Abstract
This research addresses the asphalt smoke emission in the process of asphalt pavement construction. The nano-graphene oxide (GO)/tourmaline composites were prepared to alleviate the asphalt smoke emission and improve the construction environment. The macrocharacteristics and micromorphology of the composites were analyzed, and their optimal preparation process was determined. Using material microanalysis methods, such as X-ray diffractometer, Raman spectroscopy, and Fourier transform infrared spectrometer, the structural characteristics and material composition of the composites were studied. The adsorption properties of the composites on asphalt smoke were clarified. It will provide technical support for the improvement of infrastructure construction environment. The results show that 3-aminopropyl triethoxysilane is superior than hexadecyl trimethyl ammonium bromide in surface modification of tourmaline. During the composite process, surface modifiers and GO had no significant effect on the structure of tourmaline. GO could enhance the adsorption properties of tourmaline on asphalt smoke. When the GO content was 1.5 wt%, the improvement was the largest, which is 17.42%. At that time, the emission-reduction rate of asphalt smoke reached 41.11%.
1 Introduction
Transportation infrastructure plays a vital role in human life and world economic development [1]. Asphalt pavement is the main component of transportation infrastructure. During the construction process of asphalt pavement, asphalt is heated to high temperature, which will release a large amount of asphalt smoke [2]. This endangers the health of staff and causes pollution to the surrounding environment [3,4]. In recent years, the researches on emission reduction in the construction of asphalt pavement have gradually received more attention [5,6,7,8,9]. In response to this, scholars have proposed the modifiers suitable for traditional hot-mix asphalt mixtures that can effectively adsorb asphalt smoke [10]. It provides an effective way to solve the above-stated technical problems, but it needs to be further studied.
Among many modifiers with adsorption properties, tourmaline inorganic particle is unique and has been widely studied and applied in the field of environmental protection [11,12]. Scholars have applied it to the field of road materials and studied the pavement performances and environmental effects of its modified asphalt and mixtures [13,14]. Ding et al. analyzed the microstructure, chemical composition, and thermal properties of the tourmaline-modified asphalt [15]. Ye et al. studied the rheological characteristics and aging resistance of tourmaline-modified asphalt [16]. Zhao et al. and Zhu investigated the modification mechanism of tourmaline powder on base asphalt [17,18]. Wang et al. and Chen et al. prepared tourmaline-modified asphalt mixture with environmental effects and systematically evaluated its emission-reduction efficiency [19,20,21,22]. The other study was conducted to investigate the functional, piezoelectric, and thermoelectric properties of a tourmaline-modified asphalt binder and the corresponding asphalt mixtures [23]. The above researches shows that when tourmaline is used in modified asphalt mixture, it can give its emission-reduction effect without losing pavement performance. Moreover, the higher the content of tourmaline, the better its adsorption properties to asphalt smoke. When the content of tourmaline is 20−25 wt% of asphalt, the emission-reduction rate of asphalt smoke can reach 40−50%. However, the high content (>20 wt%) of tourmaline will cause the deterioration of the service performance of asphalt pavement. Therefore, how to further improve the adsorption properties of tourmaline at low content (about 15 wt%) is a research topic worth exploring.
Li et al. discussed the efficient adsorption behavior of tourmaline and the ion exchange in the process [24]. Li et al. prepared tourmaline/g-C3N4/BiVO4 composites to enhance the adsorption effect of tourmaline on pollutants [25]. Chen et al. studied the adsorption and its mechanism of Pb(II) by tourmaline-montmorillonite composite [26]. Li et al. studied the charge transfer law in tourmaline composites [27]. Wang et al. analyzed the correlation between the amount of negative ions released by tourmaline and the band gap, carrier concentration, and mobility, respectively [28]. The current research shows that the adsorption properties of tourmaline mainly rely on the released negative ions to adsorb pollutants [29]. Its electrical properties are the key indicators and need to be further improved.
One of the key technical approaches to improve the negative ion release and adsorption properties of tourmaline is to enhance its electrical properties. Graphene (G) and graphene oxide (GO) have significant conductivity [30]. They have been widely used in composites, nano-electronic devices, catalyst carriers, sensors, and energy storage devices [31,32,33]. The modification of G or GO can effectively improve the electrical properties and surface activity of inorganic particles [34]. The prepared high-performance composites have excellent application prospects. If the excellent electrochemical properties of G or GO can be used, the adsorption properties of tourmaline will be further improved. Considering the economic cost of the material, GO is preferred as the modified material in this research.
At present, there are few reports on the preparation methods of GO/tourmaline composites, but tourmaline belongs to inorganic particles. The preparation methods of inorganic particle/GO composites can be referenced. Their main preparation methods were electrostatic self-assembly, hydrothermal synthesis, high-energy ball milling, sol–gel, melting metallurgy, and chemical deposition [35,36,37,38,39]. These studies indicated that the processes of hydrothermal synthesis and electrostatic self-assembly are similar, but the former needs high-pressure reactor and other containers. In sol–gel process, the precursor is used to form oxides and then assembled together. Hydrothermal synthesis, sol–gel, melting metallurgy, and chemical deposition have a narrow application range, high cost, and high process requirements. They are not suitable for large-scale promotion and application. Based on this, the electrostatic self-assembly and high-energy ball milling are mainly concerned.
According to the above-stated motivation, the primary objective of this research is to further enhance the adsorption properties of tourmaline on asphalt smoke, thereby significantly improving the construction environment of asphalt pavement. The surface modifier of tourmaline with obvious effect was selected by the zeta potential. The preparation method of composites was proposed, and GO/tourmaline composites were prepared. The macrocharacteristics and micromorphology of composites were analyzed, and the optimal preparation process was determined. Based on material microanalysis methods, the structural characteristics and material composition of composites were studied. The adsorption properties of asphalt smoke of composites were clarified. It laid a solid foundation for further promotion and application of tourmaline in the field of environmental friendly materials.
2 Experiment materials and methods
The surface of tourmaline was modified by modifiers, which changed the charge property of tourmaline in aqueous solution. The positively charged modified tourmaline and negatively charged GO were combined by an electrostatic attraction among particles. The exfoliated GO adhered to large-sized tourmaline particles. In addition, nano-sized tourmaline can be intercalated between GO layers. Finally, GO/tourmaline composites were prepared.
2.1 Main raw materials
Tourmaline is a kind of borosilicate mineral with a ring structure, and its chemical formula was Na(Mg,Fe,Mn,Li,Al)3Al6[Si6O18][BO3]3(OH,F)4. It was supplied by Hebei Lingshou Tourmaline Co., Ltd (Industrial Zone, Shijiazhuang City, Hebei Province, China), and its parameters are shown in Table 1.
Parameters of tourmaline
| Material | Color | Mohs hardness | Negative ion release | Emissivity (ε) | Fineness |
|---|---|---|---|---|---|
| Tourmaline | Black powder | 7.0–7.2 | 800–1,000 cm−3 | 84–94 | 2,000 mesh |
GO was prepared from natural graphite by optimized Hummers method. It was supplied by Suzhou Tanfeng Graphene Technology Co., Ltd (Suzhou City, Jiangsu Province, China), and its parameters are shown in Table 2. According to efficacy improvement and material cost, the content of GO was determined as 0.5–1.5 wt% of tourmaline [40].
Parameters of GO
| Material | Purity | Thickness | Lamella diameter | Number of plies | Specific surface area | Morphology | Color |
|---|---|---|---|---|---|---|---|
| GO | >95 wt% | 3.4–7 nm | 10–50 μm | 6–10 | 100–300 m2 g−1 | Powder | Dark brown |
In this research, the electrostatic self-assembly method was used. G materials and tourmaline have the same negative potential. Therefore, some modifiers are needed to change the potential of the material surface. The surface of tourmaline was modified by two kinds of commonly used modifiers: (1) 3-aminopropyl triethoxysilane modifier (KH-550) was colorless and transparent liquid and was sensitive to humidity and corrosive. (2) Hexadecyl trimethyl ammonium bromide modifier (cetyltrimethylammonium bromide [CTAB]) was a white powder, which is soluble in water and produced a large amount of foam during concussion. It had obvious compatibility with cationic, nonionic, and amphoteric surfactant modifiers. They were supplied by Shaanxi Xinhui Chemical Glass Instrument Co., Ltd (Xi'an City, Shaanxi Province, China), and their parameters are shown in Table 3.
Parameters of surface modifier
| Material | Alternative name | CAS NO | Molecular formula | Molecular weight |
|---|---|---|---|---|
| 3-Aminopropyl triethoxysilane | KH-550 | 919-30-2 | C9H23NO3Si | 221.37 |
| Hexadecyl trimethyl ammonium bromide | CTAB | 57-09-0 | CH3(CH2)15N(Br)(CH3)3 | 364.45 |
Anhydrous ethanol was a colorless and clear liquid. It was easily soluble in water and can be miscible in water and various organic solvents in any proportion. It was used as a solvent in the powder material-modification process or as an auxiliary agent in the ball milling process. It was supplied by Shaanxi Xinhui Chemical Glass Instrument Co., Ltd, and its parameters are shown in Table 4.
Parameters of anhydrous alcohol
| Material | Melting point | Relative density | Boiling point | Relative vapor density | Molecular weight | Ignition temperature |
|---|---|---|---|---|---|---|
| Anhydrous alcohol | −114.1°C | 0.79 g cm−3 | 78.3°C | 1.59 g cm−3 | 46.07 | 363°C |
2.2 Preparation method
The surface modifier with a better effect was selected. Based on this, GO/tourmaline composites were prepared. The preparation process of GO1.0/T100 (GO:T = 1:100, weight ratio) was taken as an example. The preparation process was as follows.
2.2.1 Assembly of suction filtration device
Buchner funnel, air compressor, filtering flask, wide-mouth bottle, and filter paper were used to assemble the suction filtration device. It was used for the filtration and purification of modified powder.
2.2.2 Surface modification of tourmaline
(1) About 100 g tourmaline was added to 800 mL absolute ethanol to obtain a suspension. It was stirred at a high speed (500 rpm) in a thermostatically heated magnetic stirrer for 20 min to obtain a uniformly mixed tourmaline suspension; (2) about 100 mL absolute ethanol and 100 mL water were mixed in a ratio of 1:1. About 20 g surface modifier was added into the mixed solution of absolute ethanol and water. After the mixture was well stirred, it was added to the tourmaline suspension in batches; (3) the above mixed suspension was stirred at 50°C for 6 h. After the reaction, the mixed solution was filtered. Absolute ethanol and deionized water were used to repeatedly wash the filtrated products; and (4) the obtained filter cake was dried at 80°C and milled by a planetary ball mill at low speed (200 rpm) for 10 min. The modified tourmaline was obtained through a 200-mesh sieve.
2.2.3 Dispersion of GO
The dispersive aqueous solution of GO with a concentration of 1 mg mL−1 was prepared. The prepared GO aqueous solution was ultrasonically vibrated at room temperature for 3 h in an ultrasonic dispersant to obtain a uniformly dispersed GO suspension, which was put into a reagent bottle for use.
2.2.4 Preparation of GO/tourmaline composites
(1) The modified tourmaline was added to deionized water and stirred at high speed to prepare a uniform suspension of modified tourmaline. The content of modified tourmaline was adjusted to 10 wt%. It was weighed 200 g for standby; (2) about 200 mL GO suspension was slowly added to the modified tourmaline suspension under high-speed vibration and stirring; (3) after adding, the stirring speed was maintained, and the reaction was set for 2 h. The filtered products were collected and repeatedly washed with deionized water; and (4) the obtained filter cake was dried at 80°C and milled by planetary ball mill at low speed (200 rpm) for 10 min to make composite powder redispersible evenly. GO/tourmaline composite was obtained through a 200-mesh sieve.
2.3 Experiment equipment and methods
2.3.1 Zeta potential tester
The total number of particles at different potentials was measured by zeta potential tester (Figure 1). After data processing, zeta potential distribution diagrams of materials in solvent were obtained. The instrument was Zetasizer Nano S90 produced by Malvern company, UK. The measurement range of zeta potential was 0–150 mV. The measurement range of particle size was 3 nm to 10 μm. The test temperature was 25°C.
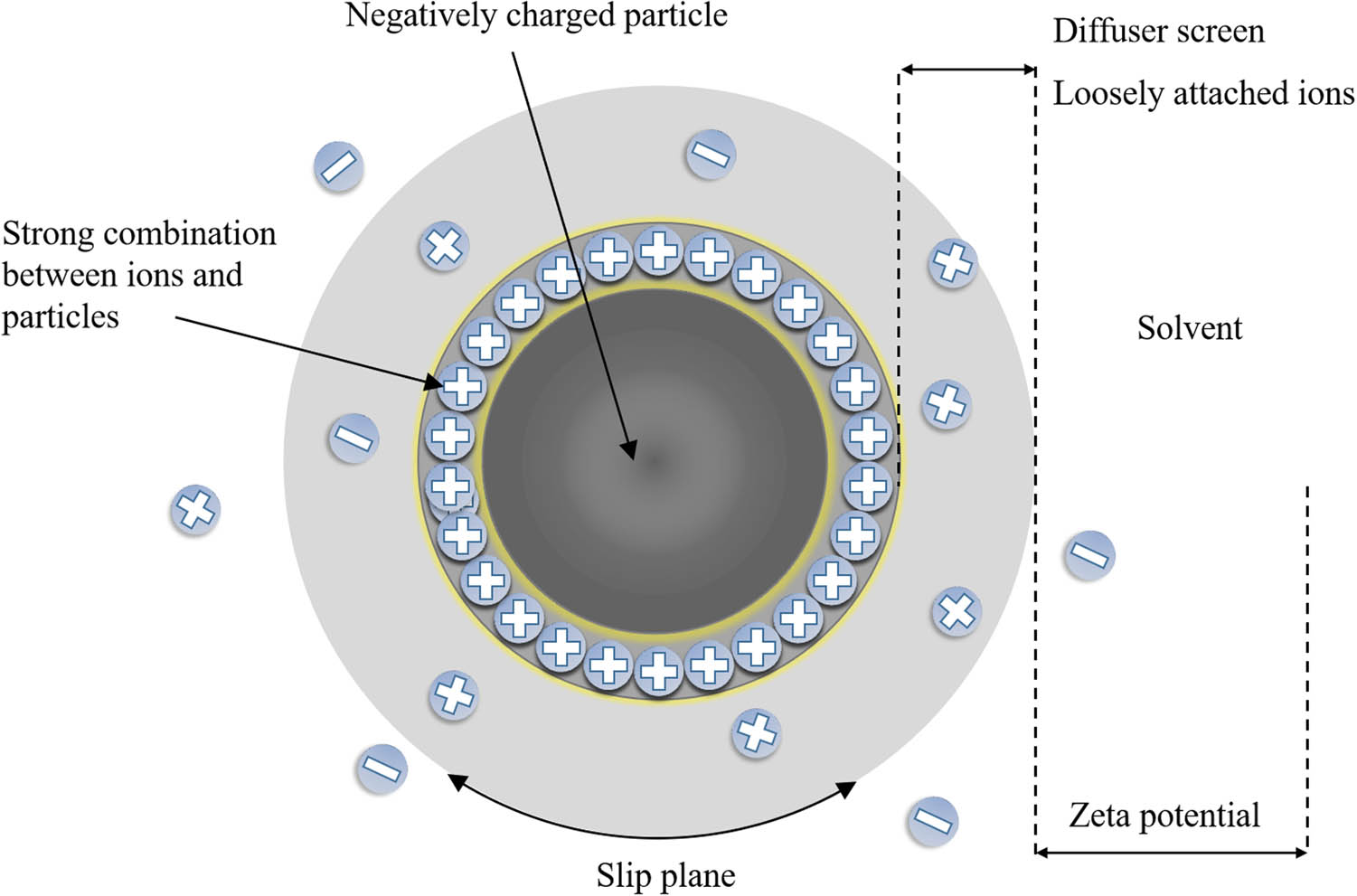
Schematic of zeta potential.
2.3.2 Field-emission scanning electron microscopy
It was mainly used to observe the fine morphology, fracture, and internal structure of materials. The instrument was Verios 460 scanning electron microscope (SEM) produced by FEI (Field Electron and Ion Co.) company, USA. The resolution of secondary electronic image was 1.0–1.4 nm, and the magnification was 20–800,000. In addition, the X-ray energy-dispersive spectrometer equipped with the instrument was used for qualitative and semiquantitative analysis of sample composition and the analysis element range was Be4-U92.
2.3.3 X-ray diffractometer (XRD)
It was mainly used for qualitative and quantitative analyses of various materials. The internal structure and morphology of materials were obtained by analyzing the diffraction patterns. To improve the accuracy of results, it was necessary to grind the powder before the test. The instrument was D8 XRD produced by Bruker AXS in Germany. The equipment used Cu target ray source. The scanning range was 10–80°. The angle accuracy was 0.0001°. The scanning speed was 4° min−1. The step size was 0.02°.
2.3.4 Laser microscopic confocal Raman spectrometry
It was mainly used for the determination and confirmation of material composition. The test material was placed under the Raman spectrometer, and the spectrum was tested from different points of the sample. The instrument type was Renishaw-inVia. The laser wavelength was 532 nm, the measurement range was 100–4,000 cm−1, and the accuracy was 2 cm−1.
2.3.5 Fourier transform infrared (FT-IR) spectrometry
FT-IR was used to determine the molecular structure of substances. The experiment materials should be dried. After drying 0.2 mg experiment materials and 150 mg KBr at 105°C for 3 h, they were ground and mixed evenly with a mortar. A small amount of mixed powder was put into the die, and the sample was prepared by holding the pressure of 10 t cm−2 for 2 min. The instrument was ALPHA II FT-IR produced by Bruker Optics in Germany. SNR was 50,000:1. The resolution was 0.8 cm−1. The wavenumber range was 7,500–375 cm−1.
2.3.6 Adsorption test of asphalt smoke
According to GB 31570–2015 [41], asphalt smoke concentration was characterized. The adsorption properties of tourmaline and its composites on asphalt smoke were evaluated by analyzing the change of asphalt smoke concentration. The recommended content of tourmaline materials was 15 wt% [21]. As the absorption solution, 20 mL benzene solution was added into the absorption bottle. Then, asphalt (SK-70#) was heated to 160°C to produce asphalt smoke. The air sampler was used to extract asphalt smoke into the absorption bottle. The collection speed was 0.5 L min−1, and the collection time was 0.5–2 h. Next, the benzene-asphalt smoke solution in the absorption bottle was poured into the evaporating dish. It was dried (80°C) and weighed. The concentration of asphalt smoke was calculated by equation (1).
where α was concentration of asphalt smoke, mg m−3; m 1 was weight of evaporating dish after drying, g; m 0 was weight of evaporating dish, g; and t was collection time of asphalt smoke, min.
3 Results and discussion
3.1 Selection of surface modifier
The key of electrostatic self-assembly was the surface modification of inorganic particles. Inorganic particles and GO should have opposite electrical properties in solution. Zeta potentials of G and GO were measured. The changes of zeta potential before and after tourmaline modification were compared. The test results were shown in Figures 2 and 3 and Table 5. The suitable surface-modification scheme of tourmaline was determined. For standard writing, KH-550-modified tourmaline was abbreviated as KT, and CTAB-modified tourmaline was abbreviated as CT.
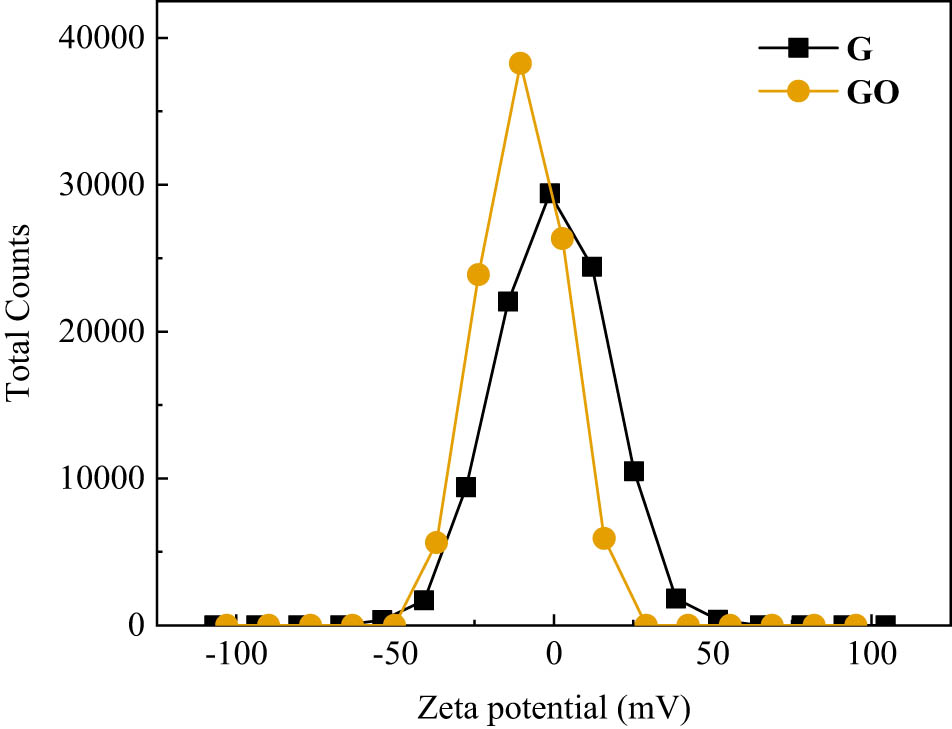
Zeta potential distribution of G and GO.
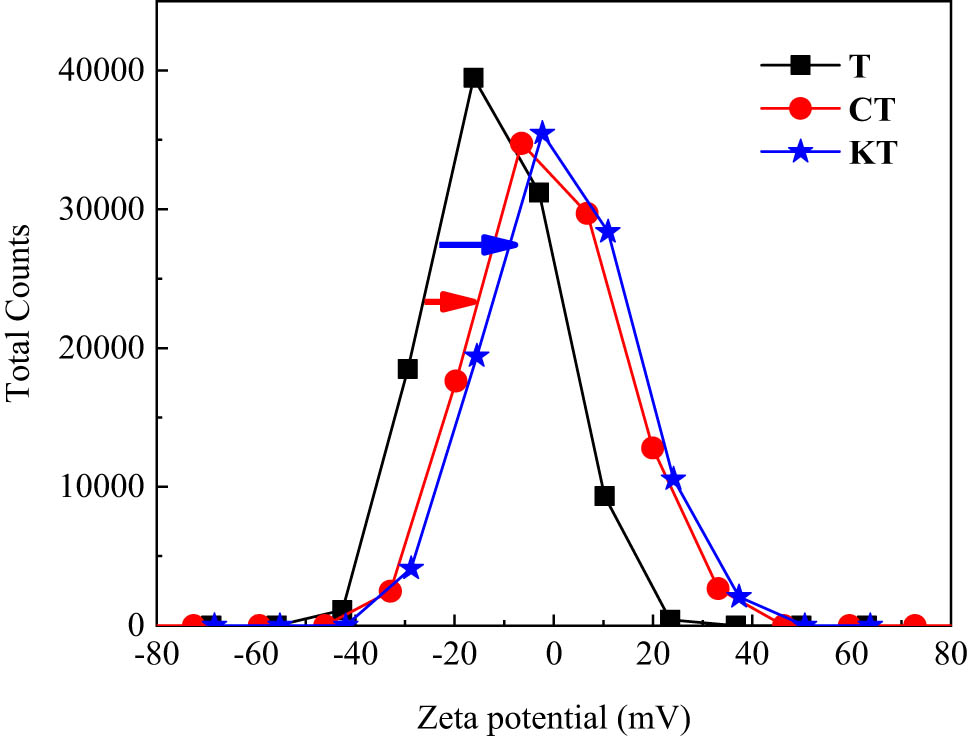
Zeta potential distribution of tourmaline before and after modification.
Zeta potential test results
| Test object | G | GO | T | CT | KT |
|---|---|---|---|---|---|
| Zeta potential (mV) | −0.54 | −10.2 | −12.22 | −1.13 | +1.41 |
According to Figure 2 and Table 5, G was approximately electrically neutral in the solvent, and the absolute value of zeta potential was relatively small. Compared with G, the potential distribution of GO shifted to the left, and the zeta potential was −10.2 mV. It indicated that G is not suitable for the preparation of composites by electrostatic self-assembly. The final zeta electrical change of KT showed that KH-550-modified tourmaline and GO meet the basic conditions for electrostatic self-assembly of composite materials. From Figure 3, the potential of tourmaline in solvent was obviously lower than 0, whereas the zeta potential after surface modification shifted to the right. KT was superior than CT in changing the whole electrical property. According to zeta potential in Table 5, KH-550 changed the surface potential of tourmaline from −12.22 to +1.41 mV. This confirmed the judgment that KH-550 has a better modification effect. It could be inferred that there is a strong electrostatic adsorption between GO and KT after mixing. The electrostatic effect made KT adsorb GO. In addition, the two were combined by the reaction of chemical groups. This allowed a single layer or a few layers of GO to be coated on KT surface, thereby obtaining GO/KT composites. Therefore, silane coupling agent (KH-550) was selected as the final surface modifier of tourmaline.
In the preparation of GO/KT composite, KH-550 was hydrolyzed in water, and the generated silanol reacted with the hydroxyl groups on the surface of tourmaline to form ether bond. This formed a strong chemical connection and changed the electrical properties of tourmaline surface. There were a large number of oxygen-containing functional groups on the surface of GO, such as carboxyl groups and phenolic hydroxyl groups. In the aqueous solution, these groups were prone to ionization, which made the surface of GO negative [42]. This made the modified tourmaline, and GO can be synthesized by electrostatic self-assembly. In addition, the methoxyl group at one end of KH-550 can be hydrolyzed to form silicon hydroxyl group, which condensed with the hydroxyl group on the tourmaline surface. The amino groups at the other end of KH-550 can react with epoxy functional groups on the GO surface [43]. KH-550 acted as a bridge to connect GO to the surface of tourmaline by a chemical bond.
3.2 Morphology characteristics of composites
3.2.1 Macrophenomenon
Figure 4 showed the powder photos of tourmaline, KH-550-modified tourmaline, and GO/tourmaline composites. The surface morphology of tourmaline was not changed by KH-550. The overall appearance was still gray and white. This was because the tourmaline samples come from raw ore, and there are gray white powder impurities in tourmaline powder. The surface color of tourmaline modified by GO was deepened and uniform. With the increase of GO content, the color of composites deepened gradually. The GO/T (or GO/KT) composites presented in the following were composed of G (or GO) and tourmaline with a weight ratio of 1:100.

Macromorphology of tourmaline before and after modification.
The GO/T powder prepared by mechanical mixing method was used as the control group, and the composite effect of GO/KT powder prepared by electrostatic self-assembly method was explored. KT, GO/T, and GO/KT were used to prepare three kinds of aqueous solutions (1 g mL−1), which were stirred and dispersed evenly and then placed in a measuring cylinder. The dispersion of the mixed solution was observed after standing for different times, as shown in Figure 5.
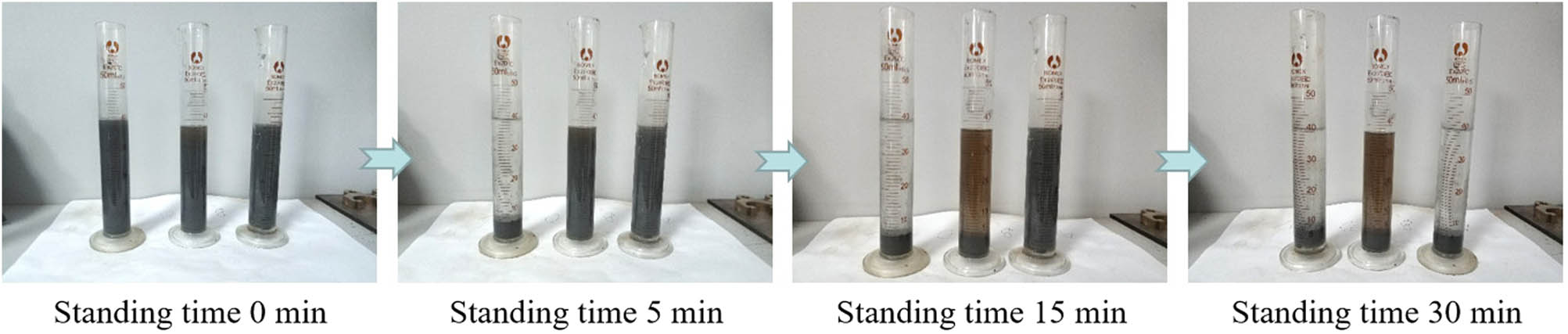
Suspension standing phenomenon (from left to right: GO/KT composites, GO/T composites, and KT).
From Figure 5, after standing for 5 min, GO/KT suspension began to stratify. The upper layer was clear liquid, and the lower layer was modified tourmaline powder. In contrast, KT and GO/T suspensions were still turbid. After standing for 15 min, GO/T suspension also showed obvious stratification phenomenon, but it was different from GO/KT suspension. The upper solution of GO/T was brown yellow, not clear. This showed that simple mechanical mixing cannot effectively load G onto the surface of tourmaline. The two separated in the ethanol solution due to their own weight. The upper brown yellow solution was GO aqueous solution. At the same time, it also indirectly proved the success of electrostatic self-assembly of GO and KT. After standing for 30 min, KT suspension showed obvious stratification. The increase of delamination time indicated that KH-550 can effectively modify the surface properties of tourmaline and improve its dispersion in aqueous solution.
3.2.2 Micromorphology
To further verify the modification effect of GO on tourmaline, the surface morphology of samples was amplified and observed.
In Figure 6(a), GO was in the form of flakes with a flake diameter between 5 and 15 μm. They were multiparticle accumulation, and their thickness was relatively thick, showing the phenomenon of multilayer accumulation. They had not been peeled off sufficiently. This kind of GO was difficult to participate in the reaction due to its own size and thickness, and it cannot demonstrate the superiority of G two-dimensional materials. In Figure 6(b), after 3 h of ultrasonic dispersion, the wrinkles on GO surface can clearly be observed. Compared with GO that is not ultrasonically dispersed, its flake diameter had not changed significantly, but its thickness was reduced. Its peeling layer number was higher than that of untreated GO, and its specific surface area improvement effect was obvious. It showed that the peel off effect of GO was significant after ultrasonic dispersion, forming a similar single-layer GO, which can effectively improve its performance.
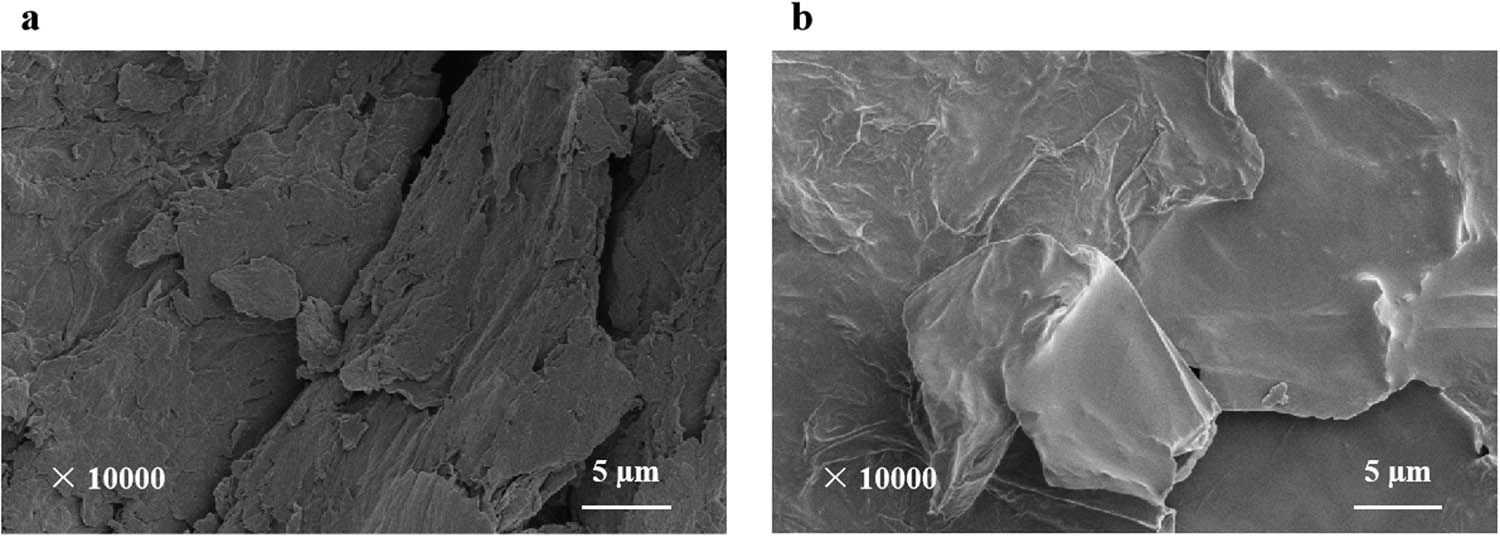
SEM images of GO: (a) before ultrasonic dispersion and (b) after ultrasonic dispersion for 3 h.
From Figure 7(a), tourmaline presented irregular distribution, with flake, blocky, and prismatic morphology. There were irregular edges and corners on the surface, and the size was mostly between 2 and 10 μm. Additionally, due to its own polarization, tourmaline surface adsorbed irregular particles, which may be fine particles in the process of tourmaline grinding. From Figure 7(b), the surface of tourmaline treated by KH-550 had no significant change. It indicated that KH-550 does not change the surface morphology of tourmaline, only changes its surface chemical characteristics.
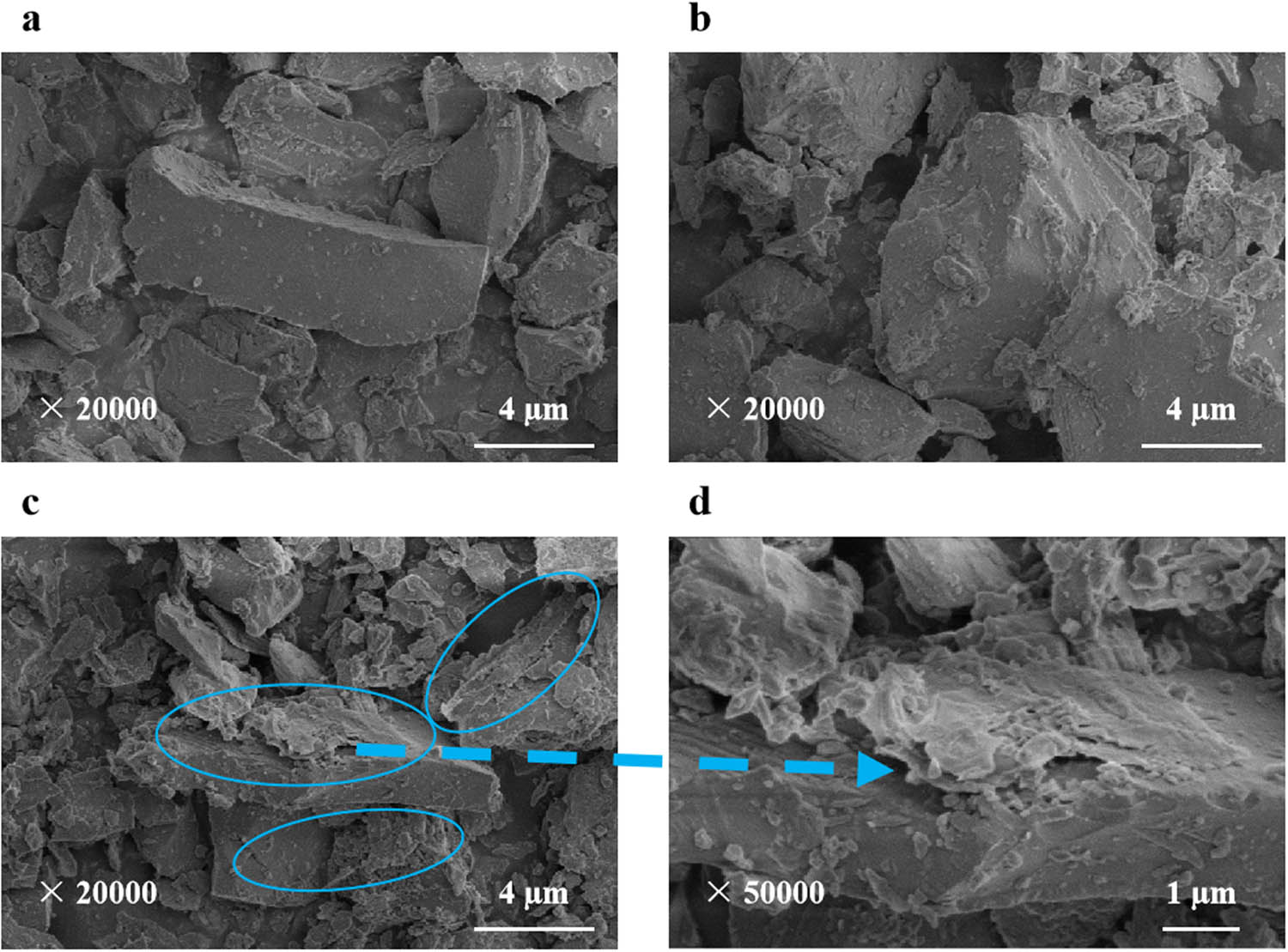
SEM images: (a) tourmaline, (b) KH-550-modified tourmaline, and (c and d) GO/KT composites.
In Figure 7(a)–(c), it was found that the preparation process of GO/KT composites does not damage the original surface morphology of tourmaline. In Figure 7(c), it was obvious that layered materials are attached to the surface of tourmaline. The surface morphology was further magnified (×50,000), and the characteristics of surface wrinkles were observed, as shown in Figure 7(d). According to Figures 6 and 7, it was determined that the above-layered materials are GO with lamellar structure. This indicated that GO is effectively attached to tourmaline surface after electrostatic assembly, and the lamellar layers are effectively peeled off. At the same time, there were few separate GO lamellar structure, which verified the interaction between GO and modified tourmaline.
3.3 Material composition of composites
3.3.1 XRD analysis
The X-ray diffraction patterns of different materials before and after the test were tested to study the composition characteristics of GO/KT composites prepared by electrostatic self-assembly method.
Figure 8 showed the XRD pattern of GO. There was a strong diffraction peak at 2θ of 11.306°, which was the characteristic peak formed by restacking GO after drying. Moreover, there was a small peak at 2θ of 42.551°, which was the amorphous peak of graphitized carbon. The peak intensity was weak. According to Bragg equation (2d sin θ = λ, X-ray wavelength = 1.5406), the interlayer spacing of GO was 0.78 nm, which was much higher than that of ordinary graphite (2θ is about 26.5°) [28]. It indicated that a large number of oxygen-containing groups are intercalated between GO flakes. Under the condition of ultrasonic dispersion, the lamellar was easier to peel off. This provided a prerequisite for the exfoliation of single-layer GO.
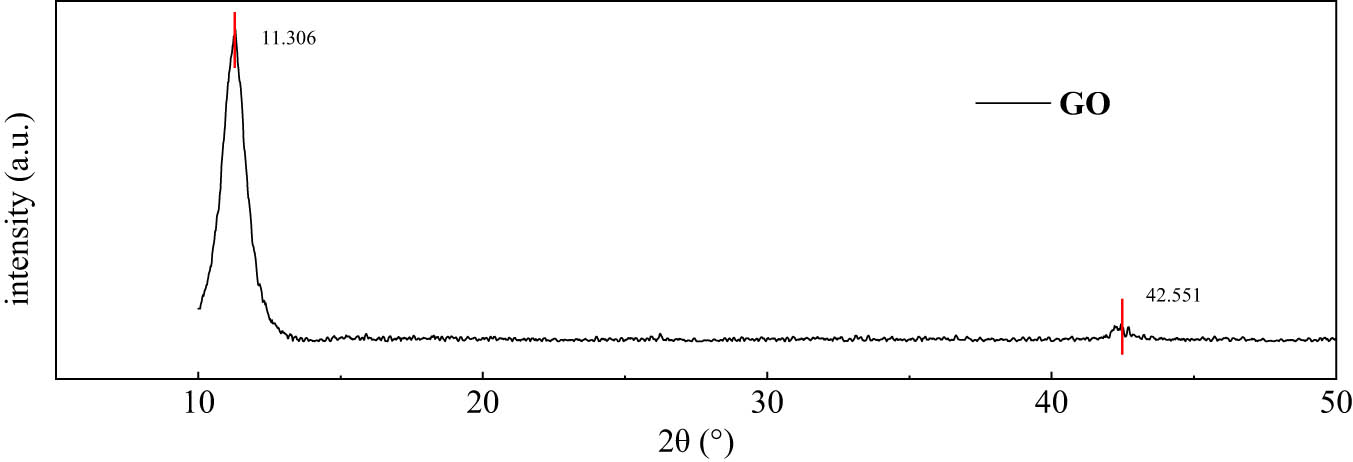
XRD pattern of GO.
Figure 9 showed the X-ray diffraction pattern of tourmaline and the phase retrieval results obtained by Jade software (Materials Data, Inc., Livermore, California, United State). According to search results, the substances with high matching value were selected. The retrieval results of standard cards were compared with the test results. In Figure 9(a)–(c), it can be seen that the diffraction peaks and their intensities of standard cards match those of tourmaline samples. Among them, the matching results of the three strongest peaks in Figure 9(b) with the tourmaline sample were the best, and their standard diffraction peaks almost coincide with those of the sample. Based on the chemical composition table obtained by X-ray fluorescence spectrometry, the main crystal image of the sample was Na0.42Fe3Al6B3O9Si6O18(OH)3.65. The retrieval number of standard PDF card was #89-6506. In addition, when the 2θ was 26.636°, the sample had a diffraction peak (101). According to Figure 9(d) and search results of X-ray photoelectron spectroscopy, it can be judged that there may be a small amount of SiO2 phase in the sample. The chemical composition of tourmaline powder was shown in Table 6.
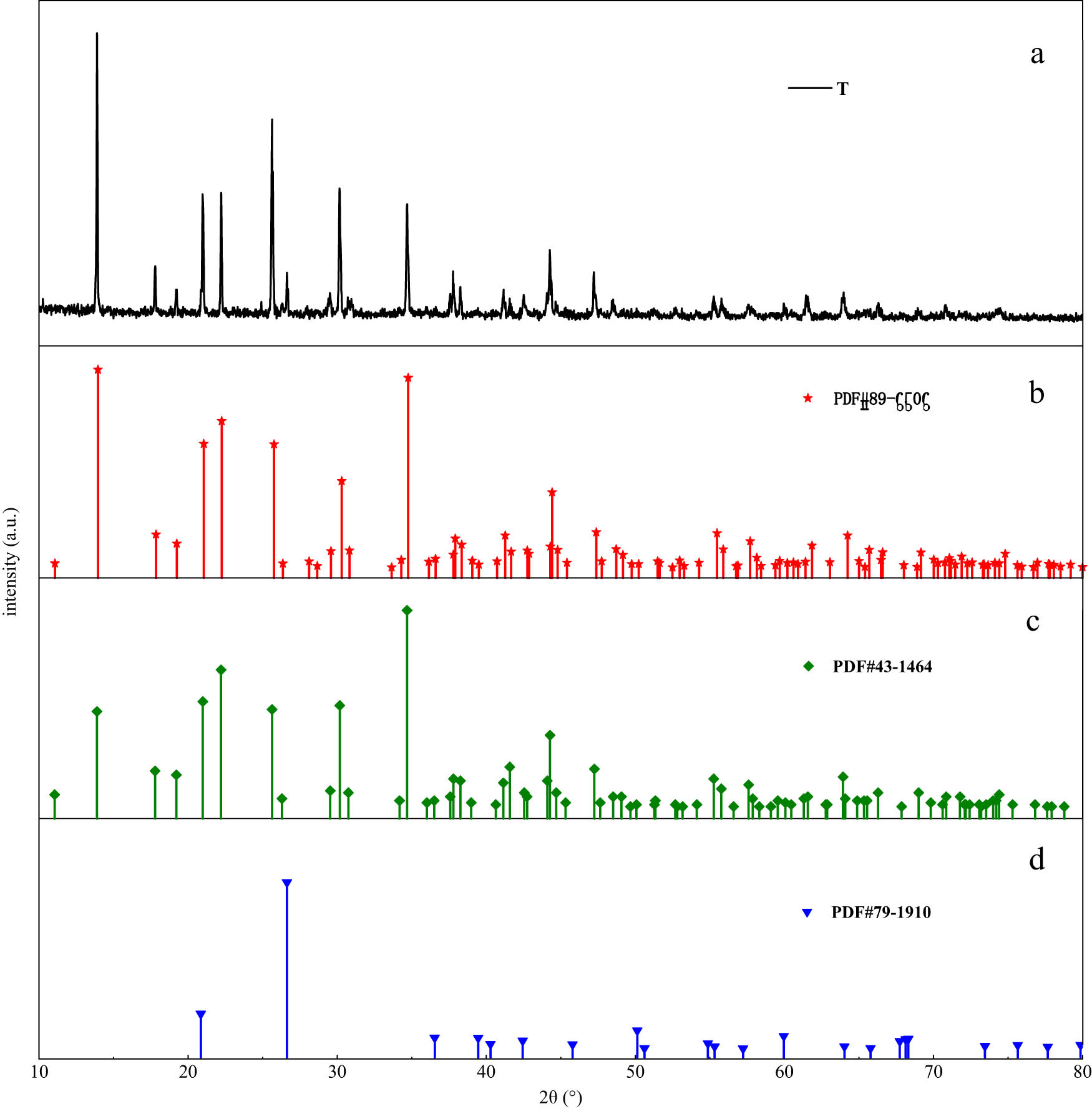
XRD patterns of tourmaline and its corresponding power diffraction file (PDF) card.
Chemical composition of tourmaline powder
| Chemical composition | SiO2 | Al2O3 | Fe2O3 | Na2O | F | MgO | CaO | MnO |
|---|---|---|---|---|---|---|---|---|
| Proportion | 40.5004 | 34.1202 | 20.4223 | 2.1322 | 1.0184 | 0.5900 | 0.3890 | 0.2638 |
| Chemical composition | K2O | TiO2 | ZnO | SnO2 | P2O5 | Ga2O3 | Y2O3 | SrO |
| Proportion | 0.2612 | 0.1500 | 0.0633 | 0.0379 | 0.0226 | 0.0153 | 0.0085 | 0.0050 |
Figure 10 showed the changes of XRD pattern of tourmaline before and after modification. The XRD patterns of tourmaline modified by KH-550 and GO did not change significantly. There was no obvious displacement or intensity change of the corresponding characteristic peak. The results showed that the structure of tourmaline is not destroyed during the process of electrostatic self-assembly. The characteristic peaks in XRD patterns were summarized in Table 7. The addition of GO had no significant effect on characteristic peak strength of tourmaline. In addition, from the abscissa of each peak, the addition of GO made the characteristic peak of tourmaline have a small angle deviation. This phenomenon may be due to the intercalation of tourmaline crystals with smaller sizes between GO flakes [44].
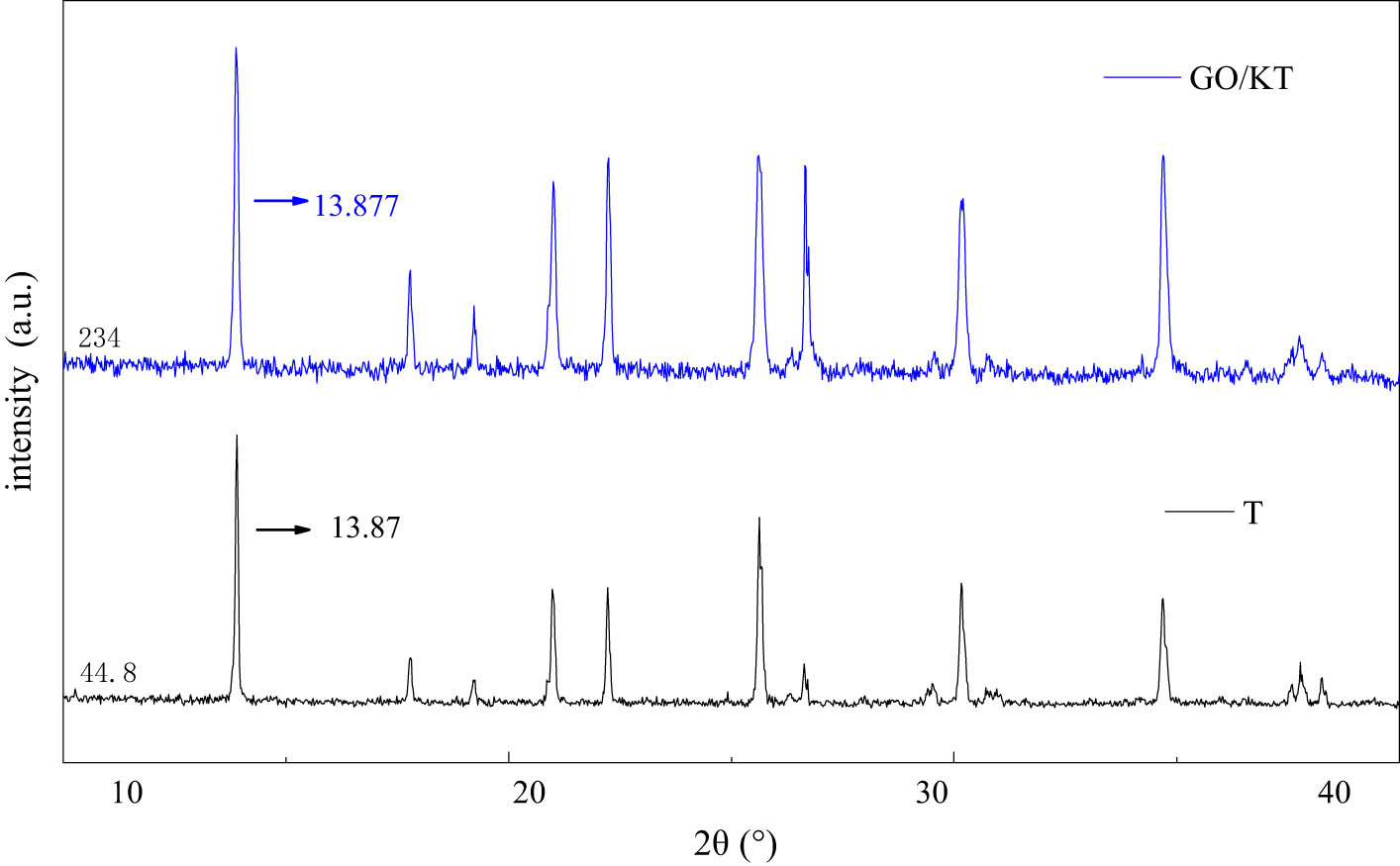
XRD pattern of GO/KT composites and T.
Change of peak line position before and after modification of tourmaline
| Materials | Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Peak 6 | Peak 7 |
|---|---|---|---|---|---|---|---|
| T | 13.87° | 34.675° | 25.589° | 22.228° | 20.975° | 30.146° | 26.65° |
| GO/KT | 13.877° | 34.685° | 25.607° | 22.238° | 20.998° | 30.194° | 26.652° |
3.3.2 Raman spectroscopy analysis
The measured Raman spectra were shown in Figure 11. The characteristic peaks of GO were summarized in Table 8, and the characteristic values of I D/I G were calculated.
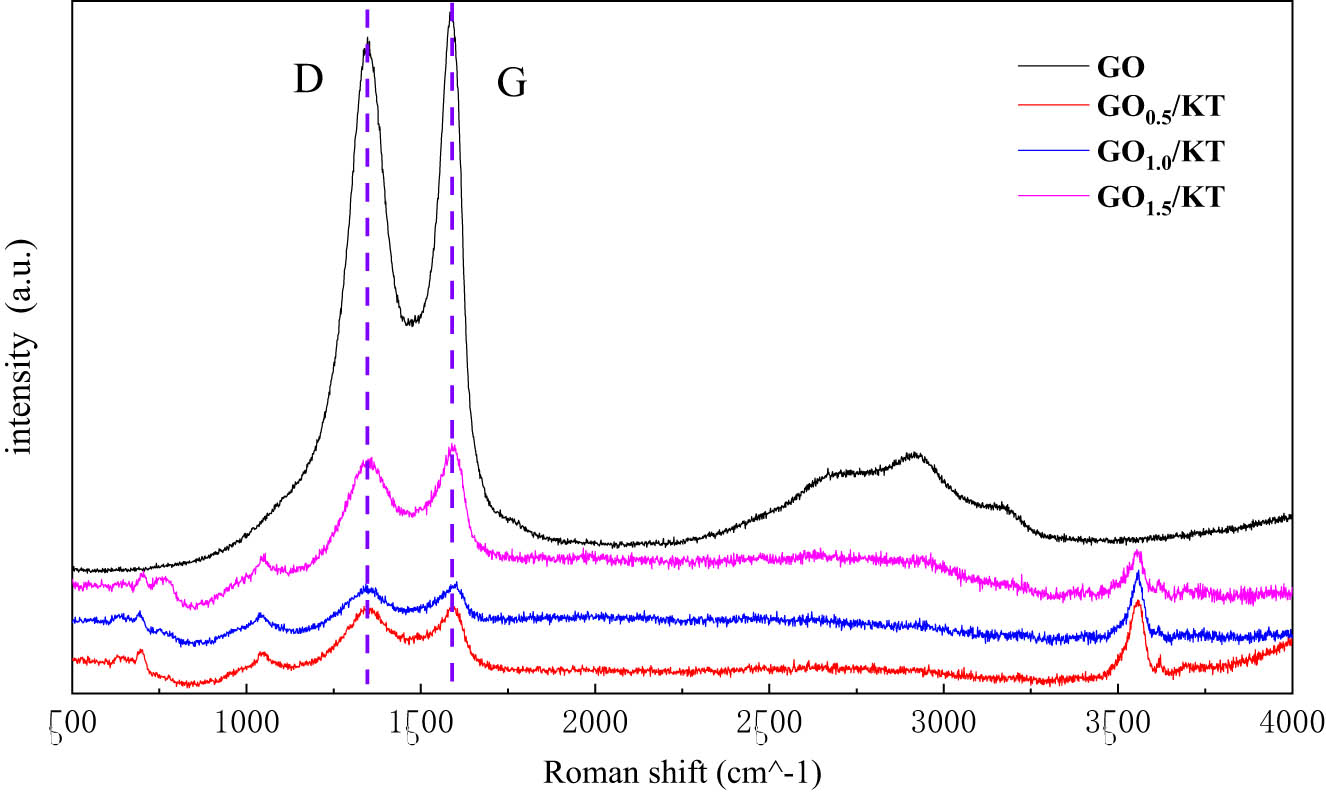
Raman spectra of GO/KT composites with different GO contents.
Characteristic peaks of Raman spectra of GO and modified tourmaline
| Materials | GO | GO0.5/KT | GO1.0/KT | GO1.5/KT | ||||
|---|---|---|---|---|---|---|---|---|
| Characteristic peak | D | G | D | G | D | G | D | G |
| Frequency | 1346.91 | 1587.29 | 1344.07 | 1591.42 | 1342.65 | 1602.42 | 1359.67 | 1588.67 |
| Strength | 21687.6 | 22599.9 | 2840.71 | 2867.82 | 2242.71 | 2335.07 | 5323.91 | 5827.03 |
| I D/I G | 0.960 | 0.991 | 0.960 | 0.914 | ||||
In Figure 11, GO clearly showed two characteristic peaks at 1346.91 and 1587.29 cm−1. D peak can reflect the degree of structural defects and disorder of carbon plane, whereas G peak can reflect the order degree of carbon plane. After GO was compounded with tourmaline, the characteristic peak intensity decreased at the positions of D peak and G peak of the original GO, but the width did not change significantly. This indicated that GO adheres to the surface of tourmaline and has a corresponding change [45]. In addition, the relative strength of D peak decreased with the increase of the GO content. The results showed that the increase of the GO content in the composites is helpful to improve its physical properties.
According to Figure 11 and Table 8, G peak did not change significantly, but D peak had a certain red shift with the increase of the GO content. The I D/I G of GO0.5/KT, GO1.0/KT, and GO1.5/KT showed a decreasing trend, which indicated that the disorder degree of materials decreased. The I D/I G in carbon materials is directly proportional to the structural defects and inversely proportional to their size. According to the change of I D/I G, the average size of GO decreased after it was compounded with tourmaline. This indicated that a large number of small size or exfoliated GO were produced in preparation process. With the increase of the GO content, these small-sized GO will not peel off from the surface of tourmaline due to drying and dispersion problems, and the defects will reduce. In addition, there were more oxygen-containing groups on the surface and edge of GO, which made it easier to form a three-dimensional structure with tourmaline, thus reducing the disorder structure.
3.3.3 FT-IR analysis
When tourmaline samples were irradiated with infrared light, the chemical bonds or functional groups in the molecules appeared stretching the vibration mode. Different chemical bonds or functional groups had various absorption frequencies and were in different positions in infrared pattern. The information about the changes of chemical bonds and functional groups can be obtained. At the same time, according to the status of characteristic peaks, the quality of modification effect was judged, and the existence forms of silane and GO on tourmaline surface were known. The changes of surface functional groups of powder before and after modification were compared. The surface modification effect of tourmaline was verified, and its mechanism was analyzed.
Figure 12 showed that tourmaline has significant infrared activity at wave numbers of 500, 750, 1,000, 1,400, and 3,560 cm−1. Among them, the characteristic absorption band of Si–O bending vibration was mainly near the wave number of 500 cm−1. The characteristic absorption band of Si–O–Si skeleton vibration was mainly near the wave number of 750 cm−1. The characteristic absorption band of O–Si–O stretching vibration was mainly near the wave number of 1,000 cm−1. The characteristic absorption band of BO3 stretching vibration was mainly near the wave number of 1,400 cm−1. The characteristic absorption band of O–H stretching vibration was mainly near the wave number of 3,560 cm−1. According to FT-IR spectrum of KT, the content of silane coupling agent in modified tourmaline was very low after washing with alcohol and deionized water. It did not affect subsequent reactions. The main characteristic peaks of tourmaline did not change significantly before and after the reaction (surface modification and compounding). This indicated that GO does not have a significant effect on the structure of tourmaline during the electrostatic self-assembly process.
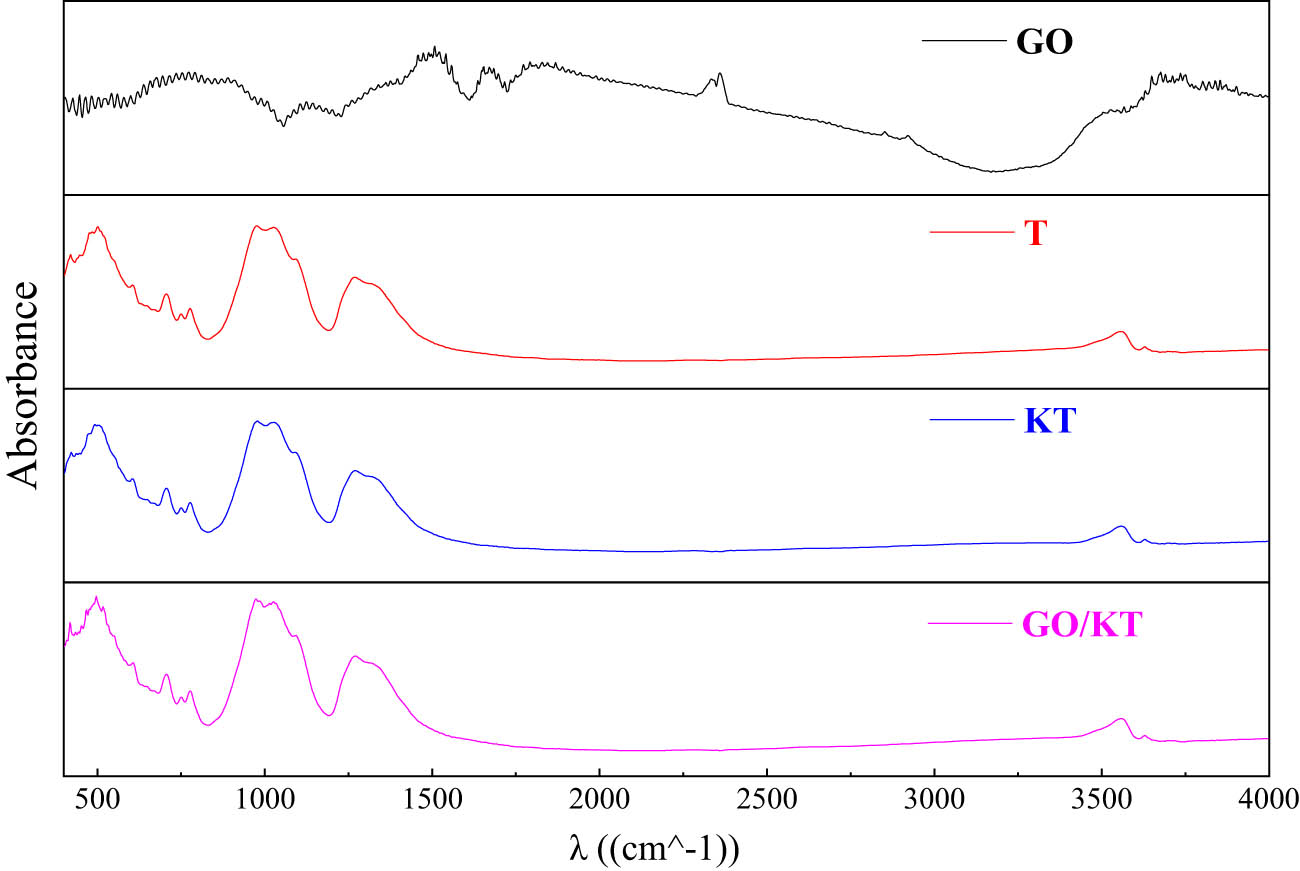
Infrared emission spectrum of different materials.
3.4 Adsorption properties of asphalt smoke
The smoke concentrations of base asphalt, KT-modified asphalt, and GO/KT-modified asphalt were tested, respectively. Their emission-reduction rates of asphalt smoke were calculated. The effect of the GO content on adsorption properties of tourmaline was assessed. The adsorption effects of different materials on asphalt smoke were shown in Figure 13.
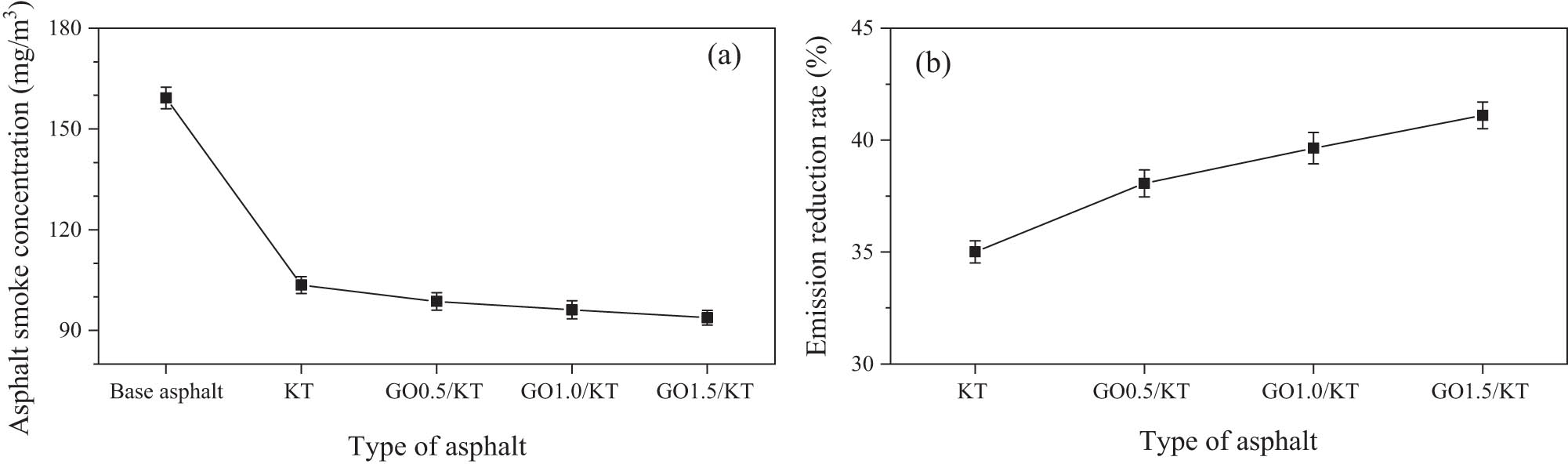
Asphalt smoke concentration (a) and emission reduction rate (b) of different types of asphalt.
From Figure 13, asphalt smoke concentration decreased sharply after the addition of tourmaline materials (KT and GO/KT). Moreover, with the increase of the GO content, asphalt smoke concentration decreased gradually. When the GO content was 1.5 wt%, the emission-reduction rate of asphalt smoke reached 41.11%. After adding GO, adsorption properties of tourmaline on asphalt smoke were improved. With the increase of the GO content, the improvement range gradually became flat. When the GO content was 1.5 wt%, the improvement was the largest, reaching 17.42%. Based on the above experimental results, it can be predicted that with the further increase of the GO content (>1.5 wt%), adsorption properties of tourmaline will continue to enhance. However, according to previous studies [46], if the content exceeds 1.5 wt%, the structure of G materials will be damaged to a certain extent during the preparation of composites. Therefore, the optimum content of GO was recommended to be 1.5 wt%.
Tourmaline has strong thermoelectric properties. When temperature changed, the crystal structure of tourmaline changed, resulting in the separation of positive and negative charges. It made tourmaline surface generate electric charge and has an adsorption effect on asphalt smoke [47]. GO had a strong ability of electron migration. When GO was compounded with tourmaline, it can promote the heteropolar charge around the tourmaline unit cell to leave the original position and promote the redistribution of positive and negative charges. The change of the whole dipole moment of tourmaline crystal was intensified. Then, the strength of surface electrostatic field increased [48]. In addition, the specific surface area of GO was much larger than that of tourmaline, which provides more adsorption sites. Therefore, the adsorption properties of tourmaline were improved.
4 Conclusion
KH-550 was superior than CTAB in surface modification of tourmaline. After tourmaline was modified by KH-550, its zeta potential distribution shifted to the right and electrical property changed. The electrical property of KH-550-modified tourmaline was opposite to that of GO, which satisfied the requirements of electrostatic self-assembly method.
According to material microanalysis methods of XRD, Raman spectroscopy and FT-IR, surface modification treatment did not change the structural characteristics of tourmaline. In addition, GO had no significant effect on the structure of tourmaline during the composite process.
GO could enhance adsorption properties of tourmaline on asphalt smoke. When the GO content was 1.5 wt%, the improvement was the largest, which is 17.42%. At this time, the emission-reduction rate of asphalt smoke reached 41.11%.
In this research, only the adsorption effect of composites on asphalt smoke has been studied, but the composition of asphalt smoke has not been analyzed. In the future, the adsorption effect and its durability of composites for nitrogen oxides and carbon oxides should be supplemented.
GO, as a special material, will lead to high engineering cost when it is applied to pavement materials at this stage. Therefore, the influence of the type and content of nano-GO/tourmaline composites on the road performance of asphalt needs to be studied further. The optimum proportion of nano-GO/tourmaline composite should be considered according to road performance, environmental efficiency, and economy.
-
Funding information: This research was sponsored by the Key Research and Development Project in Shaanxi Province (2021GY-206) and Fundamental Research Funds for the Central Universities (300102219314). Their sponsorship and interest are gratefully acknowledged.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Wang C, Wang S, Gao Z, Song Z. Effect evaluation of road piezoelectric micro-energy collection-storage system based on laboratory and on-site tests. Appl Energ. 2021;287:116581.10.1016/j.apenergy.2021.116581Search in Google Scholar
[2] Khare P, Machesky J, Soto R, He M, Presto A, Gentner D. Asphalt-related emissions are a major missing source of secondary organic aerosol precursors. Sci Adv. 2020;6(36):eabb9785.10.1126/sciadv.abb9785Search in Google Scholar PubMed PubMed Central
[3] Poulikakos L, Papadaskalopoulou C, Hofko B, Gschösser F, Falchetto A, Bueno M. Harvesting the unexplored potential of European waste materials for road construction. Resour Conserv Recy. 2017;116:32–44.10.1016/j.resconrec.2016.09.008Search in Google Scholar
[4] Obrist M, Kannan R, Schmidt T, Kober T. Decarbonization pathways of the Swiss cement industry towards net zero emissions. J Clean Prod. 2021;288:125413.10.1016/j.jclepro.2020.125413Search in Google Scholar
[5] Zhang Z, Wang C, Zhang L, Wang S, Zen W. Construction and assessment technology of green road in China. J Chang’an Univ (Nat Sci Ed). 2018;38(5):76–86.Search in Google Scholar
[6] Sun X, Yuan J, Zhang Y, Yin Y, Lv J, Jiang S. Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM. Nanotechnol Rev. 2021;10(1):1157–82.10.1515/ntrev-2021-0075Search in Google Scholar
[7] Chen Q, Lu Y, Wang C, Han B, Fu H. Effect of raw material composition on the working performance of waterborne epoxy resin for road. Int J Pavement Eng. 2020;1–2. 10.1080/10298436.2020.1856842.Search in Google Scholar
[8] Zhang L. Preparation and properties of graphene/tourmaline composite modified asphalt materials. Master Dissertation. Xi’an City, Shaanxi Province, China: Chang’an University; 2019.Search in Google Scholar
[9] Fu H, Wang C, Yu G, Chen Q, Liu L. Design optimization and performance evaluation of the open graded friction course with small particle size aggregate. Adv Civ Eng. 2021;2021:6668378.10.1155/2021/6668378Search in Google Scholar
[10] Wang C, Chen Q, Li QJ, Sun X, Li Z. A quantitative rating system for pollutant emission reduction of asphalt mixture. Math Probl Eng. 2017;2017:3761850.10.1155/2017/3761850Search in Google Scholar
[11] Ma C, Christianson L, Huang X, Christianson R, Li S. Efficacy of heated tourmaline in reducing biomass clogging within woodchip bioreactors. Sci Total Env. 2020;755:142401.10.1016/j.scitotenv.2020.142401Search in Google Scholar PubMed
[12] Liang Y, Tang X, Zhu Q, Han J, Wang C. A review: application of tourmaline in environmental fields. Chemosphere. 2021;281(11):130780.10.1016/j.chemosphere.2021.130780Search in Google Scholar PubMed
[13] Wang F, Meng J, Liang J, Fang B, Zhang H. Insight into the thermal behavior of tourmaline mineral. JOM. 2019;71:2468–74.10.1007/s11837-019-03391-1Search in Google Scholar
[14] Editorial Department of China Journal of Highway and Transport. Review on China’s pavement engineering research·2020. China J Highway Transport. 2020;33(10):1–66.Search in Google Scholar
[15] Ding H, Rahman A, Li Q, Qiu Y. Advanced mechanical characterization of asphalt mastics containing tourmaline modifier. Constr Build Mater. 2017;150:520–8.10.1016/j.conbuildmat.2017.05.203Search in Google Scholar
[16] Ye Q, Dong W, Wang S, Li H. Research on the rheological characteristics and aging resistance of asphalt modified with tourmaline. Mater. 2020;13(1):69.10.3390/ma13010069Search in Google Scholar PubMed PubMed Central
[17] Zhao Y, Wang P, Wang C, Li Y. Investigation of road performance and colloidal structure of tourmaline modified asphalt. Adv Mater Res. 2012;535–537:1731–4.10.4028/www.scientific.net/AMR.535-537.1731Search in Google Scholar
[18] Zhou X, Moghaddam T, Chen M, Wu S, Adhikari S. Biochar removes volatile organic compounds generated from asphalt. Sci Total Env. 2020;745:141096.10.1016/j.scitotenv.2020.141096Search in Google Scholar PubMed
[19] Wang C, Wang P, Li Y, Zhao Y. Laboratory investigation of dynamic rheological properties of tourmaline modified bitumen. Constr Build Mater. 2015;80:195–9.10.1016/j.conbuildmat.2014.12.105Search in Google Scholar
[20] Wang C, Fu H, Chen Q, Sun X, Guo T, Guo J. Preparation and performance of road micro-surfacing materials with exhaust purification function. KSCE J Civ Eng. 2019;23(7):2877–88.10.1007/s12205-019-0856-xSearch in Google Scholar
[21] Chen Q, Wang C, Wen P, Wang M, Zhao J. Comprehensive performance evaluation of low-carbon modified asphalt based on efficacy coefficient method. J Clean Prod. 2018;203:633–44.10.1016/j.jclepro.2018.08.316Search in Google Scholar
[22] Chen Q, Wang C, Wen P, Sun X, Guo T. Performance evaluation of tourmaline modified asphalt mixture based on grey target decision method. Constr Build Mater. 2019;205:137–47.10.1016/j.conbuildmat.2019.01.168Search in Google Scholar
[23] Zhang X, Zhou X, Xu X, Zhang F, Chen L. Enhancing the functional and environmental properties of asphalt binders and asphalt mixtures using tourmaline anion powder modification. Coatings. 2021;11(5):550.10.3390/coatings11050550Search in Google Scholar
[24] Li G, Chen D, Zhao W, Zhang X. Efficient adsorption behavior of phosphate on la-modified tourmaline. J Environ Chem Eng. 2015;3(1):515–22.10.1016/j.jece.2015.01.010Search in Google Scholar
[25] Li Y, Sun Y, Li J, Gao C. Socioeconomic drivers of urban heat island effect: empirical evidence from major Chinese cities. Sustain Cities Soc. 2020;63:102425.10.1016/j.scs.2020.102425Search in Google Scholar
[26] Chen Y, Wang S, Li Y, Liu Y, Zeng Z. Adsorption of Pb(Ⅱ) by tourmaline-montmorillonite composite in aqueous phase. J Colloid Interf Sci. 2020;575:367–76.10.1016/j.jcis.2020.04.110Search in Google Scholar PubMed
[27] Li Y, Xing X, Pei J, Li R, Wen Y, Cui S, et al. Automobile exhaust gas purification material based on physical adsorption of tourmaline powder and visible light catalytic decomposition of g-C3N4/BiVO4. Ceram Int. 2020;46(8):12637–47.10.1016/j.ceramint.2020.02.029Search in Google Scholar
[28] Wang C, Chen Q, Guo T, Li Q. Environmental effects and enhancement mechanism of graphene/tourmaline composites. J Clean Prod. 2020;262:121313.10.1016/j.jclepro.2020.121313Search in Google Scholar
[29] Sun L, Guo Y, Hu Y, Pan S, Jiao Z. Conductometric n-butanol gas sensor based on Tourmaline@ZnO hierarchical micro-nanostructures. Sens Actuat B-Chem. 2021;337:129793.10.1016/j.snb.2021.129793Search in Google Scholar
[30] Zhang X, Jing Q, Ao S, Schneider GF, Kireev D, Zhang Z, et al. Ultrasensitive field‐effect biosensors enabled by the unique electronic properties of graphene. Small. 2020;16(15):1902820.10.1002/smll.201902820Search in Google Scholar PubMed
[31] Sherlala A, Raman A, Bello MM, Asghar A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere. 2018;193:1004–17.10.1016/j.chemosphere.2017.11.093Search in Google Scholar PubMed
[32] Zhang X, Wang Y, Luo G, Xing M. Two-dimensional graphene family material: assembly, biocompatibility and sensors applications. Sensors-Basel. 2019;19(13):2966.10.3390/s19132966Search in Google Scholar PubMed PubMed Central
[33] Phasuksom K, Prissanaroon-Ouajai W, Sirivat A. A highly responsive methanol sensor based on graphene oxide/polyindole composites. RSC Adv. 2020;10(26):15206–20.10.1039/D0RA00158ASearch in Google Scholar PubMed PubMed Central
[34] Korkmaz S, Kariper A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J Energy Storage. 2020;27:101038.10.1016/j.est.2019.101038Search in Google Scholar
[35] Deng P, Liu Y, Luo P, Wang J, Liu Y, Wang D, et al. Two-steps synthesis of sandwich-like graphene oxide/LLM-105 nanoenergetic composites using functionalized graphene. Mater Lett. 2017;194:156–9.10.1016/j.matlet.2017.02.038Search in Google Scholar
[36] Wang J, Lei W, Xue Z, Qian H, Liu W. Research progress on synthesis and application of graphene reinforced metal matrix composites. J Mater Eng. 2018;46(12):18–27.Search in Google Scholar
[37] Guo T, Wang C, Chen H, Li Z, Chen Q, Han A, et al. Rheological properties of graphene-tourmaline composite modified asphalt. Pet Sci Technol. 2019;37(21):2190–8.10.1080/10916466.2019.1624375Search in Google Scholar
[38] Zhang D, Shen H, Cao X, Ye Y, Zhang X, Ye L, et al. Research progress in graphene reinforced aeronautical metal matrix composites. J Mater Eng. 2019;47(1):1–10.Search in Google Scholar
[39] Chen Q, Wang C, Qiao Z, Guo T. Graphene/tourmaline composites as a filler of hot mix asphalt mixture: preparation and properties. Constr Build Mater. 2020;239:117859.10.1016/j.conbuildmat.2019.117859Search in Google Scholar
[40] Luo Y, Chen Q, Wang C, Guo T. Preparation and improved negative ion release of graphene/tourmaline composite. Mater Res Express. 2019;6:055507.10.1088/2053-1591/ab0322Search in Google Scholar
[41] Ministry of Ecology and Environment of the People’s Republic of China. Emission standard of pollutants for petroleum refining industry. GB 31570–2015. Beijing: China Environmental Science Press; 2015.Search in Google Scholar
[42] Lin S, Sun S, Shen K, Dong F. Environmental functionalities of tourmaline and applications of its functional composites. Mater Rev. 2017;31(7):131–7.Search in Google Scholar
[43] Zhang S, Sun J, Hu D, Xiao C, Zhuo Q, Wang J, et al. Large-sized graphene oxide/modified tourmaline nanoparticle aerogel with stable honeycomb-like structure for high-efficiency PM2.5 capture. J Mater Chem A. 2018;6:16139–48.10.1039/C8TA05506HSearch in Google Scholar
[44] Huang Z, Wang T, Zhang X, Zheng L, Xue G, Liang J. Preparation of tourmaline/graphene oxide and its application in thermal interface materials. J Compos Mater. 2016;50(28):3953–60.10.1177/0021998316630393Search in Google Scholar
[45] Zhang K, Wang J, Fei G, Xia H. Properties of natural rubber filled with graphene oxide and mesoporous silica. Polym Mater Sci Eng. 2018;34(8):117–23.Search in Google Scholar
[46] Guo T, Fu H, Wang C, Chen H, Chen Q, Wang Q, et al. Road performance and emission reduction effect of graphene/tourmaline-composite-modified asphalt. Sustainability. 2021;13(16):8932.10.3390/su13168932Search in Google Scholar
[47] Yin D, Yu M, Shi J, Xu Z, Song D, Liu G. Adsorption characteristics of enrofloxacin by schorl. Chin J Environ Eng. 2017;11(4):2183–9.Search in Google Scholar
[48] Bronzova Y, Babushkina M, Frank-Kamenetskaya O, Vereshchagin O, Rozhdestvenskaya I, Zolotarev A. Short-range order in Li–Al tourmalines: IR spectroscopy, X-ray single crystal diffraction analysis and a bond valence theory approach. Phys Chem Min. 2019;46(9):815–25.10.1007/s00269-019-01042-0Search in Google Scholar
© 2021 Chaohui Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

