2117–20.10.1007/s10008-020-04703-1Suche in Google Scholar
[481] Fan Y, Chen X, Legut D, Zhang Q. Modeling and theoretical design of next-generation lithium metal batteries. Energy Storage Mater. 2019;16:169–93.10.1016/j.ensm.2018.05.007Suche in Google Scholar
[482] Zhao Y, Stein P, Bai Y, Al-Siraj M, Yang Y, Xu BX. A review on modeling of electro-chemo-mechanics in lithium-ion batteries. J Power Sources. 2019;413:259–83.10.1016/j.jpowsour.2018.12.011Suche in Google Scholar
[483] Horstmann B, Single F, Latz A. Review on multi-scale models of solid-electrolyte interphase formation. Curr Opin Electrochem. 2019;13:61–9.10.1016/j.coelec.2018.10.013Suche in Google Scholar
[484] Barré A, Deguilhem B, Grolleau S, Gérard M, Suard F, Riu D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J Power Sources. 2013;241:680–9.10.1016/j.jpowsour.2013.05.040Suche in Google Scholar
[485] Zhu J, Wierzbicki T, Li W. A review of safety-focused mechanical modeling of commercial lithium-ion batteries. J Power Sources. 2018;378:153–68.10.1016/j.jpowsour.2017.12.034Suche in Google Scholar
[486] Deng J, Bae C, Marcicki J, Masias A, Miller T. Safety modelling and testing of lithium-ion batteries in electrified vehicles. Nat Energy. 2018;3:261–6.10.1038/s41560-018-0122-3Suche in Google Scholar
[487] Soto FA, Martinez de la Hoz JM, Seminario JM, Balbuena PB. Modeling solid-electrolyte interfacial phenomena in silicon anodes. Curr Opin Chem Eng. 2016;13:179–85.10.1016/j.coche.2016.08.017Suche in Google Scholar
[488] Joshi RP, Eickholt J, Li L, Fornari M, Barone V, Peralta JE. Machine learning the voltage of electrode materials in metal-ion batteries. ACS Appl Mater Interfaces. 2019;11:18494–503.10.1021/acsami.9b04933Suche in Google Scholar PubMed
[489] Zhang Y, Tang Q, Zhang Y, Wang J, Stimming U, Lee AA. Identifying degradation patterns of lithium ion batteries from impedance spectroscopy using machine learning. Nat Commun. 2020;11:1706.10.1038/s41467-020-15235-7Suche in Google Scholar PubMed PubMed Central
[490] Wang W, Brady NW, Liao C, Fahmy YA, Chemali E, West AC, et al. High-fidelity state-of-charge estimation of li-ion batteries using machine learning; 2019. arXiv preprint arXiv:1909.02448.Suche in Google Scholar
[491] Fan J, Fan J, Liu F, Qu J, Li R. A novel machine learning method based approach for Li-ion battery prognostic and health management. IEEE Access. 2019;7:160043–61.10.1109/ACCESS.2019.2947843Suche in Google Scholar
[492] Jiang Z, Li J, Yang Y, Mu L, Wei C, Yu X, et al. Machine-learning-revealed statistics of the particle-carbon/binder detachment in lithium-ion battery cathodes. Nat Commun. 2020;11:2310.10.1038/s41467-020-16233-5Suche in Google Scholar PubMed PubMed Central
[493] Liu Y, Zhao T, Ju W, Shi S. Materials discovery and design using machine learning. J Mater. 2017;3:159–77.10.1016/j.jmat.2017.08.002Suche in Google Scholar
[494] Nonaka T, Kawaura H, Makimura Y, Nishimura YF, Dohmae K. In situ X-ray Raman scattering spectroscopy of a graphite electrode for lithium-ion batteries. J Power Sources. 2019;419:203–7.10.1016/j.jpowsour.2019.02.064Suche in Google Scholar
[495] Liu T, Lin L, Bi X, Tian L, Yang K, Liu J, et al. In situ quantification of interphasial chemistry in Li-ion battery. Nat Nanotechnol. 2019;14:50–6.10.1038/s41565-018-0284-ySuche in Google Scholar PubMed
[496] Krachkovskiy S, Trudeau ML, Zaghib K. Application of magnetic resonance techniques to the in situ characterization of li-ion batteries: a review. Mater (Basel). 2020;13:1694.10.3390/ma13071694Suche in Google Scholar PubMed PubMed Central
[497] Liu D, Shadike Z, Lin R, Qian K, Li H, Li K, et al. Review of recent development of in situ/operando characterization techniques for lithium battery research. Adv Mater. 2019;31:1806620.10.1002/adma.201806620Suche in Google Scholar PubMed
© 2021 Shailendra Chiluwal et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

![Figure 7
(a) Comparison of CO2 emission from mid-size gasoline and BEV [466]. (b) Comparison between energy required during the assembly of battery when NMP is used as the solvent [467].](/document/doi/10.1515/ntrev-2021-0114/asset/graphic/j_ntrev-2021-0114_fig_007.jpg)
![Figure 8
(a) Emissions per kW h of electricity produced in the United States and (b) increases in miles per gallon efficiency in internal combustion engine. For these calculations, the original data were taken from [470–472].](/document/doi/10.1515/ntrev-2021-0114/asset/graphic/j_ntrev-2021-0114_fig_008.jpg)
