Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
-
Muhammad Hanif Sainorudin
, Munirah Mahizan
, Zahira Yaakob
and Mahboob Alam
Abstract
The present study focused on the preparation of microcrystalline cellulose (MCC) and nanocrystalline cellulose (NCC) from pineapple (Ananas comosus L.) leaves using chemical treatments followed by acid hydrolysis. Pineapple leaves could be used in medical applications such as drug delivery carriers. Advanced spectroscopy techniques such as Fourier-transform infrared (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) were used to analyze the physical, chemical, and morphological features of the isolated MCC and NCC; the results indicated the needle-shaped form of nanostructures with good purity and high crystallinity index of 75.00 and 76.38%, respectively. In addition, inhibition of the treated MRC-5 cells with all the samples revealed that the percentage of cell viability was less than 30%, which is an interesting finding given their role in the cytotoxicity effect of MCC and NCC. It appears that MCC and NCC derived from pineapple leaves have lower toxicity. As a result, the developed MCC and NCC can be used in pharmaceutical applications as a novel drug delivery system. Molecular docking was performed to understand the non-bonding interaction of cellulose with human acid-beta-glucosidase (β-Glc) (PDB: 1OGS). The docking result shows that cellulose unit docked within the active pocket of the enzyme by forming hydrogen bonds against ASN19, THR21, and VAL17 with distances of 2.18, 1.93, and 2.92 Å, respectively, with binding energy (−5.0 kcal/mol) resulting in close interaction of cellulose unit with the receptor.
1 Introduction
The growing interest in cellulose-based products from a sustainable, renewable, and environmentally friendly source has placed cellulose on top of attractive nanomaterials for commercialization in recent years. Extensive use of nanomaterials produced from natural fibres has improved its properties and generated new commercial products. A new approach, however, is possible of the use of biodegradable and easily recyclable materials for the convenience of human life. Many of the building blocks widely used in understanding communication and electronic hardware are made from these cellulose-based materials. Still, it has also shown increasing importance in human exposure, specifically as a drug delivery carrier [1,2]. Cellulose that can be isolated from agricultural wastes needs excellent attention and it has the potential to open up new opportunities for advanced technologies in the biomedical industry [3]. It is a natural biopolymer that is most abundant on the Earth, relatively inexpensive, and widely used as a medicinal excipient aiding the release of prolonged and regulated drugs [4]. Despite the fact that earlier research has concluded that cellulose has minimal or low toxicity in the use as biomedical materials [5,6], concerns regarding cytotoxicity and biosafety should be further focused on these natural nanomaterials.
Several strategies for extracting cellulose from lignocellulosic biomass and modifying cellulosic derivatives in order to produce micro and nanocrystalline cellulose (MCC and NCC) with higher purity and crystallinity degrees have been reported [7]. On the other hand, their versatility and ability to modify physicochemical qualities increased the potential of MCC and NCC to be used in various applications. The utilization of cellulose for drug delivery is an active area of research since they own notable physical features, unique chemical surface characteristics, and even excellent biological properties [8]. Generally, cellulose is composed of β-1,4 glycosidic unit bonds. Cellulose can be purified and isolated from plant cell walls and have unique physicochemical and structural characteristics that make it possible to be used in advanced nanomaterials [9] and nanoengineering applications [3]. Linear chains of cellulose microfibrils combine to form a region consisting of disordered (amorphous) and highly ordered (crystalline) structures. Once the crystals of cellulose are removed from biomass such as pineapple leaves, terminologies such as MCC and NCC can be recognized based on the size of its molecules [10]. Exclusion of the amorphous region influences the structure and crystallinity of the cellulose fibres [11]. Natural cellulose derived from biomass can be processed into micro- and nanosized materials like MCC and NCC possessing good properties for specific applications [12,13] and expected to have low or non-toxicity.
With the increasing presence of MCC and NCC in consumer products, it is important to determine and confirm the safety of these materials to be used in pharmaceutical applications. Cellulose-based materials like MCC and NCC must be non-toxic to the human body. The finding regarding in vitro studies for MCC and NCC derived from pineapple leaves on normal cells is still lacking. The potential used as the drug carrier system is limited, as well as the development of MCC and NCC from pineapple leaves for drug delivery carriers is not fully explored. Since the pineapple plant (Ananas comosus) is among the nutrient-richest plant, they offer many benefits in biomedical applications [14]. In Malaysia, the pineapple plant is harvested annually for its fruit, and the remaining parts are disposed of as agricultural waste, such as leaves. The pineapple leaf fibre obtained from the leaves of the pineapple plant, which comes from the Bromeliaceae family [15], is very hygroscopic, widely available, and of low cost, and can be considered a natural fibre that exhibits high specific strength and rigidity.
Pineapple plants are overwhelmingly valuable species and are the most prominent tropical fruits in the world. The use of its waste as the primary source of substitute fibre production is very promising. Pineapple plantations produce many leaves during the post-harvesting that can be used for high fibre-based materials. This waste, if not used properly, will bring a negative impact on the environment. Cellulose isolated from this type of agro-wastes permits the formation of high-purity crystals[16]. The physical appearance of pineapple leaves is waxy, 50–180 cm long, sword-like, and have sharp thorns on the edges that are arranged in a rosette around the stem, and the colour varies from a uniform green to red, yellow, or ivory of the striped as shown in Figure 1. Pineapple (Ananas comosus L.) leaves are among a plant residue containing high cellulose, almost identical with coconut husk and sugarcane bagasse [17].

Photograph of Ananas comosus plant.
Alternatives cheap and readily available raw materials are needed to replace the limited source of petroleum-based products. Countries grow crops and fruits not only for agricultural purposes but also to produce raw materials for industries [18]. Many developing countries trade in cellulose fibres to improve the economic condition of poor farmers with support from the country [19]. This work aims to investigate the safety and cytotoxicity assessment of MCC and NCC derived from pineapple leaves for biomedical purposes, as the isolation of cellulose from pineapple leaves for medical use has not been widely explored. Moreover, this work can provide important information in planning further studies on MCC and NCC to see its suitability and compatibility in the application of the drug delivery system. Furthermore, molecular docking of cellulose units was carried out in order to interact with human acid-beta-glucosidase (PDB: 1OGS). The study of these interactions will be beneficial and will provide ideas for biomedical purposes, especially in order to understand human cells compatibility in tissue generation as well as for drug delivery. The MCC and NCC derived from various raw materials using different acid hydrolysis methods, including crystallinities are presented in Table 1 as documented elsewhere.
Essential sources of lignocellulosic biomass for crystalline cellulose production
| Raw materials | Methods | Types of crystalline cellulose formed with *CI% | *Ref. |
|---|---|---|---|
| Eucalyptus pulp | Sulphuric acid | MCC and NCC | [20] |
| Pomelo peel | Hydrochloric acid | MCC with 40.5 | [21] |
| Roselle fibres | Hydrochloric acid | MCC with 78 | [22] |
| Corn cob | Hydrochloric acid | MCC | [23] |
| Sulphuric acid | |||
| Cotton gin waste | Hydrochloric acid | MCC | [24] |
| Sulphuric acid | |||
| Alfa fibres | Hydrochloric acid | MCC with 73.6 | [25] |
| Oil palm biomass | Sulphuric acid | MCC with 87 | [26] |
| Bacterial cellulose from acetobacter xylinum | Sulphuric acid | MCC with 69 | [27] |
| Pineapple crown leaf | Sulphuric acid | NCC with 78.21 | [28] |
| Pineapple peel residues | Hydrochloric sulphuric acid | NCC | [29] |
| Tomato stem | Sulphuric acid | NCC with 74.4 | [30] |
| Wheat stalk | Sulphuric acid | MCC and NCC | [31] |
| Rice husk | Sulphuric acid | NCC with 65 | [31] |
| Sugarcane bagasse | Sulphuric acid | NCC with 68.28 | [32] |
| Sugar palm fibres | Sulphuric acid | NCC with 85.9 | [33] |
| Rose stem | Alkaline peroxide | MCC with 65.4 | [34] |
| Date seeds | Hydrochloric | MCC with 70 | [35] |
*CI: crystallinity index, Ref.: references.
2 Materials and methods
2.1 Materials
The pineapple leaf wastes were collected and supplied by the local village area, Dengkil (Selangor, Malaysia). RPMI 1640, penicillin/streptomycin, and foetal bovine serum (FBS) were purchased from Naqalai Tech, Japan; and phosphate-buffered saline (PBS) and 0.25% trypsin-EDTA were purchased from Sigma Aldrich, (UK). The MTT reagent was purchased from Merck (Germany). Hydrochloric acid (HCl), sulphuric acid (H2SO4), sodium hydroxide (NaOH), sodium hypochlorite (NaClO), hydrogen peroxide (H2O2), and dimethyl sulphoxide (C2H6OS) were bought from Sigma Aldrich, UK. Unless otherwise stated in the text, all other chemicals were purchased from R&M Chemicals, Germany.
2.2 Isolation of MCC and NCC
Figures 2 and 3 illustrate several procedures leading to the preparation of MCC and NCC from pineapple leaves. The raw samples of pineapple leaves were obtained and washed in distilled water for purification. The samples were then dried in an oven for over 48 h at a temperature of 60°C. Then, the dried samples were ground in a grinder machine and sieved until the required powder sizes were reached. The samples were packed in a plastic polyethylene bag and then placed at room temperature for further processing [36]. The steps outlined below and in Figure 2 were used to prepare and purify MCC from pineapple leaves. Step (1) 15 g of samples were added with 2 wt% of NaOH for 5 h at 80°C. Step (2) The material was washed three times with distilled water, filtered with Whatman paper, and dried in a hot air oven at 60–65°C until it reached a constant mass. Step (3) The oven-dry sample was bleached at a ratio of 1:1 NaClO for 15 min at 75°C followed by washing with distilled water; the sample was then treated with 12 wt% of NaOH at 80°C for 1 h. Step (4) The samples obtained in step 3 were whitened with a 1:3 NaClO solution for 1 h at 75°C and then with a 1:1 NaClO solution for 24 h at room temperature before being washed until neutral. The samples were then filtered and dried for 1 h at 80°C. Step (5) The sample derived from step 4 was hydrolyzed with 2.5 N HCl for 15 min at 100°C. The hot acid mixture was poured into cold tap water with vigorous stirring and allowed to stand overnight. The sample was filtered, washed with distilled water, dried in the oven at 60°C for 1 h, and the sample obtained from step 5 was used for chemical and morphological studies. The preparation of NCC is shown as a graphical presentation in Figure 3.
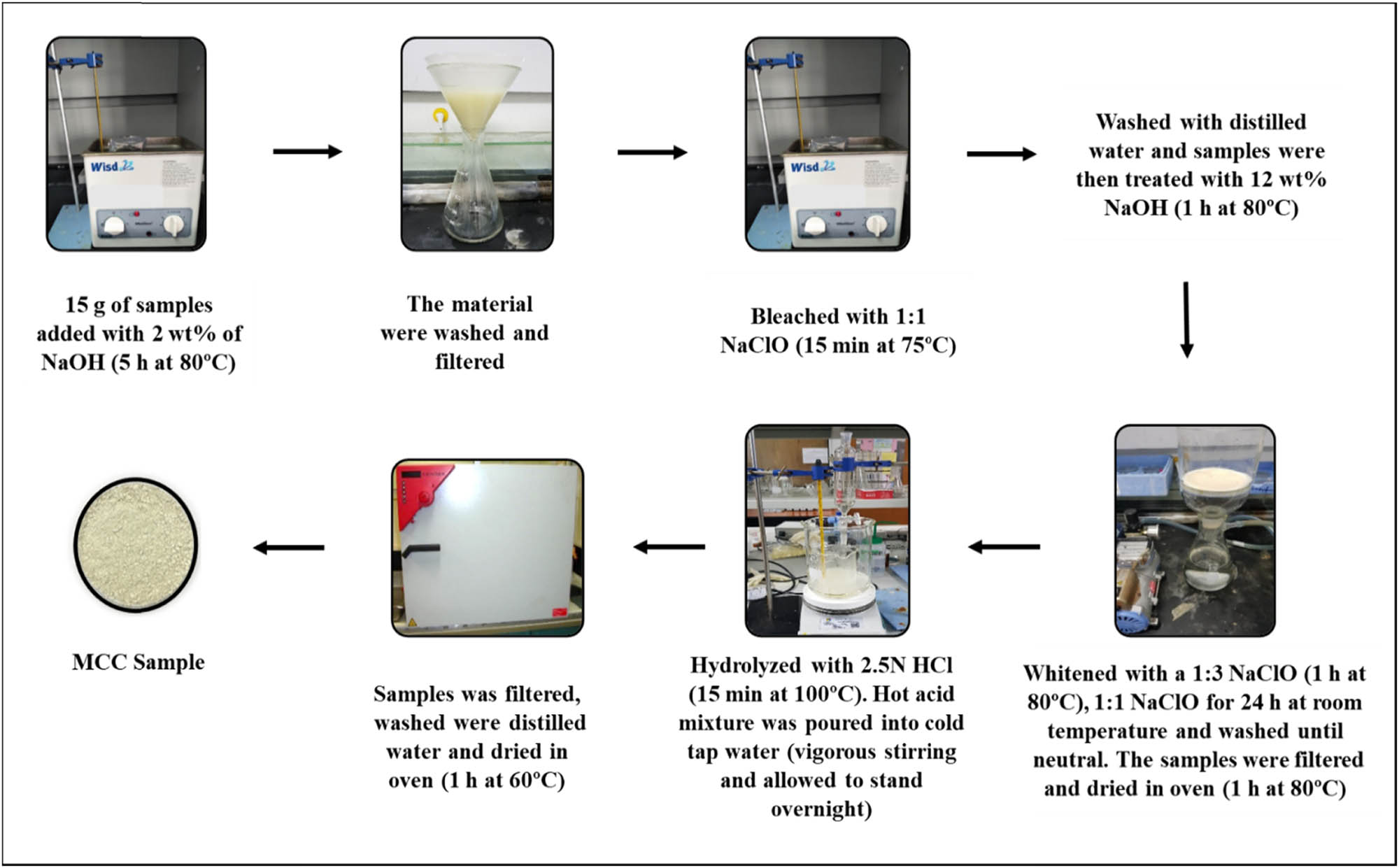
Procedures of preparation of MCC from pineapple leaves.

Procedures of preparation of NCC from pineapple leaves.
2.3 Characterization of MCC and NCC from pineapple leaves
FTIR (Shimadzu IRPrestige-21) spectroscopy was used to determine the chemical properties and the molecular structural analysis. The KBr disc (ultra-thin pellets) approach was employed while taking the IR spectra. The samples were mixed with KBr (sample/KBr ratio, 1:100). The spectra were recorded in the range 500–4,000/cm with a resolution of 4/cm and a total of 32 scans for each sample was obtained. The crystalline phase of the MCC and NCC samples was evaluated using XRD (Bruker AXS-D8 Advance) at 40 kV and 40 mA with a wavelength of 0.15406 nm (λ). For each sample, the crystallinity index was determined using the following equation [37]:
where CI is the crystallinity index (%), I cry is the maximum intensity between 22° and 24°, and I ams is the amorphous material intensity between the peaks at an angle of approximately 18° in the valley.
The crystallite size was inferred using the Scherrer equation (equation (2)) [38,39],
where k is the Scherrer constant (0.94), λ is the X-ray radiation wavelength, β is the full width at half-maximum (FWHM) of the diffraction peak, and θ is the corresponding Bragg angle.
Morphological properties were studied using SEM and TEM. The surface of the NCC sample was coated with gold/palladium using a sputter coater device of Bal-Tec (MultiCoating System MED20), suggesting an excellent sample preparation technique. The sample was subjected to SEM (JEOL-JSM-6010LA) with an acceleration voltage of 15 kV. The diluted suspension of MCC and NCC was placed on a copper grid covered with a thin carbon film, stained with a 2 wt% of uranyl acetate solution, and dried at room temperature before subjecting to TEM. The dimension images of the MCC and NCC sample were observed using a TEM (JEM-2100F) operating at 80 kV of acceleration voltage.
2.4 Cytotoxicity studies
MRC-5 cells were cultured in RPMI 1640 containing foetal bovine serum (10%) and penicillin/streptomycin (10 mL) prior to cytotoxicity assay. MCC and NCC from ananas (pineapple) leaves and commercial MCC (1,000, 100, 10, 1, and 0.1 µg/mL) were dissolved in 4% NaOH solution prepared in deionized water, as treatment solutions. A 96 well plate for the treatment was filled with 90 µL of media containing MRC-5 cells and 10 µL of the treatment solution was added prior to the cytotoxicity assay. A 96 well plate for the treatment was filled with 90 µL of media containing MRC-5 cells and 10 µL of the treatment solution. The treatments were incubated in four replicates for 24, 48, and 72 h. MTT solution (50 µL) was added to each well and incubated at 37°C for 4 h [40]. DMSO (100 µL) then was added after the cells with MTT solution in each well were pipetted out. The absorbance reading at a wavelength of 570 nm was measured by using a plate reader. The cell viability was evaluated and was plotted by using GraphPad Prism 5.0. The findings from the cytotoxicity graph and GraphPad Prism 5.0 were collected and used to assess the treatment’s absorbance reading. Using Student t-test analysis, the statistical significance between variables was tested in four replicates for each experiment, and p values
2.5 Molecular docking simulation
Molecular docking of cellobiose, the reducing unit of cellulose against human acid-beta-glucosidase (PDB: 1OGS), was carried out according to the instructions using the AutoDock 4.2 tool [41]. Briefly, the polar hydrogen atoms and the Kollman charges were assigned to the receptor protein. Partially Gasteiger charges were assigned to the ligand, and nonpolar hydrogen atoms were fused. A grid map of 40 × 40 × 40 Å with grid centre 23.05 70.77 −19.66 (x, y, and z positions, respectively) was used to cover the active site of protein structure to get the best conformational state of docking. The best docking pose was chosen based on the lowest binding energy (kcal/mol) values, and hydrogen and hydrophobic interactions were analyzed with Discovery Studio (v3.5). The LIGPLOT method was also used to build a 2-D graphical representation of the best-docked pose [42]. The amino acid residues involved in the formation of active pockets in the receptor for interaction with ligands/molecules, such as ARG2, THR21, ASN19, TYR22, ASN19, THR21, THR21, TYR22, THR21, THR21, TYR22, and VAL17, were identified based on a hetero atom co-crystallized with active sites of receptor already present in human acid beta-glucosidase (PDB: 1OGS).
3 Results and discussion
3.1 Structures of MCC and NCC from pineapple leaves
The isolated MCC and NCC powders from pineapple leaves are shown in Figure 4. Referring to the results, the MCC sample was found to be yellowish-white powder while the obtained NCC was a snowy white powder in colour. There is a difference in appearance for both samples when compared to the NCC sample. The colour of both samples changed from brown to light brown after alkaline treatment. The materials that were treated by the bleaching process appeared white for NCC and slightly yellowish-white for MCC. This visual is very important as it demonstrates that the non-cellulosic components and unwanted constituents throughout have been successfully eliminated by the chemical treatment process. The observed white colour also points out that the obtained samples are almost pure cellulosic materials. However, the texture of both samples is quite different. The difference in both MCC and NCC was influenced by the period of extraction and the suitability of the selected extraction method. Besides, referring to the literature, the processing conditions, nature of the selected plant sources, botanical origin, soil characteristics, and age of the plant also contribute differently to the amount of MCC and NCC residuals produced [43].

(a) Microcrystalline cellulose (MCC) and (b) nanocrystalline cellulose (NCC) derived from pineapple leaves.
Both the obtained samples of MCC and NCC were successfully characterized by FTIR and XRD to evaluate their purity and crystallinity. In general, FTIR characterization depends on the atom vibrations in a molecule, and it can help to classify the functional groups within each of the samples available. The analysis of chemical structure is controlled by the chemical bonds that can vibrate, stretch, and bend. The main functional groups commonly present in cellulose samples, such as aromatic, esters, alcohols, alkanes, and ketones, can be identified by this spectroscopy analysis [44,45]. With high sensitivity, speed of data collection, and improved spectral precision, FTIR spectroscopy is one of the essential characterization studies in the study of MCC and NCC by referring to the wavelength and transmittance in IR radiation.
The alteration in the cellulose crystal structure leads to a reduction or loss in the intensity of certain FTIR peaks from the cellulose crystalline domains. The samples are expected to produce an absorbance region, and the infrared spectra for both MCC and NCC samples are studied based on the literature. The variation of absorptions peaks in the spectra exposed the different functional groups influenced by the modifications in the chemical composition of the fibres during the process of isolating MCC and NCC [46]. The FTIR results for commercial MCC, MCC, and NCC samples are displayed in Figure 5. Lignin and hemicelluloses were successfully eliminated by bleaching, alkali treatment, and acid hydrolysis, as determined by the FTIR spectra. Furthermore, no significant difference was found in both extracted MCC and NCC samples at the cellulose region.
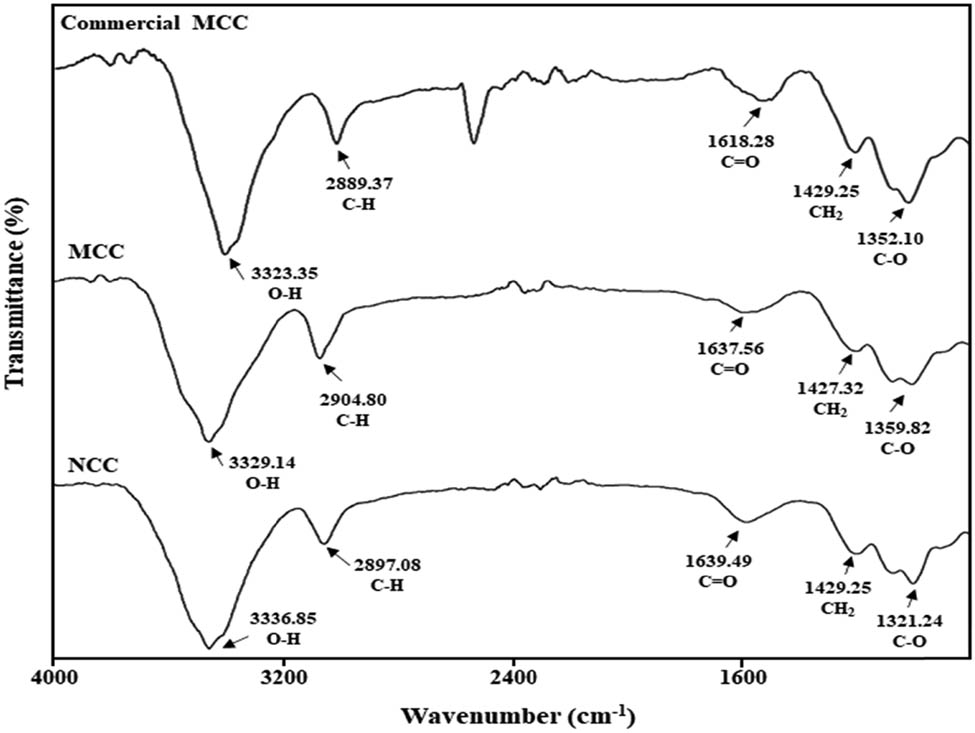
Fourier transform infrared spectra of commercial MCC, MCC, and NCC samples.
Table 2 shows the functional groups of MCC and NCC, isolated from pineapple leaves, that were found in the FTIR spectra. Both samples of MCC and NCC showed almost similar spectra compare with the standard commercial MCC. The commercial MCC has functional groups of O–H at a wavelength of 3323.35/cm, C–H was observed at 2889.37/cm, C═O at 1618.28/cm, CH2 at 1429.25/cm, and 1352.10/cm was assigned to C–O stretching. The strong dominant absorption peak associated with cellulose in extracted MCC and NCC occurs in the region from 3329.14 to 3336.85/cm, which points at the bending of the hydroxyl (O–H) groups and the stretching of hydrogen bonds to the cellulosic structure [47]. This appearance may be attributed to the absorption of moisture or water in the samples. Besides, the alkali treatment involved in the isolation of MCC and NCC reduces hydrogen bonding by removing the hydroxyl groups through a reaction with sodium hydroxide. This led to the increase in –OH concentration, evident from the increased intensity of the peak in the spectra. In both spectra, the characteristic band at about 2897.08–2904.80/cm is close to the C–H stretching vibration of alkyl groups in aliphatic bonds of cellulose, hemicellulose, and lignin [48].
Functional groups of MCC and NCC from pineapple leaves
| Samples of cellulose | −OH stretching (/cm) | C–H vibration (/cm) | C═O vibration (/cm) | CH2 vibration (/cm) | C–O stretching (/cm) |
|---|---|---|---|---|---|
| Microcrystalline cellulose (MCC) | 3329.14 | 2904.80 | 1637.56 | 1427.32 | 1359.82 |
| Nanocrystalline cellulose (NCC) | 3336.85 | 2897.08 | 1639.49 | 1429.25 | 1321.24 |
The band between 1637.56 and 1639.49/cm was due to the carbonyl group (C═O) stretching vibration of the hemicellulose acetyl and uronic ester groups, or the lignin ferulic and p-coumaric acid carboxylic group [49]. Acid hydrolysis, which is required for the extraction of MCC and NCC from pineapple leaves, eliminates hemicellulose and lignin, resulting in a decrease in the strength of this peak in the cellulose spectrum. The bands that appeared at 1427.32 to 1429.25/cm are caused by CH2 scissoring vibrating shift in MCC and NCC [50]. This peak was recognized as the crystallinity band in the samples and provides evidence of containing cellulose. Furthermore, both spectra of samples displayed the characteristic C–O stretching around 1321.24 to 1359.82/cm. The spectra of MCC and NCC, derived from pineapple leaves, revealed a comparable trend to the cellulose literature defining the O–H, C–H, C═O, CH2, and C–O functional groups [51].
Another critical analysis that is useful for assessing the thermal stability of polymeric materials is thermogravimetric analysis. In evaluating the possible uses of MCC and NCC as biomedical products, thermal stability is an important parameter to be considered. The study of thermal degradation behaviour is significant for the performance of the materials and allowed researchers to optimize the composite design and treatment conditions to produce high-performance polymers with improved thermal stability. The thermal behaviour of the lignocellulosic materials depends on the treatment involved, source of raw materials, structure, chemical composition, and degree of crystallinity [52]. All samples revealed water (moisture or chemically bonded) loss around 100°C and were attributed to dehydration during water removal [53].
Both MCC and NCC samples extracted from pineapple leaves displayed the same trend and pattern with the degradation occurring from 240 to 380°C in the second step. The exceptionally high slope for both samples is due to the depolymerization of the cellulose. The partial cross-linking of cellulose molecules reduces the degree of polymerization at this point, leading to the formation of CO, CO2, H2O, and a variety of hydrocarbon derivatives. The final steps revealed in this analysis show that rapid depolymerization occurs above a temperature of 350°C, and approximately about 70% of the weight loss. This finding is comparable with the results from Gan and co-workers that mentioned three main stages in the degradation profile [54].
The first initial weight loss component was found at about 50°C and approximately 100°C. The thermal stability of both MCC and NCC samples showed that the chemical treatment increased the temperature of degradation, influenced by the elimination of hemicelluloses and lignin. The higher degree of crystallinity also led to higher heat resistance and improved thermal degradation in MCC and NCC samples [55,56]. The residual mass at a temperature of 750°C for MCC samples is 6.13% while that of NCC samples is 14.98%. In comparison between the two samples, MCC showed a higher degradation temperature behaviour than the NCC from pineapple leaves, probably due to the variation in each MCC and NCC’s outer surface structure derived from the different extraction techniques [57].
The crystallinity was measured by XRD. For the analysis of structural properties of MCC and NCC, the XRD technique was used to analyse the degree of crystallinity and the crystal structure of the cellulose samples. The preferred orientation of crystallites, also known as texture, is a significant factor to consider while analysing XRD. The texture development of the sample is frequently influenced by the synthesis techniques, the nature of the crystallites, and pretreatment. It is well known that the relative intensities of diffraction peaks are influenced by these factors and will affect the crystallinity index accordingly [58]. The X-ray diffraction pattern was obtained for both MCC and NCC samples isolated from pineapple leaves. Based on the results, it showed that the samples peaks at 2θ for MCC are 14.5°, 22° and 34° while those for NCC were around 15.5°, 22° and 35°. The samples’ crystallinity index was calculated using the Segal method. Thompson and colleagues noted that Segal’s method allows a rapid and simple way of determining crystallinity for cellulose and its derivatives [59]. Table 3 shows the XRD analysis of the MCC and NCC samples for the crystallinity index (CI) and the crystallite size (nm).
XRD analysis for determining the crystallinity index (CI) and the crystallite size (nm) of MCC and NCC samples
| Samples | Reflection at 2θ (°) | Crystallinity index (%) | Crystallite size (nm) |
|---|---|---|---|
| Microcrystalline cellulose (MCC) | 14.5, 22, 34 | 75.00 | 4.52 |
| Nanocrystalline cellulose (NCC) | 15.5, 22, 35 | 76.38 | 4.70 |
The crystalline structure of the samples is attributed to the primary X-ray diffractogram peaks, as illustrated in Figure 6, while the low intensity is attributed to the amorphous background of the cellulose samples. The XRD properties reveal a strong peak at 2θ = 22°, indicating higher crystallinity for both MCC and NCC samples. The sharp peaks of the graph suggest greater crystallinity activity for all the samples [60]. These peaks have a similar pattern and should resemble the typical cellulose I structure. Cellulose I is the most typical crystalline type, consisting of a series of crystallites and disordered amorphous regions. The significant intensity for both samples from this work was associated with the cellulose I crystalline structure of cellulose.
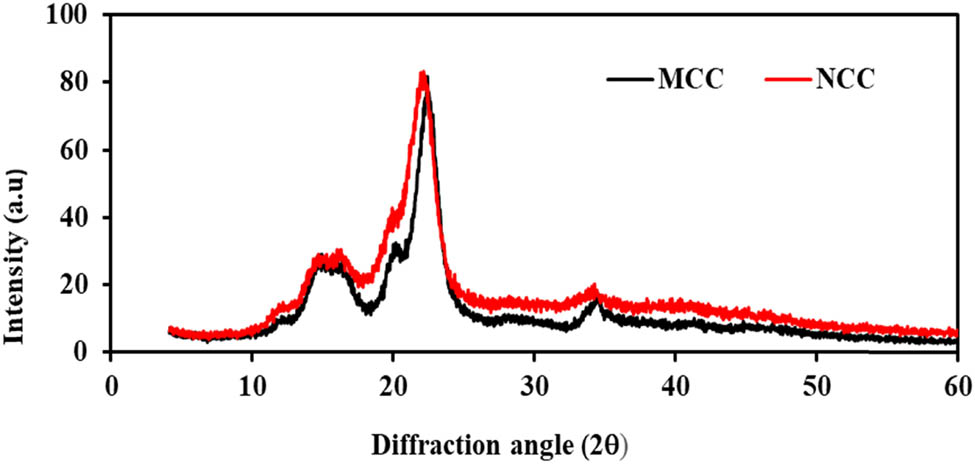
X-ray diffractograms of MCC and NCC from pineapple leaves.
The crystallinity index of MCC, from pineapple leaves, was 75.00%, while NCC has 76.38% of crystallinity. As discussed in several studies, the crystallinity index provides a quantitative analysis of the crystallinity in powder, which can be related to the strength and stiffness of fibres [61]. Low crystallinity reveals a more disordered structure that results in a more amorphous powder, whereas high crystallinity reveals an organized molecular structure that interprets small particles. The crystallite sizes of MCC and NCC that were determined by using the Scherrer equation were 4.52 and 4.70 nm, respectively. The crystallite size results support the description of the crystallinity behaviour of samples obtained.
Compounds of cellulose usually consist of lignin, hemicellulose, and α-cellulose. Cellulose is classified as crystalline in nature, while lignin is considered amorphous. The crystalline structure of cellulose is generally due to the interaction between the neighbouring molecule with the hydrogen bond and van der Waals forces [62]. This crystallinity characteristic is very important in terms of elasticity, rigidity, thermal stability, and also adsorption. According to the findings, it can be stated that the amorphous area of cellulose in MCC and NCC was targeted by the acid solution that was used in acid hydrolysis resulting in the increase of crystallinity index. The acid hydrolysis also eliminated polysaccharides, such as lignin matrix and hemicellulose that were bound in cellulose fibres, and the chains of MCC and NCC were rearranged. The results in the XRD crystallinity profile for MCC and NCC are significant to indicate the digestibility from the obtained samples [63].
3.2 Surface morphological analysis
Figure 7 shows the SEM micrograph of MCC and NCC samples isolated from pineapple leaves at x100 magnification. SEM is a good tool to investigate the morphological changes for both cellulose samples. From compositional analysis based on the literature, the raw pineapple leaves contain cellulose fibres bound by lignin and hemicellulose [64].
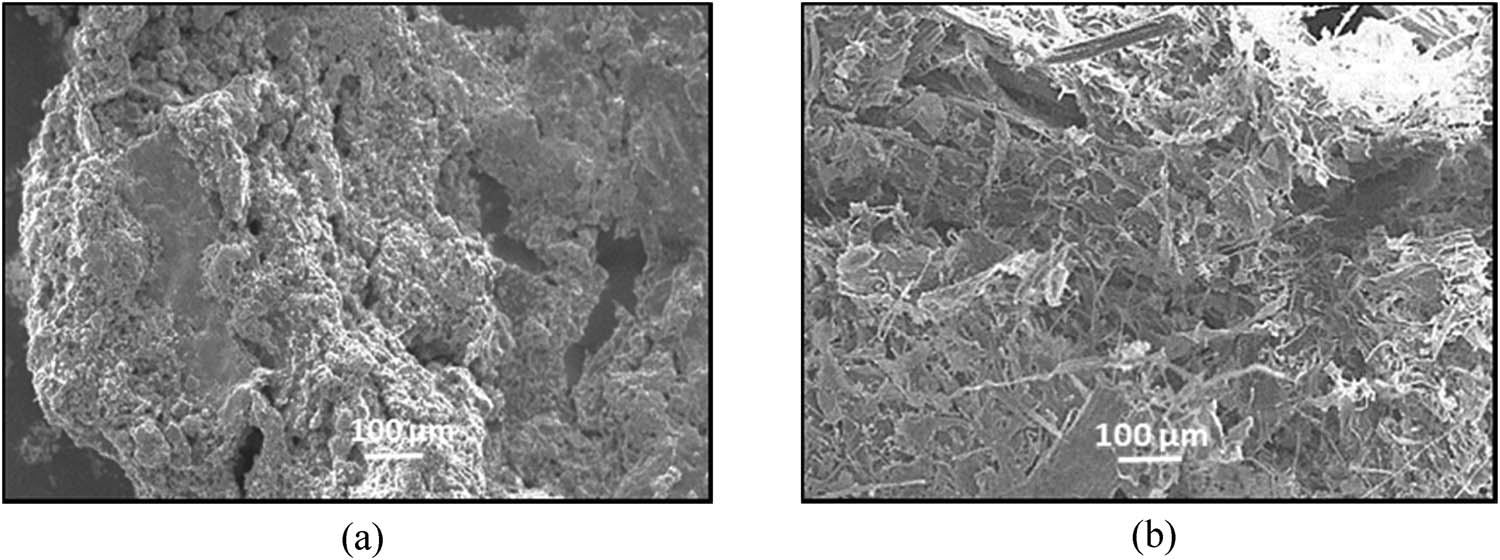
SEM micrograph of (a) MCC and (b) NCC samples isolated from pineapple leaves.
Based on the micrographs for both MCC and NCC, it appears to be composed of a compact structure. They displayed plenty of non-fibrous components spread across the fibre surface. The form of this sample shows an increase in its precise area, favours chemical response that includes acid hydrolysis. The agglomerated “rod-like” shape of the crystal structure increases the surface area and creates the reactivity of the fibres [65]. The compact agglomeration of MCC and NCC suggests intermolecular bonding of hydrogen and strong hydrophilic interaction between cellulose chains. During the bleaching treatment, most lignin is removed from the samples, and the reaction also facilitates in further defibrillation.
In this work, alkaline and bleaching treatments were involved in the isolation process of MCC and NCC for the pre-treatment of waste fibres. Bleaching leads to further defibrillation. Defibrillation occurs in the alkaline treatment and this trend increases in line with bleaching (whitening) treatment due to the expulsion of lignin and hemicellulose. Bleaching treatments can alter the surface of nanofibrils that look smoother than the untreated fibres [66]. Besides SEM analysis, TEM is also one of the good characterization techniques for analysing the morphological surface of nanometer-scale in MCC and NCC samples. The micrograph obtained by TEM allows visualization of the fibrils, which are organized in bundles by the strong attraction force of hydrogen bonds, showing further on the surface of their fibrils. The TEM micrographs as shown in Figure 8 illustrate the needle-like structure consisting mostly of single fibrils and some aggregates. A similar structure was obtained by Mahardika et al. [67] and Prado and Spinacé [68].
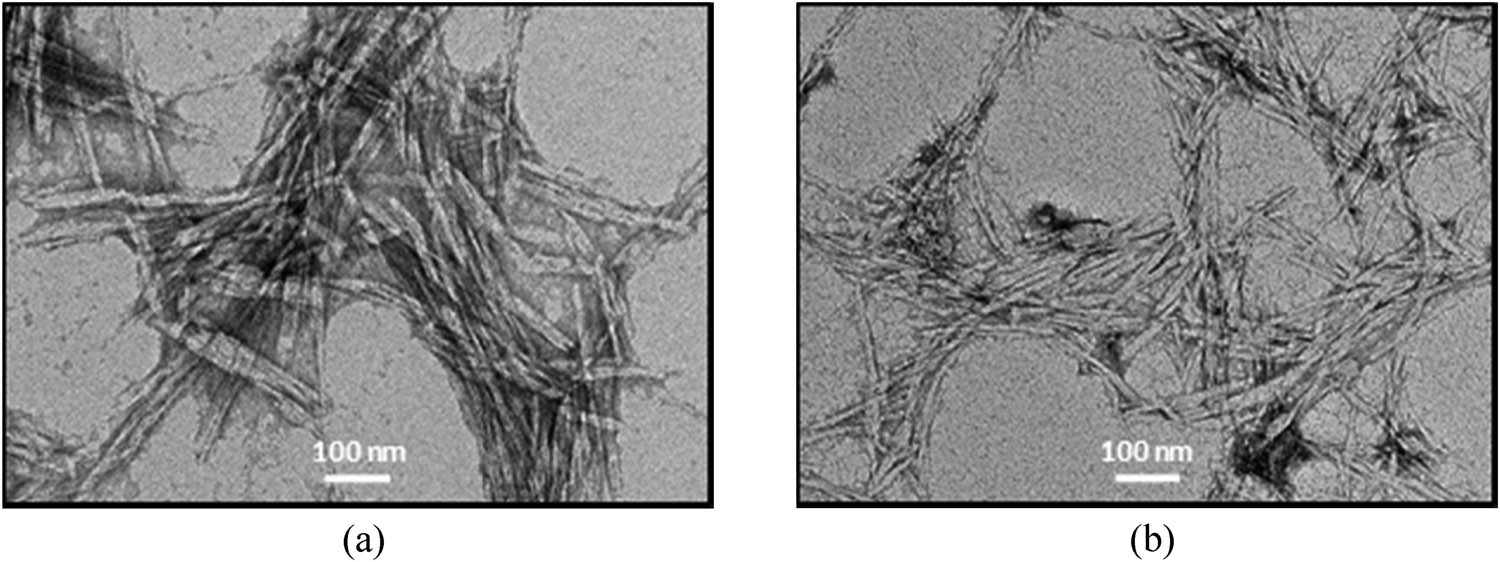
TEM micrographs of (a) MCC and (b) NCC samples isolated from pineapple leaves.
However, the size of both MCC and NCC is smaller than that reported in previous studies. This is because of the involvement of the acid hydrolysis process that removes the amorphous area of cellulosic microfibrils, maintaining intact straight crystalline domains [69]. The process of treatment inevitably decreases the size of the fibres to the nanometer scale. This finding is comparable to the work done by Teixeira and co-workers [70]. Nonetheless, the MCC and NCC agglomerates, as revealed from the TEM image, could be linked to the intermolecular hydrogen bonding between the cellulose.
Meanwhile, the hydrolysis with H2SO4 results in stable NCC aqueous suspension. The NCC surface hydroxyl groups are etherified with sulphate groups, alleviate the suspension by anionic repulsion forces, and have a low aggregation tendency [71]. Referring to the micrograph, it also depicts agglomeration of MCC and NCC bundles and points at dispersed crystallites and individual crystals. The needle-like shape of both samples is proof that the lignin and hemicellulose have been separated and eliminated by alkaline and bleaching (pre-treatment), and also acid hydrolysis causes the fibre bundles to be extracted into individual cellulose nanofibres.
3.3 Cytotoxicity effect of MCC and NCC on MRC-5 cells
Cytotoxicity test is an analysis for evaluating the cytotoxicity effect of compounds on the living organism via the cell viability assessment. This analysis is the simplest in vitro technique, which is a rapid and affordable approach for the initial biocompatibility safety evaluation of MCC and NCC materials. Since cellulose-based materials like MCC and NCC have considerable potential for medical applications, additional work is necessary to obtain further information on their toxicological and characteristics and their possible impacts on human health and safety. This cytotoxicity evaluation can be used as a vital aspect of nanomaterial characterization. MTT assays and MRC-5 cell lines were used to evaluating the cytotoxicity property of the biomaterials since this assay is largely used in the screening of toxic and harmless material compounds.
Figure 9(a–c), respectively, demonstrates the cytotoxicity activity of MCC and NCC against normal lung fibroblast cells (MRC-5). The percentage of cell viability in the medium was mainly dose-dependent. From Figure 9, it is shown that no significant difference in cell viability was observed at a concentration of 0.1 μg/mL relative to control for all cellulose types when exposed to different times. Different concentrations of MCC, NCC, and industrial MCC, ranging from 0.1, 1, 10, 100, and 1,000 μg/mL were tested on the viability of the cells and applied for 24, 48, and 72 h of treatment.
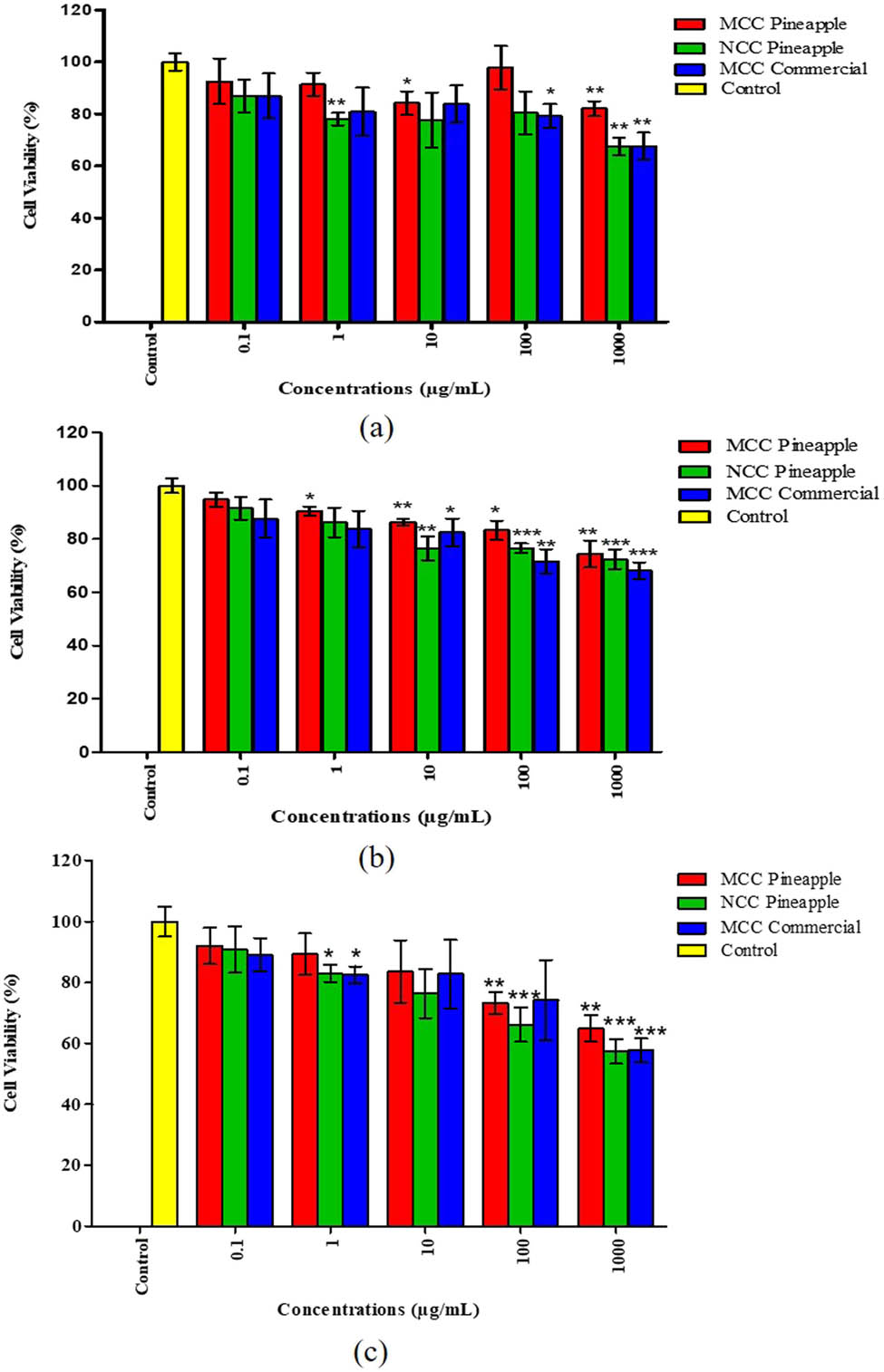
Cytotoxicity effect of MCC, NCC, and commercial MCC against human normal lung cells (MRC-5) for 24, 48, and 72 h of treatment. The analysis was performed using GraphPad Prism 5.0 and t-test analysis was done for each group. The * symbol indicates the significance level of the treatment, where *, **, and *** are p < 0.05, p < 0.01 and p < 0.001, respectively for treatments compared with the control of each group.
The results further showed that MCC and NCC had less toxicity to the MRC-5 cells after a 24 h treatment at lowest concentrations of 0.1, 1, and 10 μg/mL. On the other hand, MCC and NCC demonstrated cell inhibition below 30% at higher concentrations of 100 and 1,000 μg/mL. According to the findings, MCC, NCC, and commercial MCC significantly decreased cell viability at a concentration of 1,000 μg/mL between 70 and 52% compared to control except for MCC in 24 h treatment application. Furthermore, the research on cytotoxicity was investigated here to reveal that MCC and NCC from pineapple leaves were evaluated as less toxic compared to the commercialized MCC. The viability of MRC-5 cells in industrial MCC (1,000 μg/mL) and NCC (100 and 1,000 μg/mL) at 48 h of treatment was significantly lower (p < 0.001). The viability of MRC-5 cells for industrial MCC (100 μg/mL) and MCC (100 and 1,000 μg/mL) was significantly lower (p < 0.001) for 72 h of exposure. Besides, the viability of MRC-5 cells for commercial MCC (1,000 μg/mL) and NCC (100 and 1,000 μg/mL) was significantly different compared to the control. It also emerged from the results that the lowest concentrations (0.1, 1, and 10 μg/mL) of MCC and NCC were less toxic to MRC-5 cells with 24 h of treatment. At higher concentrations (100 and 1,000 μg/mL), the toxicity effect of MCC and NCC still showed cell inhibition below 30%. In spite of the cytotoxicity effects of MCC, NCC, and commercial MCC, the toxic effect was considered to be marginal as the inhibition of the treated MRC-5 cells did not exceed 50% inhibition concentration (IC50).
According to previous studies, different treatments with MCC samples showed negligible toxicity to cell lines used with the lowest concentrations of treatments ranging from 10 to 250 μg/mL [72]. Thus, the results are consistent with the literature findings that indicate negligible cytotoxicity at lower concentrations of the treatments, against different cell lines [73]. In this analysis, we observed that the maximum concentration (1,000 μg/mL) for both cellulose samples showed less toxicity and had major cytotoxicity effects compared to control. Compared to MCC, NCC was typically slightly toxic due to its propensity to form a suspended gel when incubated at higher concentrations that could have blocked the passage of gases through the cell membranes. Meanwhile, at 48 and 72 h of incubation with the maximum concentrations of MCC and NCC (1,000 μg/mL), the percentage of cell viability decreased to 60%. This suggested that the MRC-5 cells may be nutrient deficient because of media condensation during incubation.
3.4 Molecular docking analysis
Figure 10 shows a graphical representation of the best docking pose with the receptor and the active binding pocket region. The lowest binding energy values (kcal/mol) and hydrogen/hydrophobic interaction analyses were used to examine cellulose- β-Glc docked complex (Figure 10c). The pose with the energy values (−5.0 kcal/mol) was found to be the best and most active conformational position. The pattern of hydrogen and hydrophobic contacts between the cellulosic unit and the target protein was investigated in more detail in the best-docked energy complex (10 D). The active amino acid pockets of enzymes such as ASN19, THR21, VAL17, and TYR22 are directly involved in hydrogen bonding with distances of 2.18, 1.63, 2.92, and 2.76 Å (Figure 10c and d).
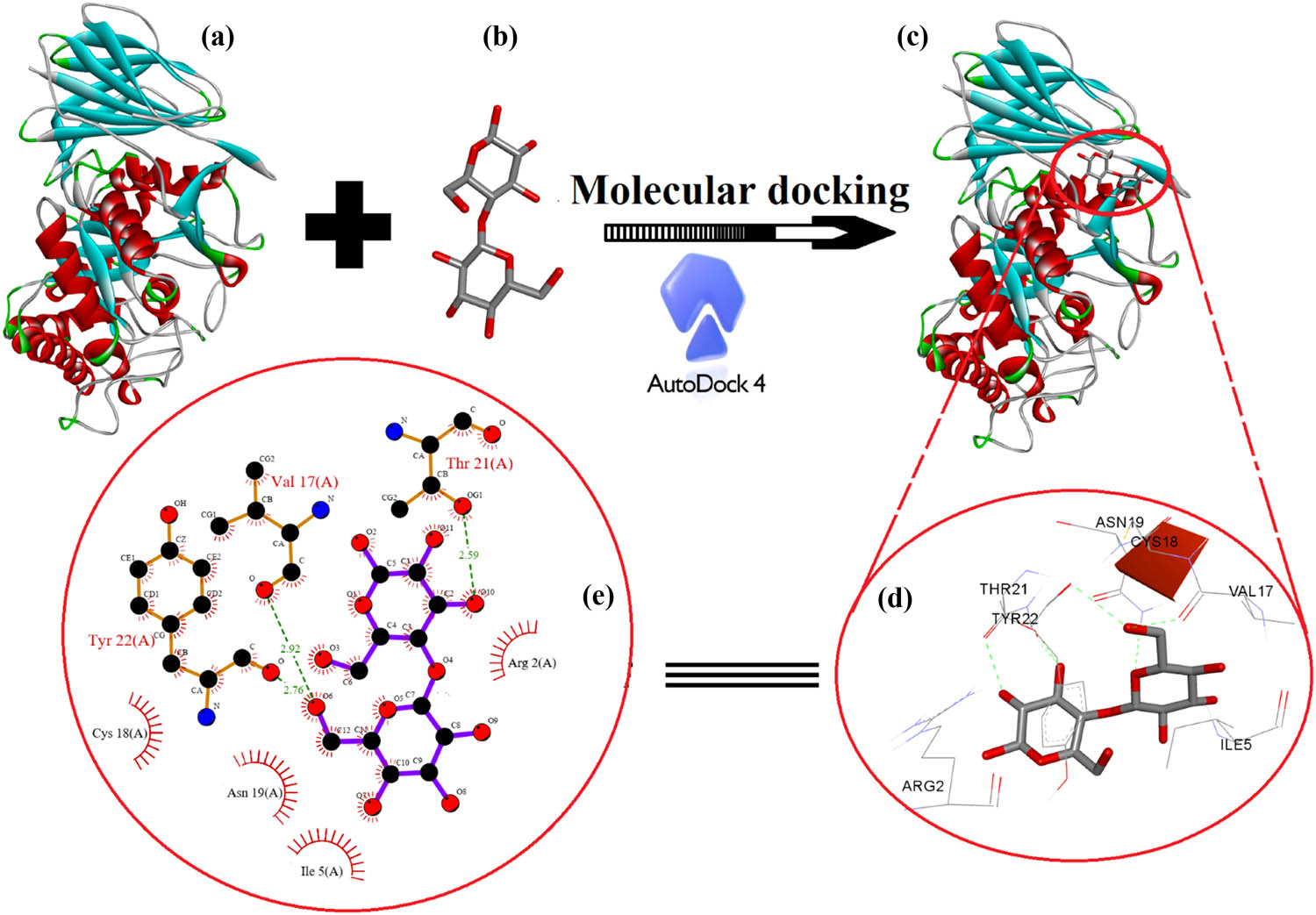
Molecular docking analysis between (a) human acid-beta-glucosidase (PDB: 1OGS) (b) cellulose unit (c), including the best-docked pose showing various interactions profiles and (d and e) 2D interaction diagram showing the active site participation in hydrogen such as hydrophobic and hydrophilic interactions.
Furthermore, hydrophobic and hydrophilic amino acids of the active site of the receptor, such as Cys18, Ile5, Asn19, and Arg2, were involved in stabilizing the docked pose of the cellulose unit and the enzyme complex in proper orientation via secondary forces such as van der Waals forces in order to use cellulose as a drug delivery carrier. Moreover, LIGPLOT was used to generate schematic 2D representations of ligand-receptor interactions for the best-docked pose, as shown in Figure 10e. The neighbouring amino acid residue in the pocket region, as shown in the 2D representation, is involved in hydrophobic and hydrophilic interactions to support the close contact of the ligand with the enzyme (red half circles) as well as the hydrogen bonding interaction between the receptor and the ligand (green bonds).
4 Conclusion
In this study, the cytotoxicity effects of MCC and NCC from pineapple leaves to normal fibroblast cells in the lungs (MRC-5) were assessed. The cytotoxicity effect of MCC and NCC revealed low toxicity for 24, 48, and 72 h of treatment at higher concentrations. The obtained MCC and NCC were of high purity and high crystallinity based on the characterization studies. The morphology of needle-like shapes proved that these materials have a nanoscale of aspect ratio in length and diameter. The crystallinity index of cellulose from the pineapple leaves gives NCC a higher crystallinity compared with the MCC samples. The difference in findings of physical properties and sample crystallinity may be due to the different pretreatment techniques and hydrolysis of acids. Overall, the obtained MCC and NCC from pineapple leaves are an effective option for sustainable natural resource production in Malaysia, which are safe and biologically compatible to be used as a drug delivery carrier in pharmaceutical applications. A molecular docking analysis will provide a deeper understanding of the relationship between cellulose and receptor proteins/enzymes, which is useful for researching drug delivery pathways in biological systems.
-
Funding information: The authors acknowledge the support from the Centre of Research and Instrument Management (CRIM), Universiti Kebangsaan Malaysia and Ministry of Higher Education Malaysia, under grants MI-2019-018 and DIP-2015-028 and Taif University Researchers Supporting Project number (TURSP-2020/45) Taif University, Taif, Saudi Arabia.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):1–33.10.1186/s12951-018-0392-8Search in Google Scholar PubMed PubMed Central
[2] Amin MCIM, Abadi AG, Ahmad N, Katas H, Jamal JA. Bacterial cellulose film coating as drug delivery system: physicochemical, thermal and drug release properties. Sains Malaysiana. 2012;41(5):561–68.Search in Google Scholar
[3] Jian W, Hui D, Lau D. Nanoengineering in biomedicine: current development and future perspective. Nanotechnol Rev. 2020;9(1):700–15.10.1515/ntrev-2020-0053Search in Google Scholar
[4] Dias F, Duarte C. Cellulose and its derivatives use in the pharmaceutical compounding practice. Cellul – Med Pharm Electron Appl. 1st ed. 2013. p. 141–62.10.5772/56637Search in Google Scholar
[5] Lin N, Dufresne A. Nanocellulose in biomedicine: current status and future prospect. Eur Polym J. 2014;59:302–25.10.1016/j.eurpolymj.2014.07.025Search in Google Scholar
[6] Halib N, Ahmad I. Nanocellulose: insight into health and medical applications. Handb Ecomater. 2019;2:1345–56.10.1007/978-3-319-68255-6_5Search in Google Scholar
[7] Yu T, Soomro SA, Huang F, Wei W, Wang B, Zhou Z, et al. Naturally or artificially constructed nanocellulose architectures for epoxy composites: a review. Nanotechnol Rev. 2020;9(1):1643–59.10.1515/ntrev-2020-0116Search in Google Scholar
[8] Plackett D, Letchford K, Jackson J, Burt H. A review of nanocellulose as a novel vehicle for drug delivery. Nord Pulp Pap Res J. 2014;29(1):105–18.10.3183/npprj-2014-29-01-p105-118Search in Google Scholar
[9] Kargarzadeh H, Ahmad I, Abdullah I, Dufresne A, Zainudin SY, Sheltami RM. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose. 2012;19(3):855–66.10.1007/s10570-012-9684-6Search in Google Scholar
[10] Santos RM, dos Flauzino Neto WP, Silvério HA, Martins DF, Dantas NO, Pasquini D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind Crop Prod. 2013;50:707–14.10.1016/j.indcrop.2013.08.049Search in Google Scholar
[11] Sheltami RM, Abdullah I, Ahmad I, Dufresne A, Kargarzadeh H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr Polym. 2012;88(2):772–79.10.1016/j.carbpol.2012.01.062Search in Google Scholar
[12] Yang J, Han CR. Mechanically viscoelastic properties of cellulose nanocrystals skeleton reinforced hierarchical composite hydrogels. Appl Mater Interfaces. 2016;8(38):25621–30. 10.1021/acsami.6b08834.Search in Google Scholar PubMed
[13] Qian S, Tao Y, Ruan YP, Fontanillo Lopez CA, Xu LQ. Ultrafine bamboo-char as a new reinforcement in poly(lactic acid)/bamboo particle biocomposites: the effects on mechanical, thermal, and morphological properties. J Mater Res. 2018;33(22):3870–79.10.1557/jmr.2018.290Search in Google Scholar
[14] Prasenjit D, Prasanta D, Abhijit C, Tejendra B. A survey on pineapple and its medicinal value. Sch Acad J Pharm. 2012;1(1):24–9.Search in Google Scholar
[15] Ibrahim NA, Azraaie N, Zainul Abidin NAM, Mamat Razali NA, Abdul Aziz F, Zakaria S. Preparation and characterization of alpha cellulose of pineapple (Ananas comosus) leaf fibres (PALF). Adv Mater Res. 2014;895:147–50.10.4028/www.scientific.net/AMR.895.147Search in Google Scholar
[16] Trilokesh C, Uppuluri KB. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci Rep. 2019;9(1):1–8.10.1038/s41598-019-53412-xSearch in Google Scholar PubMed PubMed Central
[17] Sainorudin MH, Mohammad M, Kadir NHA, Abdullah NA, Yaakob Z. Characterization of several microcrystalline cellulose (MCC)-based agricultural wastes via x-ray diffraction method. Solid state phenomena. Vol. 280. Trans Tech Publications Ltd; 2018.10.4028/www.scientific.net/SSP.280.340Search in Google Scholar
[18] Tripathy DB, Mishra A. Renewable plant-based raw materials for industry. In: Scott RA, editor. Encyclopedia of inorganic and bioinorganic chemistry. Hoboken, New Jersey: John Wiley & Sons, Ltd; 2016. p. 1–16.10.1002/9781119951438.eibc2432Search in Google Scholar
[19] Asim M, Abdan K, Jawaid M, Nasir M, Dashtizadeh Z, Ishak MR, et al. A review on pineapple leaves fibre and its composites. Int J Polym Sci. 2015;2015:1–16.10.1155/2015/950567Search in Google Scholar
[20] Boschetti WTN, Carvalho AMML, Carneiro AdCO, Vidaurre GB, Gomes FJB, Soratto DN. Effect of mechanical treatment of eucalyptus pulp on the production of nanocrystalline and microcrystalline cellulose. Sustainability. 2021;13:5888.10.3390/su13115888Search in Google Scholar
[21] Liu Y, Liu A, Ibrahim SA, Yang H, Huang W. Isolation and characterization of microcrystalline cellulose from pomelo peel. Int J Biol Macromol. 2018;111:717–21.10.1016/j.ijbiomac.2018.01.098Search in Google Scholar PubMed
[22] Kian LK, Jawaid M, Ariffin H, Alothman OY. Isolation and characterization of microcrystalline cellulose from roselle fibers. Int J Biol Macromol. 2017;103:931–40.10.1016/j.ijbiomac.2017.05.135Search in Google Scholar PubMed
[23] Agblevor FA, Ibrahim MM, El-Zawawy WK. Coupled acid and enzyme mediated production of microcrystalline cellulose from corn cob and cotton gin waste. Cellulose. 2007;14:247–56.10.1007/s10570-006-9103-ySearch in Google Scholar
[24] Trache D, Donnot A, Khimeche K, Benelmir R, Brosse N. Physico-chemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohydr Polym. 2014;104:2230–6.10.1016/j.carbpol.2014.01.058Search in Google Scholar PubMed
[25] Haafiz MM, Hassan A, Zakaria Z, Inuwa IM. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr Polym. 2014;103:119–25.10.1016/j.carbpol.2013.11.055Search in Google Scholar PubMed
[26] de Oliveira RL, da Silva Barud H, de Assunçao RM, da Silva Meireles C, Carvalho GO, Filho GR, et al. Synthesis and characterization of microcrystalline cellulose produced from bacterial cellulose. J Therm Anal Calorim. 2011;106:703–09.10.1007/s10973-011-1449-1Search in Google Scholar
[27] Aprilia SNA, Arahman N. Properties of nanocrystalline cellulose from pineapple crown leaf waste. IOP Conf Ser: Mater Sci Eng. 2020;796:012007.10.1088/1757-899X/796/1/012007Search in Google Scholar
[28] Camacho M, Corrales Ureña YR, Lopretti M, Carballo LB, Moreno G, Alfaro B, et al. Synthesis and characterization of nanocrystalline cellulose derived from pineapple peel residues. J Renew Mater. 2017;5:271–9.10.7569/JRM.2017.634117Search in Google Scholar
[29] Karakehya N, Bilgiç C. Preparation of nanocrystalline cellulose from tomato stem and commercial microcrystalline cellulose: a comparison between two starting materials. Cell Chem Technol. 2019;53:993–1000.10.35812/CelluloseChemTechnol.2019.53.97Search in Google Scholar
[30] Giri J, Lach R, Sapkota J, Susan MABH, Saiter JM, Henning S, et al. Structural and thermal characterization of different types of cellulosic fibers. BIBECHANA. 2019;16:177–86.10.3126/bibechana.v16i0.21650Search in Google Scholar
[31] Islam MS, Kao N, Bhattacharya SN, Gupta R, Choi HJ. Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production. Chin J Chem Eng. 2018;2018(26):465–76.10.1016/j.cjche.2017.07.004Search in Google Scholar
[32] Sukyai P, Anongjanya P, Bunyahwuthakul N, Kongsin K, Harnkarnsujarit N, Sukatta U, et al. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res Int. 2018;107:528–35.10.1016/j.foodres.2018.02.052Search in Google Scholar PubMed
[33] Ilyas RA, Sapuan SM, Ishak MR. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohydr Polym. 2018;181:1038–51.10.1016/j.carbpol.2017.11.045Search in Google Scholar PubMed
[34] Ventura-Cruz S, Flores-Alamo N, Tecante A. Preparation of microcrystalline cellulose from residual Rose stems (Rosa spp.) by successive delignifcation with alkaline hydrogen peroxide. Int J Biol Macromol. 2020;155:324–9.10.1016/j.ijbiomac.2020.03.222Search in Google Scholar PubMed
[35] Abu-Thabit NY, Judeh AA, Hakeem AS, Ul-Hamid A, Umar Y, Ahmad A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int J Biol Macromolecules. 2020;155:730–9.10.1016/j.ijbiomac.2020.03.255Search in Google Scholar PubMed
[36] Singanusong R, Tochampa W, Kongbangkerd T, Sodchit C. Extraction and properties of cellulose from banana peels. Suranaree J Sci Technol. 2013;21:14.Search in Google Scholar
[37] Segal L, Creely JJ, Martin AE, Conrad CM. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text Res J. 1959;29(10):786–94.10.1177/004051755902901003Search in Google Scholar
[38] He J, Tang Y, Wang SY. Differences in morphological characteristics of bamboo fibres and other natural cellulose fibres: Studies on X-ray diffraction, solid state 13C-CP/MAS NMR, and second derivative FTIR spectroscopy data. Iran Polym J (Engl Ed). 2007;16(12):807–18.Search in Google Scholar
[39] El-Denglawey A. Illumination effect on the structural and optical properties of nano meso nickel (II) tetraphenyl-21H, 23H-porphyrin films induces new 2 h photo bleached optical sensor. J Lumin. 2018;194:381–6.10.1016/j.jlumin.2017.10.070Search in Google Scholar
[40] Al-Nasiry S, Hanssens M, Luyten C, Pijnenborg R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod. 2007;22(5):1304–9.10.1093/humrep/dem011Search in Google Scholar PubMed
[41] Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and autodocktools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91.10.1002/jcc.21256Search in Google Scholar PubMed PubMed Central
[42] Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995;8:127–34.10.1093/protein/8.2.127Search in Google Scholar PubMed
[43] Shanmugam N, Nagarkar RD, Kurhade M. Microcrystalline cellulose powder from banana pseudostem fibres using bio-chemical route. Indian J Nat Prod Resour. 2015;6(1):42–50.Search in Google Scholar
[44] Mat Zain NF. Preparation and characterization of cellulose and nanocellulose from pomelo (citrus grandis) albedo. J Nutr Food Sci. 2014;5(1):10–3.10.4172/2155-9600.1000334Search in Google Scholar
[45] Wulandari WT, Rochliadi A, Arcana IM. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf Ser Mater Sci Eng. 2016;107(1):012045.10.1088/1757-899X/107/1/012045Search in Google Scholar
[46] Adel AM, El-Shafei AM, Ibrahim AA, Al-Shemy MT. Chitosan/nanocrystalline cellulose biocomposites based on date palm (Phoenix dactylifera L.) sheath fibers. J Renew Mater. 2019;7(6):567–82.10.32604/jrm.2019.00034Search in Google Scholar
[47] Hishikawa Y, Togawa E, Kondo T. Characterization of individual hydrogen bonds in crystalline regenerated cellulose using resolved polarized FTIR spectra. ACS Omega. 2017;2:1469–76.10.1021/acsomega.6b00364Search in Google Scholar
[48] Li M, Wang LJ, Li D, Cheng YL, Adhikari B. Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohydr Polym. 2014;102(1):136–43.10.1016/j.carbpol.2013.11.021Search in Google Scholar
[49] Rashid M, Gafur MA, Sharafat MK, Minami H, Miah MAJ, Ahmad H. Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method. Carbohydr Polym. 2017;170:72–9.10.1016/j.carbpol.2017.04.059Search in Google Scholar
[50] Lee KY, Aitomäki Y, Berglund LA, Oksman K, Bismarck A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos Sci Technol. 2014;105:15–27.10.1016/j.compscitech.2014.08.032Search in Google Scholar
[51] Huntley CJ, Crews KD, Curry ML. Chemical functionalization and characterization of cellulose extracted from wheat straw using acid hydrolysis methodologies. Int J Polym Sci. 2015;2015:1–9.10.1155/2015/293981Search in Google Scholar
[52] Fisher T, Hajaligol M, Waymack B, Kellogg D. 27-pyrolysis behavior and kinetics of biomass derived materials. J Anal Appl Pyrolysis. 2002;62:331–49.10.1016/S0165-2370(01)00129-2Search in Google Scholar
[53] Moreno G, Ramirez K, Esquivel M, Jimenez G. Isolation and characterization of nanocellulose obtained from industrial crop waste resources by using mild acid hydrolysis. J Renew Mater. 2018;6:362–69.10.7569/JRM.2017.634167Search in Google Scholar
[54] Gan PG, Sam ST, Faiq M, Omar MF. Thermal properties of nanocellulose-reinforced composites: a review. J Appl Polym Sci. 2020;137:48544.10.1002/app.48544Search in Google Scholar
[55] Santmartí A, Lee KY. Crystallinity and thermal stability of nanocellulose. Nanocellulose and sustainability. CRC Press; 2018. p. 67–8610.1201/9781351262927-5Search in Google Scholar
[56] Sainorudin MH, Abdullah NA, Rani MSA, Mohammad M, Abd Kadir NH, Razali H, et al. Investigation of the structural, thermal and morphological properties of nanocellulose synthesised from pineapple leaves and sugarcane bagasse. Curr Nanosci. 2021;17:1875–86.10.2174/1573413717666210216115609Search in Google Scholar
[57] Indarti E, Marwan, Wanrosli WD. Thermal stability of oil palm empty fruit bunch (OPEFB) nanocrystalline cellulose: effects of post-treatment of oven drying and solvent exchange techniques. J Phys Conf Ser. 2015;622:622.10.1088/1742-6596/622/1/012025Search in Google Scholar
[58] Flórez Pardo LM, Salcedo Mendoza JG, López Galán JE. Influence of pretreatments on crystallinity and enzymatic hydrolysis in sugar cane residues. Braz J Chem Eng. 2019;36(1):131–41.10.1590/0104-6632.20190361s20180093Search in Google Scholar
[59] Thompson L, Azadmanjiri J, Nikzad M, Sbarski I, Wang J, Yu A. Cellulose nanocrystals: production, functionalization and advanced applications. Rev Adv Mater Sci. 2019;58:1–16.10.1515/rams-2019-0001Search in Google Scholar
[60] Zhang D, Zhang Q, Gao X, Piao G. A nanocellulose polypyrrole composite based on tunicate cellulose. Int J Polym Sci. 2013;2013:1–6.10.1155/2013/175609Search in Google Scholar
[61] Azubuike CP, Okhamafe AO. Physicochemical, spectroscopic and thermal properties of microcrystalline cellulose derived from corn cobs. Int J Recycl Org Waste Agric. 2012;1(1):9.10.1186/2251-7715-1-9Search in Google Scholar
[62] Nishiyama Y. Molecular interactions in nanocellulose assembly subject areas. Phil Trans R Soc A. 2017;376:20170047.10.1098/rsta.2017.0047Search in Google Scholar PubMed
[63] Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK. Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels. 2010;3:1–10.10.1186/1754-6834-3-10Search in Google Scholar PubMed PubMed Central
[64] Sun JX, Sun XF, Zhao H, Sun RC. Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stab. 2004;84(2):331–9.10.1016/j.polymdegradstab.2004.02.008Search in Google Scholar
[65] Saba N, Jawaid M. Recent advances in nanocellulose-based polymer nanocomposites. In: Cellulose-reinforced nanofiber composites: production, properties and applications. Woodhead publishing series in composites science and engineering. Woodhead Publishing, Elsevier Ltd; 2017. p. 89–112.10.1016/B978-0-08-100957-4.00004-8Search in Google Scholar
[66] Marwah R, Ibrahim NA, Zainuddin N, Saad WZ, Razak NIA, Chieng BW. The effect of fiber bleaching treatment on the properties of poly(lactic acid)/oil palm empty fruit bunch fiber composites. Int J Mol Sci. 2014;15(8):14728–42.10.3390/ijms150814728Search in Google Scholar PubMed PubMed Central
[67] Mahardika M, Abral H, Kasim A, Arief S, Asrofi M. Production of nanocellulose from pineapple leaf fibers via high-shear homogenization and ultrasonication. Fibers. 2018;6(2):1–12.10.3390/fib6020028Search in Google Scholar
[68] Prado KS, Spinacé MAS. Isolation and characterization of cellulose nanocrystals from pineapple crown waste and their potential uses. Int J Biol Macromol. 2019;122:410–6.10.1016/j.ijbiomac.2018.10.187Search in Google Scholar PubMed
[69] Yang J, Ching YC, Chuah CH. Applications of lignocellulosic fibers and lignin in bioplastics: a review. Polym (Basel). 2019;11(5):1–26.10.3390/polym11050751Search in Google Scholar PubMed PubMed Central
[70] Teixeira JG, Gomes MG, Oliveira RR, Silva VA, Mariana M, Ortiz AB, et al. Sugarcane bagasse ash reinforced hdpe composites: effects of electron-beam radiation crosslinking on tensile and morphological properties. In International Nuclear Atlantic Conference – INAC. 2013.Search in Google Scholar
[71] Zeni M, Favero D, Pacheco K, Ana Grisa M. Preparation of microcellulose (MCC) and nanocellulose (NCC) from Eucalyptus Kraft Ssp Pulp. Polym Sci. 2015;1:1–7.10.4172/2471-9935.100007Search in Google Scholar
[72] Hanif Z, Ahmed FR, Shin SW, Kim YK, Um SH. Size- and dose-dependent toxicity of cellulose nanocrystals (CNC) on human fibroblasts and colon adenocarcinoma. Colloids Surf B Biointerfaces. 2014;119:162–5.10.1016/j.colsurfb.2014.04.018Search in Google Scholar PubMed
[73] Menas AL, Yanamala N, Farcas MT, Russo M, Friend S, Fournier PM, et al. Fibrillar vs crystalline nanocellulose pulmonary epithelial cell responses: cytotoxicity or inflammation? Chemosphere. 2017;171:671–80.10.1016/j.chemosphere.2016.12.105Search in Google Scholar PubMed PubMed Central
© 2021 Muhammad Hanif Sainorudin et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Articles in the same Issue
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

