Abstract
Graphene (G) and graphene oxide (GO) have been shown to significantly improve the mechanical properties of cement-based materials. In this study, the effect of the G/GO on cement hydration was investigated. First, the zeta potential of G/GO in simulated solutions was tested, and the interaction between G/GO’s surface and Ca2+ was explored. Subsequently, scanning electron microscopy was used to observe the morphology of C–S–H nucleation and growth on the cement surface in the cement paste containing G/GO. Furthermore, XRD and TGA analyses were carried out on the hydration products of the sample. At last, isothermal calorimetry was applied to investigate the influence of G/GO on the early hydration of cement. The results showed that the addition of G/GO significantly accelerates C–S–H nucleation and growth on the cement surface. It is indicated that the high mobility ions derived by G/GO in the cement paste dominate the reason for the accelerated hydration of cement. The presence of G, especially GO, facilitates the mobility of ions, especially Ca2+, thus enhances the interaction between the cement surface and the ions. This strong interaction promotes the C–S–H nucleation and growth, and therefore, the hydration of the cement.
Graphical abstract
(1) GO has a strong interaction with Ca2+. (2) G/GO accelerates C–S–H growth. (3) High mobility ions derived by G/GO facilitate hydration.

1 Introduction
Cement-based materials modified with nanomaterials can gain a significant performance improvement and related research has been receiving increasing attention [1,2,3]. Graphene (G) and graphene oxide (GO) are applied to optimize the performance of cement-based materials due to their unique properties [4,5]. Many studies [6,7,8] reported that GO can significantly improve the compression strength of cement-based materials. It reported that adding 1.5 wt% of GO increased the compressive strength by 53% [9]. Some studies [10,11,12] indicated that the change in compressive strength of G-modified cement-based materials is within 15%. However, Dela Vega and Vasquez [13] reported a 56% increase in compressive strength by adding 0.5 wt% of G.
The microstructure is the key to understanding the mechanical properties of cement-based materials. Liu et al. [14,15] reported a denser interfacial transition zone in mortars with G/GO, resulting in a significant increase in the strength. Zhang et al. [16] found that the porosity of GO-modified cement-based materials decreased by 31% at a GO content of 2.0 wt%. The improvement in microstructure and the decrease in porosity were attributed to the ‘filling’ and ‘nucleation’ effect of G/GO [17,18]. The addition of G/GO can not only refine the pore structure, but also regulate the formation of hydration products [19].
C–S–H is the most important hydration product [20]. Lu and Liu [21,22,23] found that the C–S–H becomes a regular flower in shape due to the nucleation and regulatory of G/GO in the formation of C–S–H. Wang et al. [24] reported that the incorporation of 0.01–0.05% GO accelerated the formation of C–S–H, and the arrangement of C–S–H was more regular. Most studies [25,26,27] reported that G/GO provides the C–S–H nucleation sites to enhance the cement hydration. In addition, Guo et al. [28,29,30,31] indicated that the interface of GO/C–S–H generates a new chemical bond. However, Kong et al. [32] did not find C–S–H on the surface of nanomaterials and believed that nanoparticles have no nucleation effect based on classical nucleation theory.
The effect of G/GO on cement hydration is still uncertain. These studies focused more on the morphology of C–S–H and less on the effect of G/GO on the formation process of C–S–H. The process of C–S–H formation is that the aggregations of ions or molecules become large and stable enough to develop nuclei in the saturated state and then grow gradually. G/GO has excellent electrical conductivity, which will motivate the ions in the solution [33]. The analysis of the effect of G/GO on the C–S–H morphology from the perspective of the C–S–H formation process would provide a deeper understanding of the role of G/GO in cement hydration.
Ca2+ plays an important role in the kinetic, morphological, and structural characteristics of C–S–H [34]. In this study, the interaction between G/GO and Ca2+ was first characterized with the zeta potential test. Then, SEM was applied to observe the hydrates in cement paste incorporating G/GO at 15 min, 1, 4, and 7 h of hydration. The hydration products of the samples were analyzed by XRD and TGA. Furthermore, isothermal calorimetry was used to measure the heat of cement hydration during the first 24 h. Finally, the role of G/GO in the early hydration of cement was discussed.
2 Materials
2.1 Materials and mixture
The materials used in this study include cement, G, and GO. The cement is Portland cement type I produced by Fushun Cement Co., Ltd. in China. The chemical content of the cement was tested by X-ray fluorescence (XRF), and the result is shown in Table 1. G/GO was purchased from Suzhou Carbon Fun Technology Co., Ltd. in China. The properties and characterization of G/GO are listed in Table 2. To further characterize G/GO, microscopic observation was performed by scanning electron microscopy (SEM) and the morphology of G/GO is shown in Figure 1. The red frame is the enlarged zone. The mixing ratio is detailed in Table 3. PC is a control sample with 100% cement paste. CG is a cement paste incorporating G. CGO is a cement paste containing GO.
Chemical content of cement (wt%)
| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|
| 63.51 | 20.86 | 5.3 | 3.56 | 2.67 | 1.81 | 0.631 | 0.387 | 0.332 |
Physical parameters of G and GO
| Types | Purity (wt%) | Thickness (nm) | Diameter of lamellae (nm) | Specific surface area (m2/g) |
|---|---|---|---|---|
| G | >98 | ∼2 | 5–10 | 1,000–1,217 |
| GO | >95 | ∼1 | 10–50 | >400 |

SEM image of G (a) and (b) and GO (c) and (d).
Mix design
| Sample code | Cement* (%) | Blending amount* (%) | w/b |
|---|---|---|---|
| PC | 100 | 0 | 0.4 |
| CG | 100 | 0.4 | 0.4 |
| CGO | 100 | 0.4 | 0.4 |
*Percentage of the total mass of cement by weight.
2.2 Sample preparation
First, G/GO was added to water to produce a suspension of nanoscale particles. To disperse the G/GO uniformly in the aqueous solution, the suspension was sonicated in an ultrasonic meter (Power of 400 W) for 15 min. Water temperature was controlled within 30°C in the sonicate process. Then, cement was put into the G/GO suspension and stirred evenly by a whisk at a slow speed for 2 min, then at a fast speed for 2 min. After that, all samples were placed in the standard conditioning chamber for 15 min, 1, 4, and 7 h, respectively. The samples were maintained to a specified age and then soaked in anhydrous ethanol to terminate the hydration. The samples in the anhydrous ethanol were filtered and dried in a 35°C oven for 12 h. Finally, the dried samples were collected for investigations.
2.3 Zeta potential test
The zeta potential of G/GO was tested with a Malvern Zetasizer Nano (Malvern Instruments Ltd., UK) in a simulated solution. The liquid phase of early cement pastes is mainly composed of Na+, K+,
2.4 SEM
Before SEM observation, the conductive film was attached to the sample stage, and then each specimen (about 1 g) was fixed on the conductive film. To keep the sample stably fixed on the conductive film, it was sprayed with high-pressure gas. Due to the poor conductivity of the hardened paste, the powder samples were sprayed with gold before the test. Then, the samples were observed using a Phenom ProX electron microscope (Phenom, FEI). The specific details of this test method can be found in the literature [37].
2.5 X-ray diffraction (XRD)
An X-ray diffractometer (Japan Smartlab) was used to analyze the hydrated products of the samples. The radiation of the test was CuKα, the voltage was 40 kV, and the current was 40 mV. The diffraction angle was 10–80° with a step of 2°/min. The target material was copper. The samples were kept dry in a vacuum until testing.
2.6 Thermogravimetric (TGA)
TGA analysis of the samples was carried out by a term synchronous thermal analyzer (Germany NETZSCH-STA449F5). The set temperature rise rate was 10°C/min and the maximum temperature was 800°C. Argon was used as a protective gas. The Ca(OH)2, CaCO3, and C–S–H contents were calculated by the following equation [38]:
Note: LOI(CH) is the percentage of Ca(OH)2 losing H2O in the TG curve. LOI(CC) is the percentage of CaCO3 losing CO2 in the TG curve. LOI(C–S–H) is the percentage of the C–S–H losing H2O in the TG curve.
2.7 Isothermal calorimetry
The exothermic rate of hydration and the total exothermic heat of hydration of samples were automatically measured and recorded by the TAM Air eight-channel isothermal calorimeter. The instrument temperature was set to 20°C, and the next step was performed after the instrument temperature stabilized. Ampoules containing samples were placed in the channel and the exothermic hydration data were tested continuously for 24 h. Each recorded value was derived from the average of two measurement results.
3 Results and discussion
3.1 Zeta potential
The variation pattern of zeta potential of G/GO with Ca2+ concentration in Ca(OH)2 solution is shown in Figure 2. The variation pattern of zeta potential for limestone powder (LP) and quartz powder (QP) in Ca(OH)2 solution with Ca2+ concentration was tested by Ouyang et al. [36]. This result was compared with the zeta potential of C–S–H particles measured by Nachbaur et al. [39] and Viallis-Terrisse et al. [40]. As can be seen from Figure 2, for a certain Ca2+ concentration, the zeta potentials of QP and C–S–H particles were similar, while the zeta potentials of LP particles were relatively more positive. LP particles have stronger interaction with Ca2+ compared to QP and C–S–H particles [36].

The variation pattern of zeta potential of G, GO, LP, QP, and C–S–H particles with Ca2+ concentration in Ca(OH)2 solution.
As can be seen from Figure 2, the zeta potential of G is −15.3 mV at a Ca2+ concentration of 0.2 mmol/L. With the increase of Ca2+ concentration, the zeta potential of G is almost linear and increases due to the adsorption of Ca2+ on particles’ surfaces. The isoelectric point (IEP) corresponds to a point where the zeta potential is 0, indicating that the electrophoretic mobility is 0. G particles reach IEP at a Ca2+ concentration of about 2 mmol/L, which is similar to QP and C–S–H particles. The potential value of G is positive after a Ca2+ concentration of about 2 mmol/L. And the maximum value of the potential of G is 24.55 mV at a Ca2+ concentration of 6 mmol/L. In general, the trend of the potential of G with Ca2+ concentration is similar to that of QP and C–S–H. It is indicated that G particles have similar interaction with Ca2+ as QP and C–S–H particles.
For GO particles, the potential of suspension exhibits a negative value at a Ca2+ concentration of 0.2 mmol/L. It may be caused by carboxylate groups on the surface [41]. GO particles reach IEP at a Ca2+ concentration of below 2 mmol/L, which is about 0.9 mmol/L. The zeta potential increased with increasing Ca2+ concentration from 0.9 to 6 mmol/L. The maximum value of potential is 32.77 mV at a Ca2+ concentration of 6 mmol/L. After Ca2+ concentration continued to increase, the high concentration solution of Ca2+ resulted in an irregular decrease of G/GO potential [42]. The potential of GO is higher than that of G, QP, and C–S–H for the same Ca2+ concentration. It is possible due to the oxygen-containing groups which have a stronger interaction with Ca2+ [41].
3.2 Morphology of hydrates
Figure 3 presents the SEM image of hydration products on the surface of cement particles at 15 min and 1 h hydration. The red frame is the enlarged zone. SEM image of the surface of cement particles in PC paste after hydration for 15 min is shown in Figure 3a and b (partial enlarged). It can be seen that some rod-shaped C–S–H particles appear on cement surface. Figure 3c and d (partial enlarged) demonstrate that many needle-shaped C–S–H particles are distributed on the surface of cement particles in CG paste after hydration for 15 min. The surface of cement particles is covered by C–S–H of similar dimensions in CGO paste after hydration for 15 min (Figure 3e and f). For CG and CGO past, the C–S–H on the cement surface appears denser and more uniform than that in PC paste. In addition, as can be observed in Figure 3d and f, the C–S–H on the surface of cement particles in the CGO paste is larger and a little denser than that in CG paste.

Morphology of hydration products on the surface of cement particles at 15 min in PC (a and b), CG (c and d), CGO (e and f), and at 1 h in PC (g and h), CG (i and j), CGO (k and l).
In Figure 3g and h, the size of the rod-shaped C–S–H started to grow on the surface of cement particles in PC paste after hydration for 1 h. As can be observed from Figure 3i and j, the surface of cement particles in CG paste has needle-shaped C–S–H after hydration for 1 h. These C–S–H were numerous and evenly distributed. Figure 3k and l (partial enlarged) also show a large number of needle-shaped C–S–H covering on the surface of cement particles in CGO paste after hydration for 1 h. Compared to PC paste, the surface of cement particles has more and finer needle-shaped C–S–H in CG and CGO paste.
Figure 4 presents the morphology of hydration products on the surface of cement particles at 4 and 7 h. As can be seen in Figure 4a and b, some rod-shaped C–S–H particles become larger on the surface of cement particles in PC paste after hydration for 4 h. The size is still not uniform. As shown in Figure 4c and d (partial enlarged), there are a large number of fine C–S–H and some calcium hydroxide (CH) particles on the surface of cement particles in CG paste after hydration for 4 h. Meanwhile, many fine C–S–H particles on the surface of cement particles were also observed in CGO paste after hydration for 4 h. These results show that the C–S–H on the surface of cement particles in CG and CGO is finer and has more quantity than that in PC paste.

Morphology of hydration products on the surface of cement particles at 4 h in PC (a and b), CG (c and d), CGO (e and f), and at 7 h in PC (g and h), CG (i and j), CGO (k and l).
As demonstrated in Figure 4g and h (partial enlarged), overlapping rod-shaped C–S–H can be identified and their growth orientation is varied on the surface of cement particles in PC paste after hydration for 7 h. Figure 4i and j (partial enlarged) show that the mesh structure formed by the interweaving of C–S–H completely covers the surface of cement particles in CG paste. As can be observed from Figure 4k and l (partial enlarged), the surface of cement particles is also covered by the C–S–H of the mesh structure after hydration for 7 h. The difference in the morphology of C–S–H between the PC paste and CG and CGO paste is significant. Compared to PC paste, C–S–H with a higher amount and finer dimensions appears on the surface of cement particles in CG and CGO paste.
3.3 XRD analysis
XRD data are shown in Figure 5, mainly identifying C2S, C3S, CH, and CaCO3. CG and CGO pastes do not show significantly different diffraction peaks. This indicates that the addition of G/GO to the cement paste does not produce new substances. The diffraction peaks of CH do not change significantly after the addition of G/GO. However, the diffraction peak of CaCO3 was found. It is possible due to the carbonation of Ca(OH)2.

XRD patterns of the sample at 15 min (a), 1 h (b), 4 h (c), and 7 h (d).
3.4 TGA analysis
The TG and DTG curves of PC, CG, and CGO obtained by TGA analysis are presented in Figure 6. The weight loss at 50–105°C is caused by the evaporation of adsorbed water. The weight loss at 110–170°C is caused by dehydration of the gel phase, such as AFt, C–S–H, etc. The weight loss at 400–500°C and 600–700°C is caused by the dehydration of Ca(OH)2 and the decomposition of CaCO3. The presence of CaCO3 suggests that the Ca(OH)2 was carbonized, verifying the XRD results.
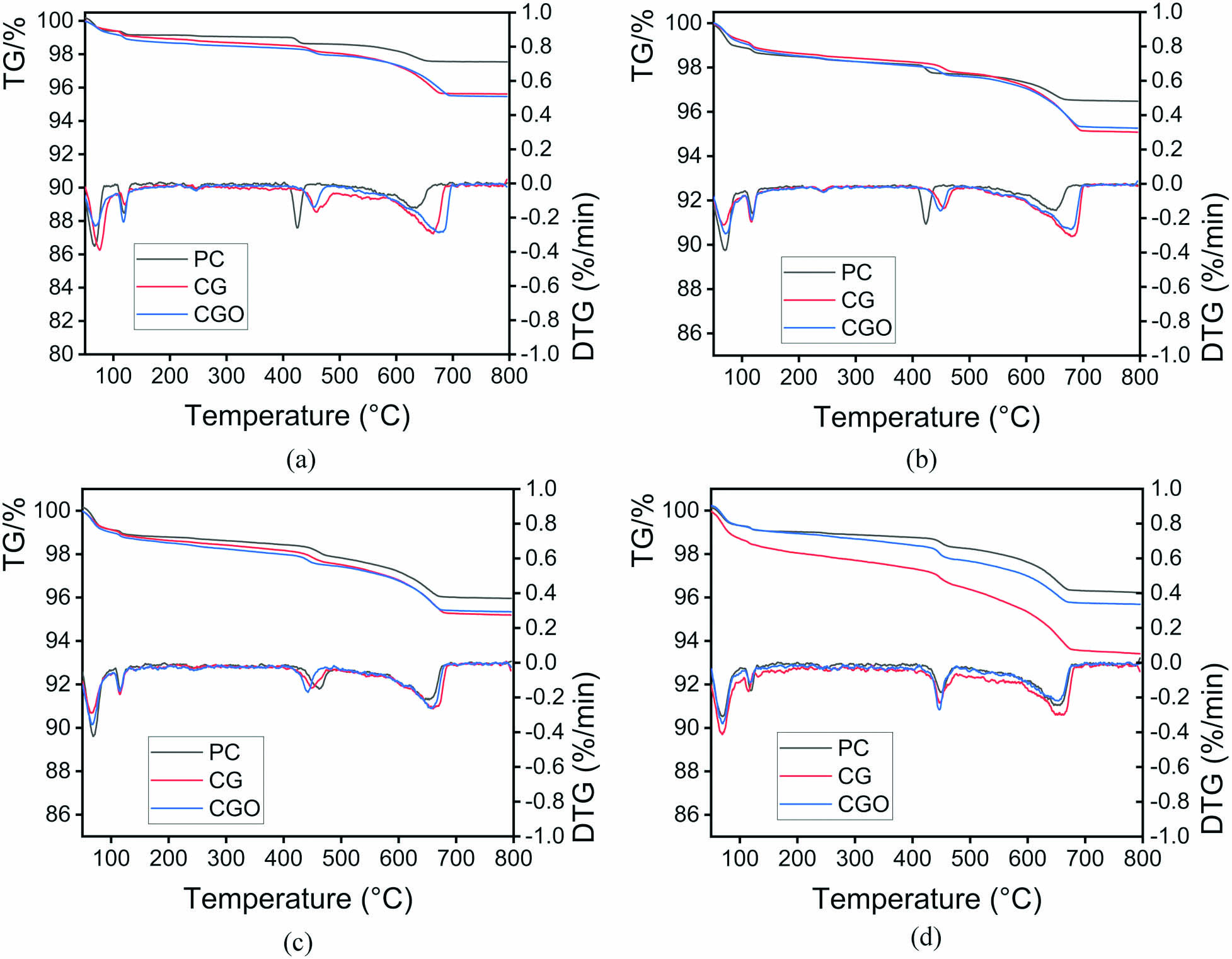
TG-DTG analyses of PC, CG, and CGO pastes at 15 min (a), 1 h (b), 4 h (c), and 7 h (d).
The hydration degree of the sample can be quantified by the content of Ca(OH)2, CaCO3, and C–S–H. The contents of Ca(OH)2, CaCO3, and C–S–H were calculated and shown in Figure 7. It can be seen that the total contents of Ca(OH)2, CaCO3 in the CG and CGO pastes are higher than those in the PC paste. Moreover, the content of C–S–H in CG and CGO pastes was higher than that in PC pastes. It further indicates that the addition of G/GO can promote the hydration of cement, and thus generate more hydration products at the same hydration time. The content of C–S–H in the CGO paste is significantly less than that in CG in Figure 7(d). It is most likely due to partial C–S–H filtered out in the sample preparation.

The relative content of the hydration products of the samples at 15 min (a), 1 h (b), 4 h (c), and 7 h (d).
3.5 Cement hydration heat calorimetry analysis
Figure 8 shows the exothermic rate and total accumulated heat obtained from the isothermal calorimetry test. The exothermic rate of hydration is presented in Figure 8a. It can be found that the induction period of CG/CGO paste is longer than PC paste. The peak of heat flow for PC, CG, and CGO paste is 1.93, 2.02, and 2.11 mW/g, respectively. Peak heat flow increased by 5.1% and 9.8% for CG and CGO pastes, respectively, compared to PC pastes. The data obtained indicate that the presence of G/GO significantly facilitates the exothermic rate of cement hydration. The total accumulated heat of hydration is demonstrated in Figure 8b. At 24 h of hydration, the total accumulated heat of PC, CG, and CGO paste is 84.57, 85.10, and 89.64 J/g, respectively. The total accumulated heat for CG and CGO pastes increased by 0.63% and 6.0%, respectively, compared to PC pastes. These data show that the incorporation of GO accelerates the hydration of the cement paste. However, the total accumulated heat of CG paste is similar to that of PC paste. Similar results have been found in previous studies [18,43].

The heat flow (a) and accumulated heat (b) of PC, CG, and CGO pastes.
3.6 Discussion
The formation of C–S–H undergoes the process of nucleation and growth [44]. Classical theory suggests that nucleation and growth are related to the concentration of ions and are dynamic equilibrium processes of dissolution and recrystallization of cement particles [45]. The steps are as follows: (1) cement in solution dissolves a large number of ions. Due to irregular motion or interatomic attraction, ions move to the matrix surface and are adsorbed to form ion clusters (nuclei). (2) Ionic clusters may adsorb surrounding ions and grow. These ion clusters will continue to grow until their size exceeds the limit of the critical range. After that, the nuclei will enter the growth process. (3) Before reaching critical clusters, the ion clusters may also dissolve and reenter the solution [46]. After that step (1) is repeated.
When cement comes in contact with water, the cement dissolves, releasing large amounts of ions. Ca2+ ions are soon absorbed on the surface of G/GO, increasing zeta potential value. As described in the previous section 3.1, G particles adsorb a large amount of Ca2+ on their surface, while the adsorption of Ca2+ ions on the surface of GO particles is higher than that of G, QP, and C–S–H particles. After a while, the Ca2+ concentration in the vicinity of G/GO gradually increased. With a high surface area and strong affinity to Ca2+, G/GO can greatly promote the mobility of ions in cement paste, facilitating the development of ionic clusters and thus generating a large quantity of needle-shaped C–S–H particles at the cement surface, as demonstrated in Figures 3 and 4. Many studies [11,14,25,26,27,47,48] have suggested that the high surface area of G/GO may provide additional sites for C–S–H nucleation, enhancing the nucleation effect, thus facilitating the hydration process. However, the nucleation effect on the G/GO surface is difficult to be observed experimentally. In this study, the high-density C–S–H covered on the surface of cement particles in CG/CGO paste is not due to the nucleation effect of G/GO. This is possible because G/GO increases the mobility of ions in cement paste, which greatly increases the interaction of Ca2+ with the surface of cement particles, thus promoting the nucleation and growth process of C–S–H on cement surface. In other words, the high mobility ions derived by G/GO in the cement paste dominate the reason for the accelerated hydration of cement.
4 Conclusions
The zeta potential of G is similar to QP and C–S–H particles in Ca(OH)2 solutions. The higher zeta potential of GO indicates a stronger interaction between the GO surface and the Ca2+ ions.
The C–S–H on the surface of cement particles in CG and CGO past is denser and more uniform than that in PC paste at the same hydration time.
The exothermic peak value in CG and CGO paste is 5.1 and 9.8% higher than that in PC paste. The total accumulation exothermic of CGO paste was 6.0% higher than that of PC paste.
The presence of G, especially GO, facilitates the mobility of ions, especially Ca2+, and thus enhances the interaction between the cement surface and the ions. This strong interaction promotes the C–S–H nucleation and growth, and therefore, the hydration of the cement.
-
Funding information: The authors would like to acknowledge the financial support from the National Nature Science Foundation of China (Grant No. 51925802 and 52008119), the Natural Science Foundation of Guangdong Province (Grant No. 2019A1515110799 and 2021A1515012624), and the 111 Project (Grant No. D21021).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Zhao Z, Qi T, Zhou W, Hui D, Xiao C, Qi J, et al. A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials. Nanotechnol Rev. 2020;9(1):303–22.10.1515/ntrev-2020-0023Search in Google Scholar
[2] Liew KM, Kai MF, Zhang MW. Carbon nanotube reinforced cementitious composites: an overview. Compos Part A Appl Sci Manufact. 2016;91(1):301–23.10.1016/j.compositesa.2016.10.020Search in Google Scholar
[3] Fouaidi M, Jamal M, Zaite A, Belouaggadia N. Bending analysis of functionally graded graphene oxide powder-reinforced composite beams using a meshfree method. Aerosp Sci Technol. 2021;110:106479–80.10.1016/j.ast.2020.106479Search in Google Scholar
[4] Yan Y, Nashath FZ, Chen S, Manickam S, Lim SS, Zhao H, et al. Synthesis of graphene: potential carbon precursors and approaches. Nanotechnol Rev. 2020;9(1):1284–314.10.1515/ntrev-2020-0100Search in Google Scholar
[5] Scrivener K, Ouzia A, Juilland P, Kunhi Mohamed A. Advances in understanding cement hydration mechanisms. Cem Concr Res. 2019;124:105823.10.1016/j.cemconres.2019.105823Search in Google Scholar
[6] Wang J, Xu Y, Wu X, Zhang P, Hu S. Advances of graphene- and graphene oxide-modified cementitious materials. Nanotechnol Rev. 2020;9(1):465–77.10.1515/ntrev-2020-0041Search in Google Scholar
[7] Zhao L, Guo XL, Song L, Song Y, Dai GZ, Liu JP. An intensive review on the role of graphene oxide in cement-based materials. Constr Build Mater. 2020;241(2020):117939.10.1016/j.conbuildmat.2019.117939Search in Google Scholar
[8] Zhao Y, Liu Y, Shi T, Gu Y, Zheng B, Zhang K, et al. Study of mechanical properties and early-stage deformation properties of graphene-modified cement-based materials. Constr Build Mater. 2020;257:119498–507.10.1016/j.conbuildmat.2020.119498Search in Google Scholar
[9] Gopalakrishnan R, Kaveri R. Using graphene oxide to improve the mechanical and electrical properties of fiber-reinforced high-volume sugarcane bagasse ash cement mortar. Eur Phys J Plus. 2021;136(2):1–15.10.1140/epjp/s13360-021-01179-4Search in Google Scholar
[10] Liu Q, Wu W, Xiao J, Tian Y, Chen J, Singh A. Correlation between damage evolution and resistivity reaction of concrete in-filled with graphene nanoplatelets. Constr Build Mater. 2019;208:482–91.10.1016/j.conbuildmat.2019.03.036Search in Google Scholar
[11] Krystek M, Pakulski D, Patroniak V, Gorski M, Szojda L, Ciesielski A, et al. High-performance graphene-based cementitious composites. Adv Sci (Weinh). 2019;6(9):1801195–210.10.1002/advs.201801195Search in Google Scholar PubMed PubMed Central
[12] Ying G-G, Song C, Ren J, Guo S-Y, Nie R, Zhang L. Mechanical and durability-related performance of graphene/epoxy resin and epoxy resin enhanced OPC mortar. Constr Build Mater. 2021;282:122644–59.10.1016/j.conbuildmat.2021.122644Search in Google Scholar
[13] Dela Vega MSDC, Vasquez MR. Plasma-functionalized exfoliated multilayered graphene as cement reinforcement. Compos Part B Eng. 2019;160:573–85.10.1016/j.compositesb.2018.12.055Search in Google Scholar
[14] Liu C, Huang X, Wu Y-Y, Deng X, Zheng Z. The effect of graphene oxide on the mechanical properties, impermeability and corrosion resistance of cement mortar containing mineral admixtures. Constr Build Mater. 2021;288:123059–73.10.1016/j.conbuildmat.2021.123059Search in Google Scholar
[15] Pei C, Ueda T, Zhu J. Investigation of the effectiveness of graphene/polyvinyl alcohol on the mechanical and electrical properties of cement composites. Mater Struct. 2020;53(3):1–15.10.1617/s11527-020-01508-6Search in Google Scholar
[16] Zhai S, Pang B, Liu G, Zhang Y, Xu K, She W, et al. Investigation on preparation and multifunctionality of reduced graphene oxide cement mortar. Constr Build Mater. 2021;275:122119–32.10.1016/j.conbuildmat.2020.122119Search in Google Scholar
[17] Wang B, Pang B. Mechanical property and toughening mechanism of water reducing agents modified graphene nanoplatelets reinforced cement composites. Constr Build Mater. 2019;226:699–711.10.1016/j.conbuildmat.2019.07.229Search in Google Scholar
[18] Baomin W, Shuang D. Effect and mechanism of graphene nanoplatelets on hydration reaction, mechanical properties and microstructure of cement composites. Constr Build Mater. 2019;228:116720.10.1016/j.conbuildmat.2019.116720Search in Google Scholar
[19] Indukuri CSR, Nerella R. Enhanced transport properties of graphene oxide based cement composite material. J Build Eng. 2021;37:102174.10.1016/j.jobe.2021.102174Search in Google Scholar
[20] Pellenq RJM, Van Damme H. Why does concrete set? The nature of cohesion forces in hardened cement-based materials. MRS Bull. 2011;29(5):319–23.10.1557/mrs2004.97Search in Google Scholar
[21] Liu Y, Jia M, Song C, Lu S, Wang H, Zhang G, et al. Enhancing ultra-early strength of sulphoaluminate cement-based materials by incorporating graphene oxide. Nanotechnol Rev. 2020;9(1):17–27.10.1515/ntrev-2020-0002Search in Google Scholar
[22] Wang L, Li Q, Song J, Liu S. Effect of graphene oxide on early hydration and compressive strength of Portland cement-copper tailing powder composite binder. Powder Technol. 2021;386:428–36.10.1016/j.powtec.2021.04.006Search in Google Scholar
[23] Chintalapudi K, Pannem RMR. The effects of graphene oxide addition on hydration process, crystal shapes, and microstructural transformation of ordinary portland cement. J Build Eng. 2020;32:101551–61.10.1016/j.jobe.2020.101551Search in Google Scholar
[24] Wang Q, Wang J, Lu CX, Liu BW, Zhang K, Li CZ. Influence of graphene oxide additions on the microstructure and mechanical strength of cement. Carbon. 2015;95:1083–4.10.1016/j.carbon.2015.08.089Search in Google Scholar
[25] Ho VD, Ng C-T, Ozbakkaloglu T, Goodwin A, McGuckin C, Karunagaran RU, et al. Influence of pristine graphene particle sizes on physicochemical, microstructural and mechanical properties of Portland cement mortars. Constr Build Mater. 2020;264(2020):120188–200.10.1016/j.conbuildmat.2020.120188Search in Google Scholar
[26] Ho VD, Ng C-T, Ozbakkaloglu T, Karunagaran RU, Farivar F, Goodwin A, et al. Investigating the reinforcing mechanism and optimized dosage of pristine graphene for enhancing mechanical strengths of cementitious composites. RSC Adv. 2020;10(70):42777–89.10.1039/D0RA07639BSearch in Google Scholar
[27] Yan S, Yan S, Tang J, Wang X. Effect of graphene on the mechanical properties of the cement paste. J Appl Mech Tech Phys. 2020;61(6):972–8.10.1134/S0021894420060103Search in Google Scholar
[28] Zhao L, Guo X, Liu Y, Zhao Y, Chen Z, Zhang Y, et al. Hydration kinetics, pore structure, 3D network calcium silicate hydrate, and mechanical behavior of graphene oxide reinforced cement composites. Constr Build Mater. 2018;190:150–63.10.1016/j.conbuildmat.2018.09.105Search in Google Scholar
[29] Wan H, Zhang Y. Interfacial bonding between graphene oxide and calcium silicate hydrate gel of ultra-high performance concrete. Mater Struct. 2020;53(2):1–12.10.1617/s11527-020-01467-ySearch in Google Scholar
[30] Fan D, Yang S, Saafi M. Molecular dynamics simulation of mechanical properties of intercalated GO/C–S–H nanocomposites. Comput Mater Sci. 2021;186:110012–23.10.1016/j.commatsci.2020.110012Search in Google Scholar
[31] Wang P, Qiao G, Hou D, Jin Z, Wang M, Zhang J, et al. Functionalization enhancement interfacial bonding strength between graphene sheets and calcium silicate hydrate: Insights from molecular dynamics simulation. Constr Build Mater. 2020;261:120500–9.10.1016/j.conbuildmat.2020.120500Search in Google Scholar
[32] Kong D, Huang S, Corr D, Yang Y, Whether SPShah. do nano-particles act as nucleation sites for C–S–H gel growth during cement hydration. Cem Concr Compos. 2018;87:98–109.10.1016/j.cemconcomp.2017.12.007Search in Google Scholar
[33] Alamdarlo FV, Solookinejad G, Zahakifar F, Jalal MR, Jabbari M. Study of kinetic, thermodynamic, and isotherm of Sr adsorption from aqueous solutions on graphene oxide (GO) and (aminomethyl)phosphonic acid–graphene oxide (AMPA–GO). J Radioanal Nucl Chem. 2021;329:1033–43.10.1007/s10967-021-07845-2Search in Google Scholar
[34] Garrault-Gauffinet S, Nonat A. Experimental investigation of calcium silicate hydrate (C–S–H) nucleation. J Cryst Growth. 1999;200(3/4):565–74.10.1016/S0022-0248(99)00051-2Search in Google Scholar
[35] Ouyang X, Koleva DA, Ye G, van Breugel K. Understanding the adhesion mechanisms between C–S–H and fillers. Cem Concr Res. 2017;100:275–83.10.1016/j.cemconres.2017.07.006Search in Google Scholar
[36] Ouyang X, Koleva DA, Ye G, van Breugel K. Insights into the mechanisms of nucleation and growth of C–S–H on fillers. Mater Struct. 2017;50(5):213.10.1617/s11527-017-1082-ySearch in Google Scholar
[37] Ouyang X, Wang L, Fu J, Xu S, Ma Y. Surface properties of clay brick powder and its influence on hydration and strength development of cement paste. Constr Build Mater. 2021;300(7):123958.10.1016/j.conbuildmat.2021.123958Search in Google Scholar
[38] Aono Y, Matsushita F, Shibata S, Hama Y. Nano-structural changes of C–S–H in hardened cement paste during drying at 50°C. J Adv Concr Technol. 2007;5(3):313–23.10.3151/jact.5.313Search in Google Scholar
[39] Nachbaur L, Nkinamubanzi P-C, Nonat A, Mutin JC. Electrokinetic properties which control the coagulation of silicate cement suspensions during early age hydration. J Colloid Interface Sci. 1998;202:261–8.10.1006/jcis.1998.5445Search in Google Scholar
[40] Viallis-Terrisse H, Nonat A, Petit JC. Zeta-potential study of calcium silicate hydrates interacting with alkaline cations. J Colloid Interface Sci. 2001;244(1):58–65.10.1006/jcis.2001.7897Search in Google Scholar
[41] Park S, Ruoff RS. Chemical methods for the production of graphenes. Nat Nanotechnol. 2009;4(4):217–24.10.1038/nnano.2009.58Search in Google Scholar PubMed
[42] Ouyang X, Wang L, Xu S, Ma Y, Ye G. Surface characterization of carbonated recycled concrete fines and its effect on the rheology, hydration and strength development of cement paste. Cem Concr Compos. 2020;114:103809.10.1016/j.cemconcomp.2020.103809Search in Google Scholar
[43] Meng W, Khayat KH. Effect of graphite nanoplatelets and carbon nanofibers on rheology, hydration, shrinkage, mechanical properties, and microstructure of UHPC. Cem Concr Res. 2018;105:64–71.10.1016/j.cemconres.2018.01.001Search in Google Scholar
[44] Goldberg SJGECA. Review of chemistry of the solid-water interface. Processes at the mineral-water and particle-water interface in natural systems, by Werner Stumm. J Colloid Interface Sci. 1992;57(3):205–19.10.1097/00010694-199309000-00010Search in Google Scholar
[45] Gauffinet S, Finot E, Nonat AJM. Structures, experimental study and simulation of C–S–H nucleation and growth. J Colloid Interface Sci. 1997;62(2):1–16.Search in Google Scholar
[46] Vekilov PG. Nucleation. Cryst Growth Des. 2010;10(12):5007–19.10.1021/cg1011633Search in Google Scholar PubMed PubMed Central
[47] Lin C, Wei W, Hu YH. Catalytic behavior of graphene oxide for cement hydration process. J Phys Chem Solids. 2016;89:128–33.10.1016/j.jpcs.2015.11.002Search in Google Scholar
[48] Lu Z, Li X, Hanif A, Chen B, Parthasarathy P, Yu J, et al. Early-age interaction mechanism between the graphene oxide and cement hydrates. Constr Build Mater. 2017;152:232–9.10.1016/j.conbuildmat.2017.06.176Search in Google Scholar
© 2021 Shaoqiang Meng et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

