A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
-
Zahra Rahimzadeh
, Esfandyar Askari
Abstract
In this paper, we use a simple and cheap approach for the synthesis of herceptin-conjugated graphene biosensor to detect the HER2-positive breast cancer cells. The bifunctional graphene-herceptin nanosheets are prepared from graphite by a simple ultrasonic-mediated technique. The prepared protein-mediated graphene is fully characterized. The results show the exfoliation of graphene layers in herceptin solution. Moreover, herceptin is effectively conjugated into the surface of graphene nanosheets. The synthesized herceptin-conjugated graphene is applied for breast cancer detection. The linear range of this biosensor is 1–80 cells, which is significant. The biosensor shows an excellent selectivity performance for detection of HER2-positive cancer cells. Likewise, the stability and functionality of the biosensor is about 40 days. Based on the results, this device is a promising candidate for rapid and selective detection of cancer cells.
1 Introduction
Cancer is one of the most dangerous diseases in the world [1,2,3,4] and the most common cancer among women is breast cancer [5]. Based on the existed challenges, there is still required a nonaggressive, safe, rapid, and easy method to detect breast cancer [5,6]. Electrochemical biosensing is one of those methods that can detect and quantify the biologic species without destroying the system [7,8,9] due to its high specificity, sensitivity, portability, low-cost preparation, and low response time [10].
Various nanomaterials have been used in biomedical engineering [11,12,13,14,15,16] as well as immunosensors and biosensors such as metal oxide nanoparticles [17], carbon-based nanomaterials [18,19], noble metal nanoparticles [20], and metal nitride nanoparticles [21]. Graphene is one of the most powerful 2D nanomaterials for biosensing applications [22]. Beside high surface area and π conjugation structure of graphene that allow a high capability for binding to other molecules, this material possesses cavities and also has a tunable structure which makes it suitable for electroanalysis [23]. Beside high electrical conductivity, graphene has more properties such as ultralight weight, superior mechanical strength, optical absorption properties, high thermal conductivity, superior elasticity, etc. [24,25,26,27], which make graphene a promising material for generating versatile signals in different biological matrixes [28]. Thus, graphene has been greatly used for several biomolecules stabilization [29,30]. Some recent successful cytosensors are based on several cell detections such as biosensors for detection of MCF-7 [31], MKN 45, MCF-7 and HT 29 [32], DU 145 [33], andLO2 [34] cells. A comparison of the research in different kinds of breast cancer biomarker detection methods based on graphene materials was recently reported and discussed [28].
As a brief report of recent research on circulating tumor cells (CTCs) counting in breast cancer, researchers used gold microelectrodes and Anti-EpCAM-modified LC-SPDP monolayer, which lead to a detection limit of 105 cells/mL for MCF-7 breast cancer cells [35]. Yang et al. [36] used DNA-labeled biosensor and reached a LOD 80 cells/mL. Also, Salahandish et al. [37] could fabricate a biosensor with a LOD of 2 cells/mL using silver nanoparticles/graphene/polyaniline. Beside, a biosensor based on peptide-aptamer/polyaniline was fabricated by Liu et al. [38], demonstrating a LOD of 20 cells/mL. Recently, Nasrollahpour et al. [39] could prepare a biosensor with a linear range of 20–2,000 cells/mL by an electrochemiluminescence cytosensor in 2021.
We already used a complex procedure for detection of breast cancer cells with minimum quantification of 2 cells [37]. In this work, we used a simple biosensor consisting of herceptin-conjugated graphene which utilized the special antibody of HER2 (turns this biosensor to an immunosensor) for single cell detection. Therefore, we used a graphene-based immunosensor on the surface of a glassy carbon electrode to quantify the amount of SK-BR-3 cancer cells. One of the advantages of this biosensor is simple and low-cost preparation of the electrode, beside an appropriate linear range for cancer cell level diagnosis. An exciting property of this biosensor is that this biosensor could quantify low concentrations of SK-BR-3 cells even to 1 cell number, which can be useful for real samples and early detection.
2 Materials and methods
2.1 Materials
Graphite prepared from Merck and Herceptin was obtained from Motamed Cancer Institute (Iran). Also, SK-BR-3, MCF-7, G-292, and HUVEC cell lines were cultured in Motamed Cancer Institute. The electrodes used in this project were glassy carbon electrodes and Fe(CN)6 solution was used as the electrolyte. Also, the water used in preparing aqueous solutions was deionized water.
2.2 Graphene-herceptin synthesis
In order to synthesize the material, we first mixed a 30 mL of 20 mg/mL concentration of herceptin with 0.5 g of graphite to reach the primer solution. Then, we stir the solution using a magnetic stirrer for half an hour to disperse the graphites in the solution. After that, the stirred dispersion was ultrasonicated for 3 h in an ice bath. This was the major step to slice the graphites in order to reach the graphene sheets. The ice bath was crucial because of the presence of proteins in the dispersed solution. It should be then preserved in a steady and cold situation for 24 h in order to let remained and unreacted graphites sediment. After one day, we collected the upper parts of dispersion and centrifuged them two times in 3,000 rpm and one time in 4,000 rpm in order to wash the graphene sheets and reach the slightly graphene-herceptin. Before every biosensing test, the graphene-herceptin dispersion should be ultrasonicated for 15 min in ice bath [40].
2.3 Biosensing methods
This biosensing method was based on a three-electrode platform. Glassy carbon electrodes were used as working electrodes in this case. Before each step, in order to prepare the electrodes, the electrodes were wet-polished with alumina powder. Then, cyclic voltammetry (CV) analysis was carried out to characterize the materials and confirm the biosensor fabrication. In order to prepare electrodes for the tests, the dispersed graphene-herceptin solution was drop-coated on the electrodes and kept in the cold situation to dry for 24 h. For both selectivity and calibration tests, square wave (SQW) analysis was taken before and after putting cells on the electrodes. To make cells’ attachment to the antibodies on the electrodes, the solution was again drop-coated and preserved in the refrigerator for an hour. The reference and counter electrodes used in the tests were Ag/AgCl and Platine, respectively. Finally, to complete the electrochemical cell, a solution was prepared using ferrocyanide and KNO3 as the electrolyte.
2.4 Cell culture
SK-BR-3, a human breast cancer cell line, that overexpresses Her2 antibody (Neu/ErbB-2), human caucasian osteosarcoma (G-292), human breast adenocarcinoma (MCF-7), and human umbilical vein endothelial cells (HUVECs) utilized here were provided from Pasteur Institute of Iran (IPI). The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) at pH 7.4 supplemented with 10% (v/v) heat-inactivated at 50°C for 30 min from fetal bovine serum (FBS), 2 mM l-glutamine, 100 units/mL of penicillin, and 100 mg/mL of streptomycin at 37°C and 5% CO2 in a humidified incubator. Then, the cells were trypsinized (0.025% trypsin, 0.02% EDTA) after they were grown until 70–80% confluent [41].
2.5 Characterization and equipment
The graphene-herceptin was provided by the ultrasonic probe (Q 500 system with standard probe) and was dried in the Freeze Dryer GAMMA 1–16 LSC. Ultraviolet visible (UV) absorption of the material was obtained by T80 + UV-Vis spectrometer instruments Ltd. Morphology characteristics were investigated by a field emission scanning electron microscopy (TE-SCAN). Micro/nanostructure evaluations were studied by transmission electron microscopy (TEM) 100 kV Philips TEM. Fourier-transform infrared spectra (FTIR) of graphene-herceptin were measured by IR spectrometer (8500S SHIMADZU) (400–4,000/cm). Raman spectra were tested in the range of 500–400/cm (excitation wavelength of 642 nm) by RFS-100/s Raman spectrophotometer. All electrochemical tests were scrutinized using a Potentiostat/Galvanostat model PGSTAT302N boards (Eco Chemie, Utrecht, Netherlands).
3 Results and discussion
3.1 Biosensor fabrication
The glassy carbon electrodes were cleaned with alumina powder and alcohol. The herceptin-conjugated graphene (bio-graphene) was synthesized by ultrasonic method and casted on the prepared electrodes. An electrochemical procedure based on the three-electrode platform was used to evaluate the biosensing performance and breast cancer detection (CTCs in blood). A schematic representation of the biosensor fabrication is shown in Figure 1.
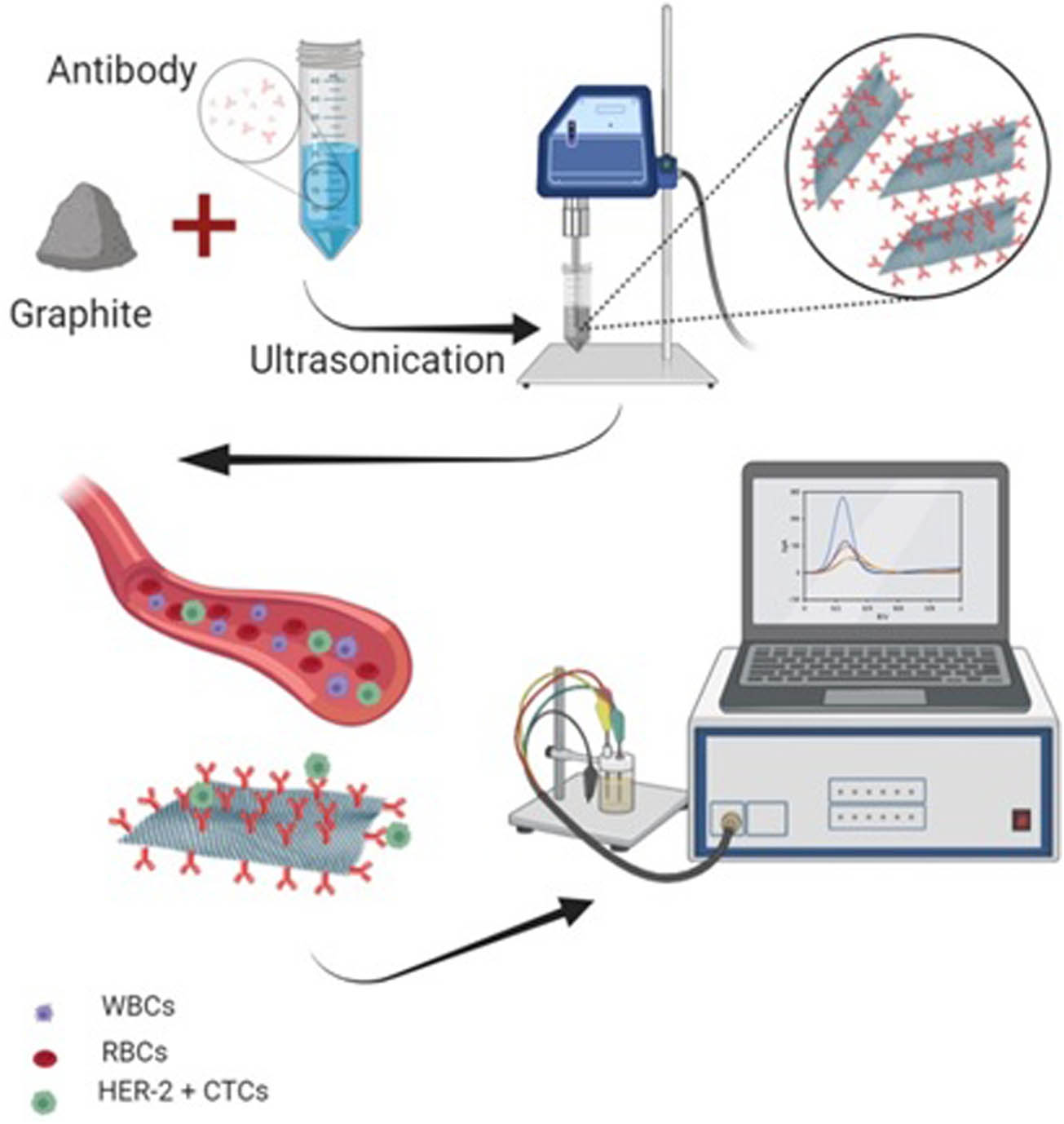
Schematic illustration of the herceptin-conjugated graphene synthesis and the immunosensor fabrication.
3.2 Morphological and physicochemical characteristics
The morphologies and microstructures of the simple herceptin-conjugated graphene nanosheets are presented in Figure 2. The FESEM results of the herceptin-conjugated graphene showed that graphene was the main material in the sample and the side dimensions of the herceptin-conjugated graphene nanosheets were in the range of 10 nm to several micrometers (Figure 2a). The non-dispersion method covered the strategy for the economically and environmentally friendly synthesis of graphene nanostructures. In Figure 2b, the TEM result of the herceptin-conjugated graphene exhibited that the prepared graphene was effectively exfoliated and one of the main features of the synthesized herceptin-mediated graphene was high surface area. TEM result showed the sonicating procedure for reducing the flake size (the flake dimensions are scaled as t −1/2). Moreover, TEM and FE-SEM results demonstrated large graphene nanosheets on the surface and their resemblance of crumpled silk veil waves scrolled and corrugated was the nature of graphene nanosheets. These typical characteristics of graphene and its derivatives were already confirmed by many researchers [42,43,44,45]. The protein-mediated graphene nanosheets were transparent and demonstrated an excellent stability under the electron beam. The distinct diffraction marks approved the structural properties (crystallites) of graphene nanosheets synthesized with a simple protein-mediated procedure. In the ultrasonic condition, ultrasonic waves establish acoustic cavitation, leading to creation, growth, and collapse of bubbles in the solution [46]. This event leads to shock waves onto the graphite and graphene layers, leading to exfoliation and isolation of graphene layers. This process relied on low-power sonicating procedure for long time. Stabilizer helps forming the few-layer graphene sheet without sheets aggregation which may be established because of the hydrophobic nature of graphene surface. Therefore, herceptin (protein) was used as a stabilizer with ultrasonic procedure to address this critical challenge. Moreover, herceptin could diffuse into the graphene layers and act as a bioreceptor for biosensing. Herceptin is a biological element that can establish selectivity in the graphene biosensor. Dong et al. reported a water-phase and non-dispersion exfoliation approach for producing large scale of high concentration graphene [47]. Khan et al. investigated exfoliation of graphite dispersions and preparation of graphene at high concentrations [48]. This technique facilitated graphene synthesis for many applications including biosensor, drug delivery, tissue engineering, and lab on a chip. The prepared hybrid material was deposited on the glassy carbon electrodes. The electrodes were cleaned completely. Then, the biosensing activity, selectivity, and calibration tests were established.
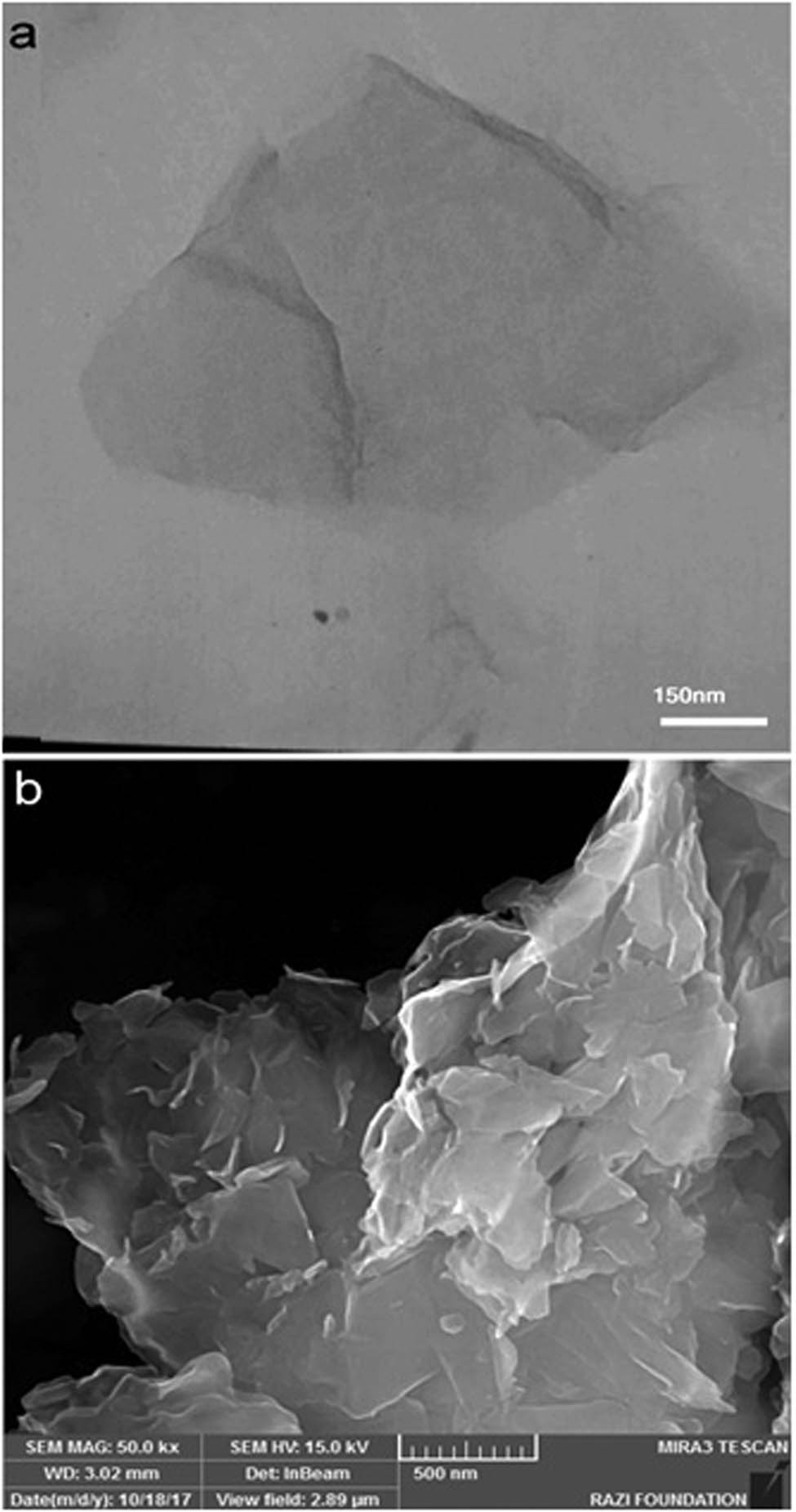
Micro/nanostructure and morphology of the herceptin-conjugated graphene prepared by sonication process: (a) TEM and (b) FE-SEM images.
Fourier-transform infrared (FTIR) spectroscopy of the herceptin-conjugated graphene was analyzed to scrutinize the in situ forming of the novel functional groups existed in bio-graphene under ultrasonication (Figure 3). The peaks at 1,098 and 1,550/cm were related to C–N and C═N stretching vibration, respectively. It seems that the nature of these peaks is ascribed to the hydrophobic residue of herceptin that reacts with the graphene layers. 1,720, 1,200, and 3,430/cm peaks are attributed to the C═O, C–OH, and O–H stretching vibration, respectively. Also, the peak at 1,600/cm was ascribed to C═C aromatic vibration [40].
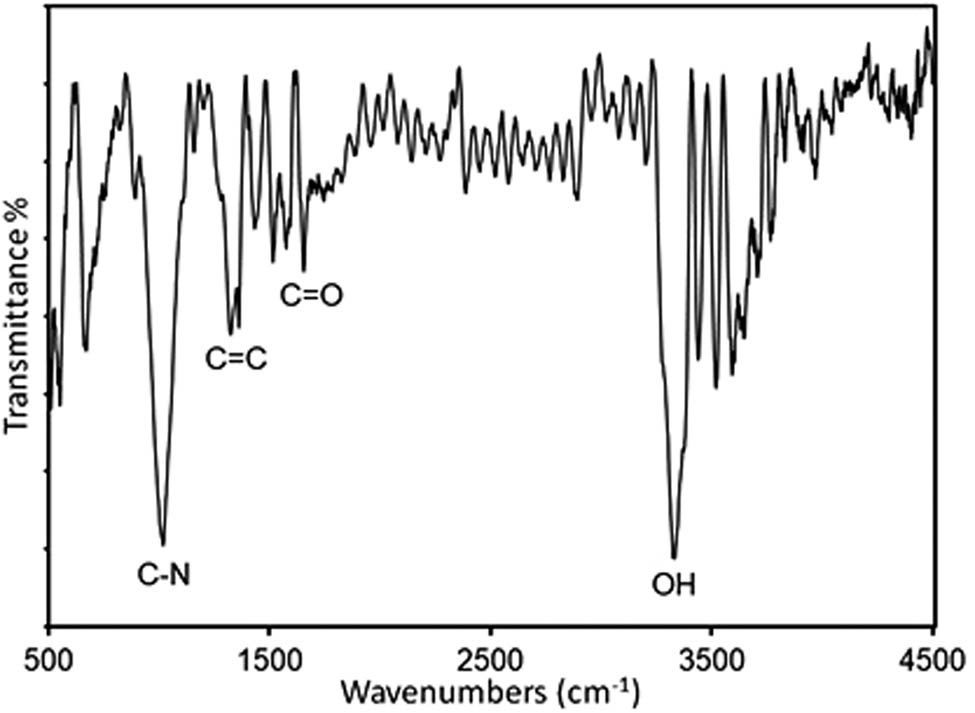
Fourier-transform infrared (FTIR) spectrum of the herceptin-conjugated graphene synthesized under ultrasonication in the presence of the antibody, herceptin.
The UV-Vis spectroscopy analysis of the herceptin-conjugated bio-graphene is shown in Figure 4. As seen, a peak is observable at 270 nm, which is ascribed to the bio-graphene characteristic [49]. An increment was observed in the intensity of this peak which was attributed to concentration of the pristine antibody, herceptin. Therefore, the herceptin-conjugated bio-graphene was successfully synthesized.
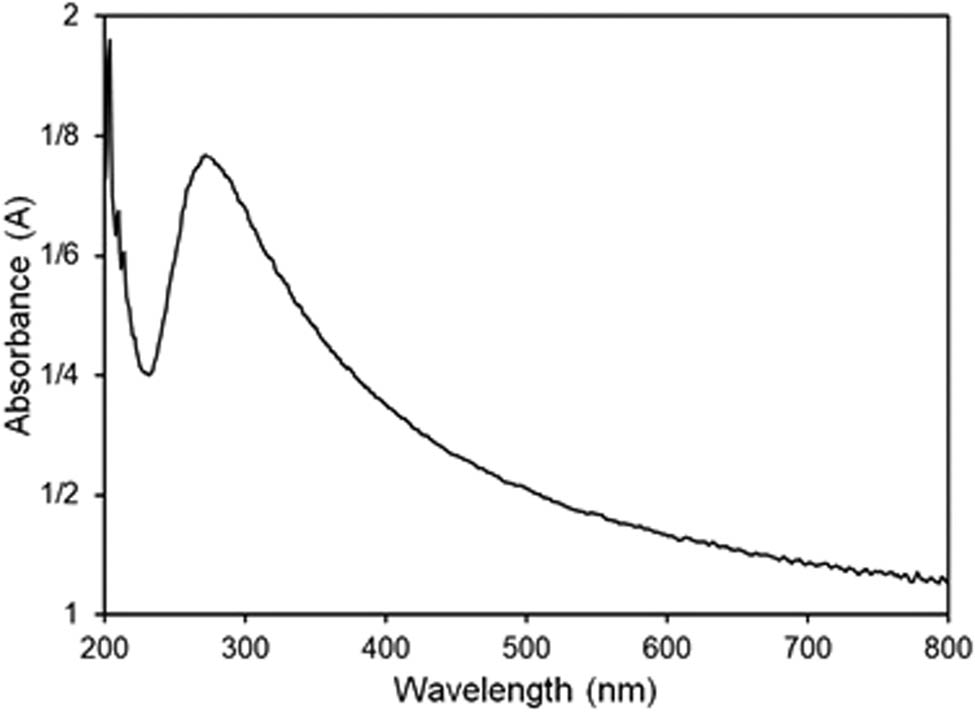
UV-Vis spectrum of the herceptin-conjugated graphene synthesized under ultrasonication in presence of the antibody, herceptin.
Raman spectroscopy analysis was used to validate carbon structure in the nanosheets of the herceptin-conjugated graphene (Figure 5). The specific bands of the functionalized bio-graphene in Raman analysis are D, G, and 2D. These bands are located at ∼1,350, ∼1,570, and ∼2,680/cm for D, G, and 2D, respectively. The shape and position of these bands in the Raman analysis include critical information in association with the quality of the bio-graphene. The intensity ratio of D/G for the herceptin-conjugated graphene and graphite was 0.32 and 0.02, respectively, revealing that the aromatic domain number was increased in the bio-graphene micro/nanostructure. The intensity ratio of 2D/G was 0.42 for the herceptin-conjugated graphene, demonstrating successful synthesis of the bio-graphene.
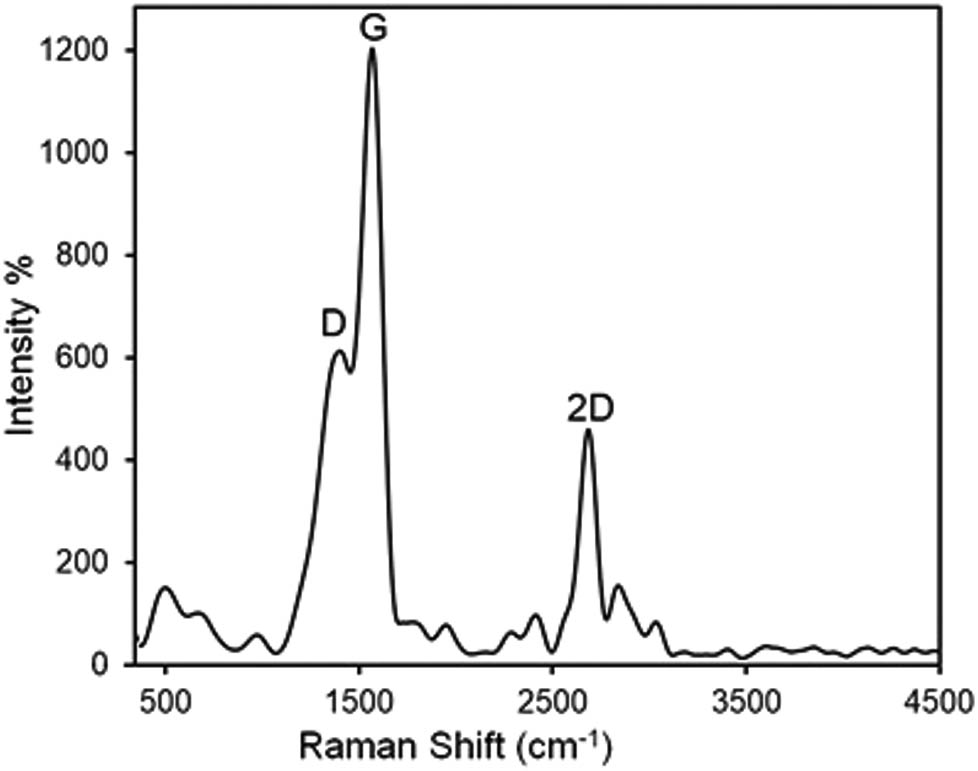
Raman spectrum of the herceptin-conjugated graphene synthesized in situ under ultrasonication in presence of the antibody, herceptin.
3.3 Electrochemical characteristics
The CV and Nyquist analysis were conducted for the bare electrode and the functionalized electrode (herceptin-conjugated graphene) in 0.01 M phosphate-buffered saline (PBS) (pH 7.4) containing 5 mM K3Fe(CN)6 (these electrochemical analyses were commonly applied to the investigation of the characterization of sensors and biosensors). These two tests were used to explore the electrochemistry and electrochemical features of the herceptin-conjugated graphene biosensor (Figures 6 and 7).
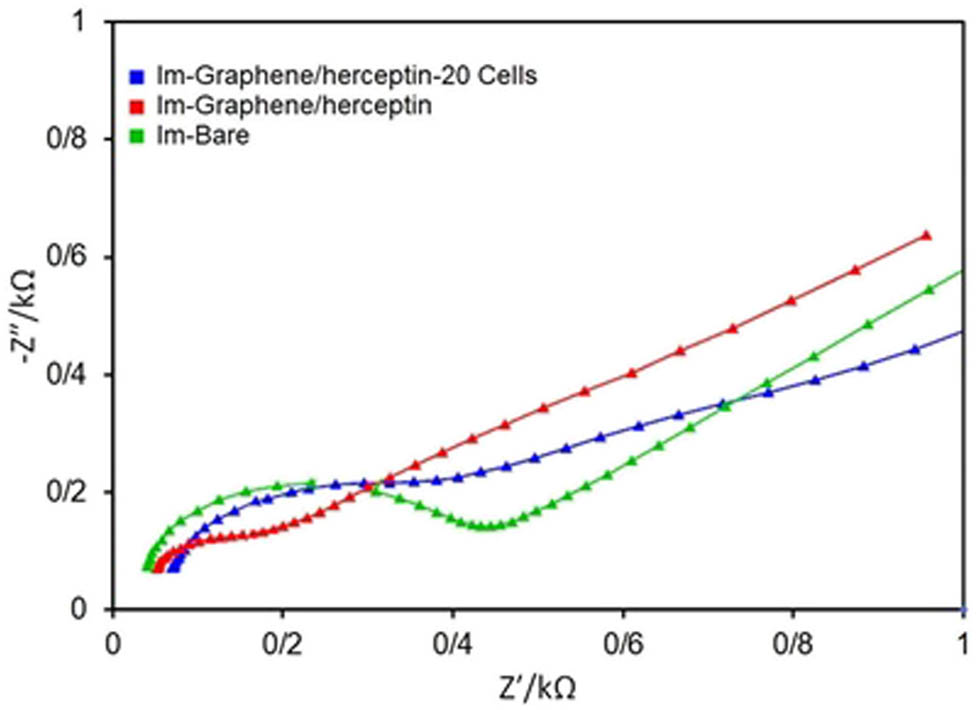
Nyquist plots for the bare electrode (green), herceptin-conjugated graphene (red), and herceptin-conjugated graphene with 20 cells (blue), respectively. All tests were done in 0.01 M PBS (pH = 7.4) containing 5 mM K3Fe(CN)6.
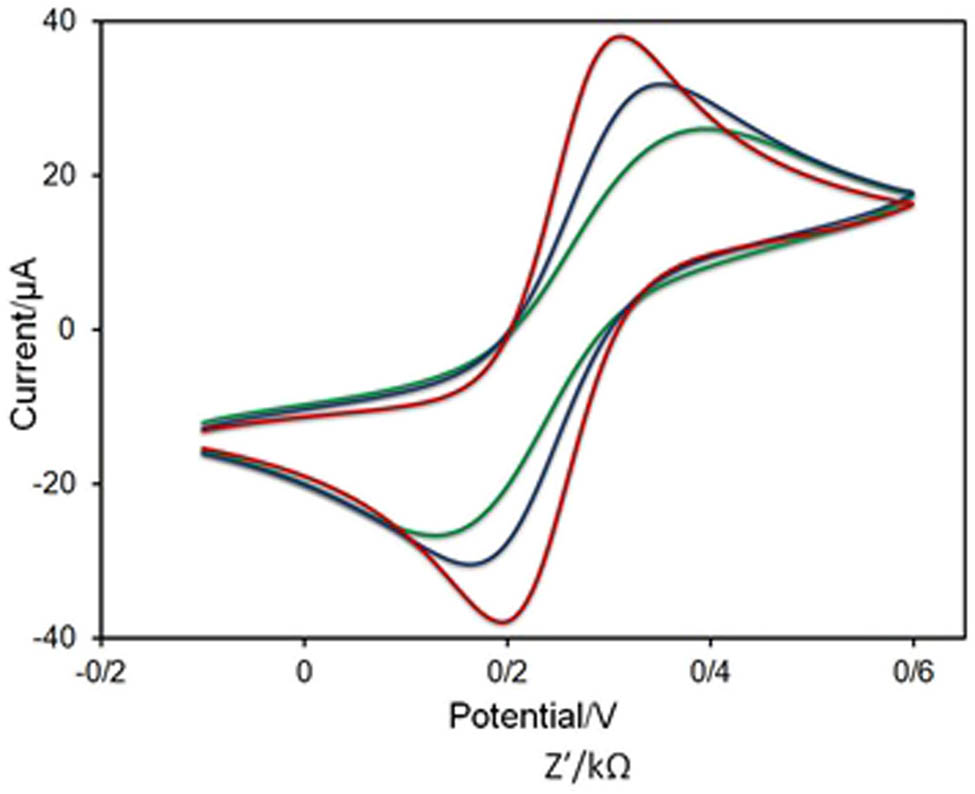
Cyclic voltammograms (CVs) for the bare electrode (red), herceptin-conjugated graphene (blue), and herceptin-conjugated graphene with 20 cells (green), respectively. All analyses were established in 0.01 M PBS (pH 7.4) containing 5 mM K3Fe(CN)6.
As can be seen in Figure 6, herceptin-conjugated graphene substrate decreased the charge transfer resistance (R
ct) in comparison with the bare electrode and enhanced the conductivity and surface area of the conventional electrode (glassy carbon), presenting a highly conductive and inexpensive substrate for biosensing applications. The herceptin-conjugated graphene substrate enriched the reaction kinetics and decreased the Rct because of great surface area and porosity that were established by its pellet/flake-like graphene structure. Owing to the rise in the surface conductivity, the electron transfer and conductivity were increased with the
As can be seen in Figure 7, there is a satisfactory agreement between Nyquist plot and CV where the peak height in CV was decreased with the functionalization of the electrode with herceptin-conjugated graphene substrate. The redox peaks of the herceptin-conjugated graphene and herceptin-conjugated graphene with 20 cells were reduced in comparison with the bare electrode which could ascribe to the good electron mediation of herceptin-conjugated graphene and the glassy carbon electrode.
Impedance spectroscopy technique is used for electrochemical characterization. However, the use of impedance technique is semi-qualitative for biosensing evaluation and detection. Moreover, this technique uses resistance to sense the analyte, which allows the interferences to affect the results decreasing the selectivity. The square wave voltammetry was used as a strong technique for biosensing measurements [50]. Actually, we established a single cell detection platform for early breast cancer.
3.4 Selectivity analysis
The main analysis for sensing performance of a biosensor is the selectivity test and response of the sensor in real biological samples containing electroactive species. In order to perform this analysis, we used different kinds of cell lines including MCF-7, SK-BR-3, HUVEC, and G-292. In every step, we drop-coated a solution of cells with a 2,000 cells/mL concentration. In each one, SQW analysis was performed before and after putting cells on the electrode surface. In order to remove the parameter of difference of the electrodes from the selectivity results, we divided the difference of the SQW peaks intensity on the primitive intensity (before depositing the cells) as the parameter ∆I/I. The concerned curves and final comparison of the data are shown in Figure 8.
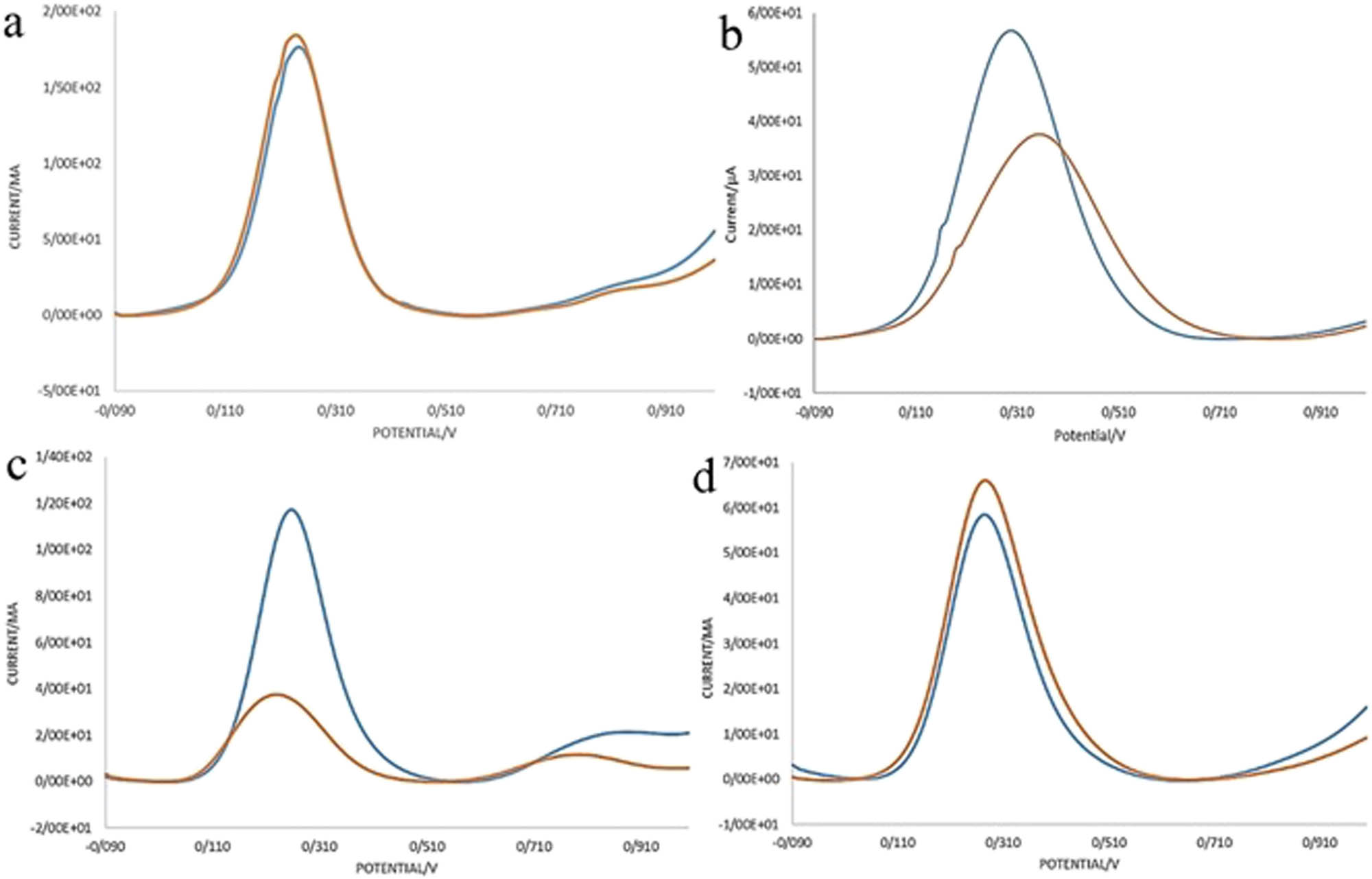
Selectivity of the herceptin-conjugated graphene biosensor and the comparison of different cell lines on the sensing performance of the bioelectrode: (a) MCF-7, (b) G-292, (c) SK-BR-3, and (d) HUVEC. In each graph, the blue and red curves are related to electrodes before and after depositing cells on them, respectively.
Apparently, the parameter ∆I/I for SK-BR-3 cells is more than 0/45 which is more than these data for other cell lines. This means in the SK-BR-3, cells adhered to the electrodes were more than other cells which showed the excellent selectivity and good affinity of the herceptin antibodies existed in the synthesized graphene to HER-2 receptors of SK-BR-3 cells (Figure 9).
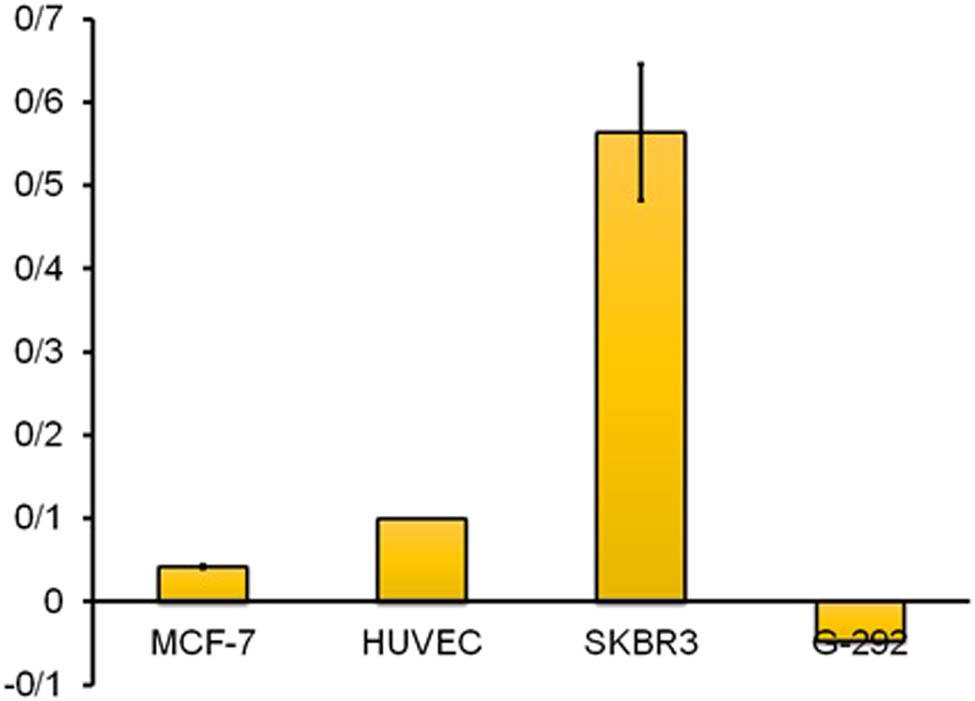
Effect of several cells on the electrode response and a comparison of the biosensor activity in the presence of the cells: MCF-7, G-292, SK-BR-3, and HUVEC.
3.5 Cancer biosensing analyses
The linear range of a biosensor usually shows the analyte concentration range in which the biosensor exhibits a good linear correspondent response. The amended electrochemical cell and bio-graphene electrode were applied to achieve the linear dynamic range of the immunosensor for detection of HER2+ overexpressed on the surface of SK-BR3 cell line (as a sample of breast cancer cells). To find the linear range of the biosensor, we put different concentrations of SK-BR-3 cells on the electrode surface so that the following results were reached by comparing the SQW graphs (Figure 10).
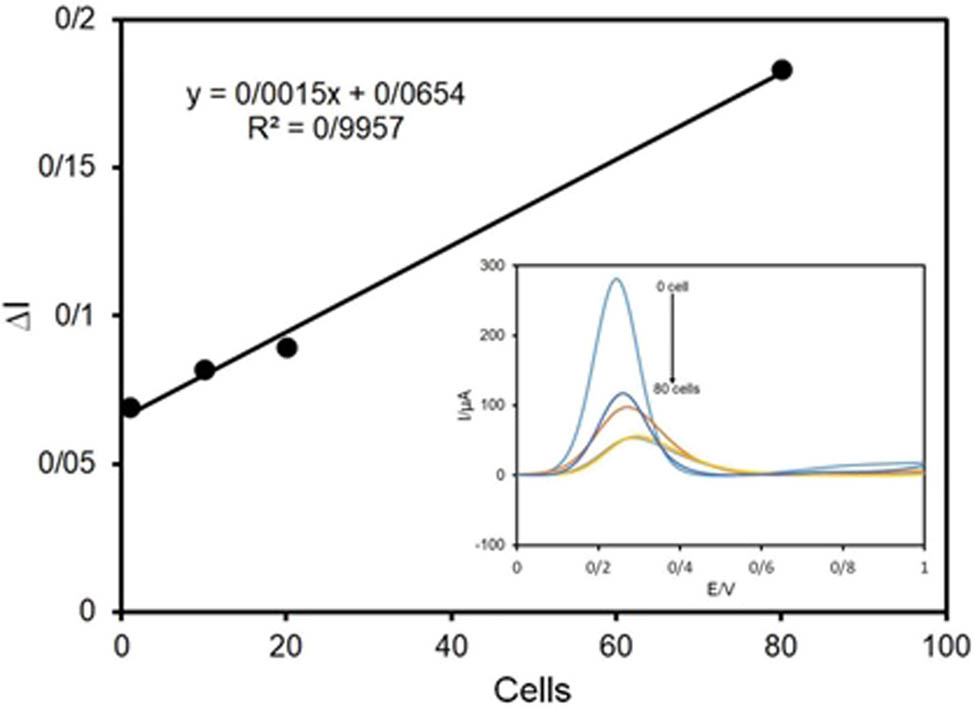
Calibration graph of the graphene-herceptin biosensor in the range of 1–80 cells.
As can be seen in Figure 10, this biosensor can detect cancer cells linearly with a R 2 = 0.9957 in the range of 1–80 cells which is a very successful result for developing this biosensor in real sample analysis (because in real samples (blood) of patients, there is low concentration of cancerous cells). Also, this biosensor had the potential to be tested in more concentrations above 80 cells.
Beside, in the case of reproducibility of this biosensor, six results in the concentration of 20 cells were compared. As a result, we found that the parameter ∆I/I is more than the numerical quantity of 0/45 for four of six tests. Moreover, the functionality or durability of this synthesized material was estimated for 40–50 days. As a result, beside simple and low-cost synthesis of the used hybrid material, this biosensor was able to be used in real sample detections and developments for better performances.
Differential pulse voltammetry (DPV) technique is used for biosensing measurements. However, the results of DPV technique are very similar to square wave voltammetry for biosensing evaluation and detection. Moreover, both of these techniques use the same procedure to sense the analyte. As mentioned, square wave voltammetry was used, which is a noble technique for biosensing measurements [50]. In biosensors, linear range is very important. In most biosensors such as glucometer, the maximal limit of quantification is very critical, because the amount of the analyte (glucose) is very vital to consider the diet and medication. But, in cancer detection, only minimal limit of quantification is very important for medical diagnosis and this platform is more sensitive than other devices in literature (the ability to detect single cell). Moreover, there is almost 15–20 cell/mL in blood of metastatic breast cancer (the worst case of cancer) [51]. Our platform can detect more than 80 cells, which is suitable for clinical applications.
4 Conclusion
The quick advances of nanotechnology and nano-science facilitate the fabrication of nanostructures-mediated biosensing devices with amended biosensing features for cancer diagnosis. Because of the increasing need to detect and quantify the chemical and biological species, electrochemical biosensors have been widely developed. These devices have been used for diagnosis and therapy of cancer owing to their properties such as portability, high sensitivity, specificity, fast response, and being user-friendly. Additionally, graphene, due to its unique features such as high surface area, conductivity in room temperature, and brilliant optical, mechanical, and thermal properties, is one of most used materials in electrochemical biosensors, which significantly increases the sensitivity of biosensors. However, detecting the single cell needs a new class of nanostructure-based device with excellent sensitivity and specificity. Biosensors with low limit of detections and fast responses can make this result feasible. In this project, we used a facile in situ synthesis of graphene-herceptin hybrid for applying this material for a SK-BR-3 cell biosensor. Selectivity tests were successfully carried out which showed excellent results over the potential electroactive cells presented in real samples. The calibration test exhibited a linear dynamic detection in the range of 1–80 cells. Moreover, it was observed that for SK-BR-3 cells, the parameter ∆I/I was more than 0/45. Reproducibility of the biosensor was estimated about 66% which can be passed by programming the processor to ignore data with ∆I/I less than 0/45. Also, stability and functionality of this material were about 40–50 days. The obtained results proved that this material is a promising candidate for rapid and selective detection of cancer cells.
Acknowledgements
The authors would like to acknowledge and thank the Cancer Control Research Center, Cancer Control Foundation, Iran University of Medical Sciences, Tehran, Iran for supporting and helping this study (project No.: CCF-97052).
-
Funding information: This study has been funded by the Cancer Control Research Center, Cancer Control Foundation, Iran University of Medical Sciences, Tehran, Iran, as project. No.: CCF-97052.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Hasanzadeh M, Shadjou N, de la Guardia M. Early stage screening of breast cancer using electrochemical biomarker detection. TrAC Trends Anal Chem. 2017;91:67–76.10.1016/j.trac.2017.04.006Search in Google Scholar
[2] Kazemi F, Naghib SM, Zare Y, Rhee KY. Biosensing applications of polyaniline (PANI)-based nanocomposites: a review. Polym Rev. 2020;61:1–45.10.1080/15583724.2020.1858871Search in Google Scholar
[3] Haghiralsadat F, Amoabediny G, Naderinezhad S, Nazmi K, De Boer JP, Zandieh-Doulabi B, et al. EphA2 targeted doxorubicin-nanoliposomes for osteosarcoma treatment. Pharm Res. 2017;34:2891–900.10.1007/s11095-017-2272-6Search in Google Scholar PubMed
[4] Haghiralsadat F, Amoabediny G, Naderinezhad S, Forouzanfar T, Helder MN, Zandieh-Doulabi B. Preparation of PEGylated cationic nanoliposome-siRNA complexes for cancer therapy. Artif Cell Nanomed Biotechnol. 2018;46:684–92.10.1080/21691401.2018.1434533Search in Google Scholar PubMed
[5] Ranjan P, Parihar A, Jain S, Kumar N, Dhand C, Murali S, et al. Biosensor-based diagnostic approaches for various cellular biomarkers of breast cancer: a comprehensive review. Anal Biochem. 2020;610:113996.10.1016/j.ab.2020.113996Search in Google Scholar PubMed
[6] Cardoso AR, Moreira FTC, Fernandes R, Sales MGF. Novel and simple electrochemical biosensor monitoring attomolar levels of miRNA-155 in breast cancer. Biosens Bioelectron. 2016;80:621–30.10.1016/j.bios.2016.02.035Search in Google Scholar PubMed PubMed Central
[7] Kazemi F, Naghib SM, Mohammadpour Z. Multifunctional micro-/nanoscaled structures based on polyaniline: an overview of modern emerging devices. Mater Today Chem. 2020;16:100249.10.1016/j.mtchem.2020.100249Search in Google Scholar
[8] Naseri M, Fotouhi L, Ehsani A. Recent progress in the development of conducting polymer-based nanocomposites for electrochemical biosensors applications: a mini-review. Chem Rec. 2018;18:599–618.10.1002/tcr.201700101Search in Google Scholar PubMed
[9] Rahimzadeh Z, Naghib SM, Zare Y, Rhee KY. An overview on the synthesis and recent applications of conducting poly (3, 4-ethylenedioxythiophene)(PEDOT) in industry and biomedicine. J Mater Sci. 2020;55:1–37.10.1007/s10853-020-04561-2Search in Google Scholar
[10] Mittal S, Kaur H, Gautam N, Mantha AK. Biosensors for breast cancer diagnosis: a review of bioreceptors, biotransducers and signal amplification strategies. Biosens Bioelectron. 2017;88:217–31.10.1016/j.bios.2016.08.028Search in Google Scholar PubMed
[11] Askari E, Rasouli M, Darghiasi SF, Naghib SM, Zare Y, Rhee KY. Reduced graphene oxide-grafted bovine serum albumin/bredigite nanocomposites with high mechanical properties and excellent osteogenic bioactivity for bone tissue engineering. Bio-Design Manuf. 2021;4:243–57.10.1007/s42242-020-00113-4Search in Google Scholar
[12] Askari E, Naghib SM, Zahedi A, Seyfoori A, Zare Y, Rhee KY. Local delivery of chemotherapeutic agent in tissue engineering based on gelatin/graphene hydrogel. J Mater Res Technol. 2021;12:412–22.10.1016/j.jmrt.2021.02.084Search in Google Scholar
[13] Kalantari E, Naghib SM, Iravani NJ, Esmaeili R, Naimi-Jamal MR, Mozafari M. Biocomposites based on hydroxyapatite matrix reinforced with nanostructured monticellite (CaMgSiO4) for biomedical applications: Synthesis, characterization and biological studies. Mater Sci Eng C. 2019;105:109912. 10.1016/j.msec.2019.109912.Search in Google Scholar PubMed
[14] Kalantari E, Naghib SM. A comparative study on biological properties of novel nanostructured monticellite-based composites with hydroxyapatite bioceramic. Mater Sci Eng C. 2019;98:1087–96.10.1016/j.msec.2018.12.140Search in Google Scholar PubMed
[15] Kalantari E, Naghib SM, Naimi-Jamal MR, Aliahmadi A, Iravani NJ, Mozafari M. Nanostructured monticellite for tissue engineering applications – Part I: Microstructural and physicochemical characteristics. Ceram Int. 2018;44:12731–38.10.1016/j.ceramint.2018.04.076Search in Google Scholar
[16] Kalantari E, Naghib SM, Iravani NJ, Aliahmadi A, Naimi-Jamal MR, Mozafari M. Nanostructured monticellite for tissue engineering applications – Part II: molecular and biological characteristics. Ceram Int. 2018;44:14704–11.10.1016/j.ceramint.2018.05.098Search in Google Scholar
[17] George JM, Antony A, Mathew B. Metal oxide nanoparticles in electrochemical sensing and biosensing: a review. Microchim Acta. 2018;185:1–26.10.1007/s00604-018-2894-3Search in Google Scholar PubMed
[18] Naghib SM, Rabiee M, Omidinia E. Electroanalytical validation of a novel nanobiosensing strategy and direct electrochemistry of phenylalanine dehydrogenase for clinical diagnostic applications. Int J Electrochem Sci. 2014;9:2301–15.10.1016/S1452-3981(23)07928-2Search in Google Scholar
[19] Naghib SM, Rabiee M, Omidinia E, Khoshkenara P, Zeini D. Biofunctionalization of dextran-based polymeric film surface through enzyme immobilization for phenylalanine determination. Int J Electrochem Sci. 2012;7:120–35.10.1016/S1452-3981(23)13325-6Search in Google Scholar
[20] Doria G, Conde J, Veigas B, Giestas L, Almeida C, Assunção M, et al. Noble metal nanoparticles for biosensing applications. Sensors. 2012;12:1657–87.10.3390/s120201657Search in Google Scholar PubMed PubMed Central
[21] Amani J, Khoshroo A, Rahimi-Nasrabadi M. Electrochemical immunosensor for the breast cancer marker CA 15–3 based on the catalytic activity of a CuS/reduced graphene oxide nanocomposite towards the electrooxidation of catechol. Microchim Acta. 2018;185:79.10.1007/s00604-017-2532-5Search in Google Scholar PubMed
[22] Naghib SM. Fabrication of nafion/silver nanoparticles/reduced graphene nanosheets/glucose oxidase nanobiocomposite for electrochemical glucose biosensing. Anal Bioanal Electrochem. 2016;8:453–65.Search in Google Scholar
[23] Zhou Z, Li C, Zhu R, Wang D, Liu T, Jia J, et al. Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide. Nanotechnol Rev. 2020;9:17–27.10.1515/ntrev-2020-0002Search in Google Scholar
[24] Du S, Chen H, Hong R. Preparation and electromagnetic properties characterization of reduced graphene oxide/strontium hexaferrite nanocomposites. Nanotechnol Rev. 2020;9:105–14.10.1515/ntrev-2020-0010Search in Google Scholar
[25] Behdinan K, Moradi-Dastjerdi R, Safaei B, Qin Z, Chu F, Hui D. Graphene and CNT impact on heat transfer response of nanocomposite cylinders. Nanotechnol Rev. 2020;9:41–52.10.1515/ntrev-2020-0004Search in Google Scholar
[26] Liang W, Cai K, Chen C, Chen H, Chen Q, Fu J, et al. Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide. Nanotechnol Rev. 2020;9:155–69.10.1515/ntrev-2020-0014Search in Google Scholar
[27] Du M, Jing H, Gao Y, Su H, Fang H. Carbon nanomaterials enhanced cement-based composites: advances and challenges. Nanotechnol Rev. 2020;9:115–35.10.1515/ntrev-2020-0011Search in Google Scholar
[28] Basu S, Sen K. A review on graphene-based materials as versatile cancer biomarker sensors. Front Mater Sci. 2020; 14:1–20.10.1007/s11706-020-0530-8Search in Google Scholar
[29] Hossain MB, Akib TBA, Abdulrazak LF, Rana MM. Numerical modeling of graphene-coated fiber optic surface plasmon resonance biosensor for BRCA1 and BRCA2 genetic breast cancer detection. Opt Eng. 2019;58:37104.10.1117/1.OE.58.3.037104Search in Google Scholar
[30] Song Y, Luo Y, Zhu C, Li H, Du D, Lin Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens Bioelectron. 2016;76:195–212.10.1016/j.bios.2015.07.002Search in Google Scholar PubMed
[31] Tian L, Qi J, Qian K, Oderinde O, Cai Y, Yao C, et al. An ultrasensitive electrochemical cytosensor based on the magnetic field assisted binanozymes synergistic catalysis of Fe3O4 nanozyme and reduced graphene oxide/molybdenum disulfide nanozyme. Sens Actuators B Chem. 2018;260:676–84.10.1016/j.snb.2018.01.092Search in Google Scholar
[32] Soleymani J, Hasanzadeh M, Somi MH, Ozkan SA, Jouyban A. Targeting and sensing of some cancer cells using folate bioreceptor functionalized nitrogen-doped graphene quantum dots. Int J Biol Macromol. 2018;118:1021–34.10.1016/j.ijbiomac.2018.06.183Search in Google Scholar PubMed
[33] Yadegari A, Omidi M, Yazdian F, Zali H, Tayebi L. An electrochemical cytosensor for ultrasensitive detection of cancer cells using modified graphene–gold nanostructures. RSC Adv. 2017;7:2365–72.10.1039/C6RA25938CSearch in Google Scholar
[34] Ruiyi L, Fangchao C, Haiyan Z, Xiulan S, Zaijun L. Electrochemical sensor for detection of cancer cell based on folic acid and octadecylamine-functionalized graphene aerogel microspheres. Biosens Bioelectron. 2018;119:156–62.10.1016/j.bios.2018.07.060Search in Google Scholar PubMed
[35] Arya SK, Wang KY, Wong CC, Rahman ARA. Anti-EpCAM modified LC-SPDP monolayer on gold microelectrode based electrochemical biosensor for MCF-7 cells detection. Biosens Bioelectron. 2013;41:446–51.10.1016/j.bios.2012.09.006Search in Google Scholar PubMed
[36] Yang Y, Fu Y, Su H, Mao L, Chen M. Sensitive detection of MCF-7 human breast cancer cells by using a novel DNA-labeled sandwich electrochemical biosensor. Biosens Bioelectron. 2018;122:175–82.10.1016/j.bios.2018.09.062Search in Google Scholar PubMed
[37] Salahandish R, Ghaffarinejad A, Naghib SM, Majidzadeh AK, Zargartalebi H, Sanati-Nezhad A. Nano-biosensor for highly sensitive detection of HER2 positive breast cancer. Biosens Bioelectron. 2018;117:104–11.10.1016/j.bios.2018.05.043Search in Google Scholar PubMed
[38] Liu N, Song J, Lu Y, Davis JJ, Gao F, Luo X. Electrochemical aptasensor for ultralow fouling cancer cell quantification in complex biological media based on designed branched peptides. Anal Chem. 2019;91:8334–40.10.1021/acs.analchem.9b01129Search in Google Scholar PubMed
[39] Nasrollahpour H, Mahdipour M, Isildak I, Rashidi MR, Naseri A, Khalilzadeh B. A highly sensitive electrochemiluminescence cytosensor for detection of SKBR-3 cells as metastatic breast cancer cell line: a constructive phase in early and precise diagnosis. Biosens Bioelectron. 2021;178:113023.10.1016/j.bios.2021.113023Search in Google Scholar PubMed
[40] Askari E, Naghib SM, Seyfoori A, Maleki A, Rahmanian M. Ultrasonic-assisted synthesis and in vitro biological assessments of a novel herceptin-stabilized graphene using three dimensional cell spheroid. Ultrason Sonochem. 2019;58:104615.10.1016/j.ultsonch.2019.104615Search in Google Scholar PubMed
[41] Shamsipur M, Chabok A, Molaabasi F, Seyfoori A, Hajipour-Verdom B, Shojaedin-Givi B, et al. Label free phosphate functionalized semiconducting polymer dots for detection of iron(iii) and cytochrome c with application to apoptosis imaging. Biosens Bioelectron. 2019;141:111337.10.1016/j.bios.2019.111337Search in Google Scholar PubMed
[42] Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, et al. Improved synthesis of graphene oxide. ACS Nano. 2010;4:4806–14.10.1021/nn1006368Search in Google Scholar PubMed
[43] Choi W, Lahiri I, Seelaboyina R, Kang YS. Synthesis of graphene and its applications: a review. Crit Rev Solid State Mater Sci. 2010;35:52–71.10.1080/10408430903505036Search in Google Scholar
[44] Bhuyan MSA, Uddin MN, Islam MM, Bipasha FA, Hossain SS. Synthesis of graphene. Int Nano Lett. 2016;6:65–83.10.1007/s40089-015-0176-1Search in Google Scholar
[45] Guo H-L, Wang X-F, Qian Q-Y, Wang FB, Xia XH. A green approach to the synthesis of graphene nanosheets. ACS Nano. 2009;3:2653–9.10.1021/nn900227dSearch in Google Scholar
[46] Suslick KS. Sonochemistry. Science (80-). 1990;247:1439–45.10.1126/science.247.4949.1439Search in Google Scholar
[47] Yang L, Tucker D, Dong Y, Wu C, Lu Y, Li Y, et al. A non-dispersion strategy for large-scale production of ultra-high concentration graphene slurries in water. Nat Commun. 2018;299:9–96.10.1038/s41467-017-02580-3Search in Google Scholar
[48] Khan U, O'neill A, Lotya M, De S, Coleman JN. High-concentration solvent exfoliation of graphene. Small. 2010;6:864–71.10.1002/smll.200902066Search in Google Scholar
[49] Liu J, Fu S, Yuan B, Li Y, Deng Z. Toward a universal “adhesive nanosheet” for the assembly of multiple nanoparticles based on a protein-induced reduction/decoration of graphene oxide. J Am Chem Soc. 2010;132:7279–81.10.1021/ja100938rSearch in Google Scholar
[50] Crowley K, Cassidy J. Trace analysis of lead at a nafion-modified electrode using square-wave anodic stripping voltammetry. Electroanalysis. 2002;14:1077–82.10.1002/1521-4109(200208)14:15/16<1077::AID-ELAN1077>3.0.CO;2-3Search in Google Scholar
[51] Miller MC, Doyle GV, Terstappen LWMM. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421.10.1155/2010/617421Search in Google Scholar
© 2021 Zahra Rahimzadeh et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

