Abstract
Objectives
The overlapping neural networks of social and physical pain have been investigated intensively in recent years. It was postulated that triggering social pain might result in greater physical pain. Nonetheless, how this affects somatoform pain disorder has not yet been considered. Since an increased pain processing activity is reported in these patients, the investigation of social exclusion and its effect on this group seems interesting. Hence, the aim of this study was to compare the influence of social exclusion on healthy controls and patients with somatoform pain disorder.
Methods
Nineteen patients with somatoform pain disorder and 19 healthy controls were examined. Cyberball, a virtual ball-tossing paradigm, was used to experimentally induce social exclusion and inclusion. To measure effects on pain perception, pressure pain thresholds and heart rate variability (HRV) were recorded after each round of cyberball. Demographic data, pain medication, and potential psychosocial moderators were collected by questionnaires.
Results
After social exclusion, pressure pain thresholds were significantly reduced in healthy controls (p < 0.01) as well as somatoform pain patients (p < 0.05), while HRV increased only in patients with somatoform pain disorder (p < 0.05) indicating increased parasympathetic activity.
Conclusion
This study is the first to analyse the effects of social exclusion on pain perception in somatoform pain disorder. While the reduction in pressure pain thresholds is in line with the social pain literature, the effects on HRV could be interpreted as a form of pain regulation mechanism. However, further research is needed to investigate the role of parasympathetic activity in socially excluded somatoform pain patients.
1 Introduction
Pain is defined as an unpleasant sensory and emotional experience typically caused by actual or potential tissue injury; however, a broad influence of psychosocial factors in the development of pain is generally accepted [1]. Based on that, the idea of a distinction between two kinds of pain developed. On the one hand, physical pain is triggered by tissue damage and represents the common understanding of the word pain [2]. On the other hand, the threatening loss of close relationships or social devaluations can provoke something called social pain [3]. Over the last two decades, the examination of social pain was an area of increasing research interest [4]. The overlap theory of physical and social pain proposes the existence of shared neural regions responsible for the processing of physical and social pain [2,5]. Although shared pathways in the brain for emotional and pain processing are not specifically connected to the idea of social pain, many studies have investigated the physical–social pain overlap [4]. The most important evidence is provided by neuroimaging studies, which identified the dorsal anterior cingulate cortex (ACC) and the anterior insula as key areas for the physical–social pain overlap [6,7]. It should be noted, that the modern view of how the brain works is moving away from specific mind–brain aspects. Rather, the focus is placed on closely connected neuronal networks that cannot simply be broken down into segregated parts [8,9]. Nevertheless, it was postulated against the background of overlapping neural processing of physical and social pain, that external factors which change the current state of one pain form should influence the other pain form in the same direction [2]. Hence, triggering social pain might result in greater physical pain. There is convincing evidence for this assumption [10–13]. However, some studies found contradictory results [11,14–16]. Besides research within the general population, the effect of social exclusion on pain perception was examined in different clinical samples [12,13,17–19].
Interestingly, an analysis based on patients with somatoform pain disorder, to the best of our knowledge, has not yet been conducted. Prolonged and recurrent pain is characterizing for patients with somatoform pain disorder. In general, pain in this disorder is not sufficiently explained by physiological reasons, which does not mean that it must be purely psychological in nature. However, patients mostly exhibit serious psychosocial stress factors and a long medical history [20]. Neurophysiological aspects seem to play an important part in the development of somatoform pain disorders as well. The circuit network model of somatosensory describes enhanced neuronal pain processing activity in the ACC and the insular cortex [21]. This fact is particularly interesting because parts of the ACC and the insula play a key role in terms of the physical–social pain overlap [6]. Another noteworthy fact is that patients with somatoform pain disorder are frequently stigmatized and devaluated by their social environment [22,23]. For example, pain patients without sufficient physical explanation are more often socially excluded than pain patients with an adequately diagnosed organic cause [24].
In recent years, heart rate variability (HRV) has become a well-established, non-subjective parameter in pain research [25]. It describes natural alterations in the time interval between consecutive heartbeats. HRV is regarded as a precise assessment of autonomic input to the heart and can display activity in the sympathetic as well as the parasympathetic branch of the autonomic nervous system [26]. More interestingly, brain areas like the ACC, the insula, and others participate in autonomic control. Since these systems are as well related to pain perception, HRV can be considered as a valid indicator of autonomic reactivity to painful stimulation [25,27–29].
As mentioned above, there is no study examining the effect of social exclusion on pain perception in somatoform pain patients. Considering the described neuronal changes in the ACC and the insular cortex, however, a strong reciprocal interference of social and physical pain seems most likely, especially in somatoform pain patients. This is of clinical relevance regarding the described stigmatization and social exclusion of somatoform pain patients. Consequently, the purpose of this study was to fill this gap in the current state of research.
2 Methods
2.1 Participants
To estimate the required sample size, an a priori power analysis with G*Power Version 3.1 was conducted [30]. It was based on a significant effect of social exclusion on pain ratings in patients with borderline personality disorder of
2.2 Cyberball paradigm
The latest version of the cyberball paradigm was used to induce social inclusion and exclusion [33,34]. Subjects were informed that they will play a virtual ball-tossing game with two other players on the internet to bridge the time between the pain measurements. The players could select by a simple mouse-click which player would receive the next throw. Actually, the fellow players were simulated, and the course of play was predetermined. Every round consisted of 80 tosses [35]. In the inclusion condition the test persons received a well-balanced number of throws, whereas in the exclusion condition they were excluded after the tenth caught ball. The duration of each round was approximately 5 min.
2.3 Pressure pain threshold
To measure the effects of social inclusion and exclusion on pain perception pressure pain thresholds were determined as described elsewhere [36,14,11]. Therefore, a force gage (FDN 100, Wagner Instruments, Greenwich, USA) was used. It was vertically positioned on the thenar as well as a tender point 2 cm distal of the epicondylus lateralis humeri of the subjects’ right hand. The pressure was increased in steps of 5 N per second. Before testing, participants were familiarized with the pressure pain stimuli and briefed to say immediately “now” when they felt pain for the first time. The pressure pain threshold was determined by computing the mean of the measurements.
2.4 HRV
In addition, an electrocardiogram (ECG) was derived to detect the non-subjective HRV. Therefore, BrainVision Recorder, Version 1.24 (Brain Products GmbH, Gilching, Germany) was used. To avoid disturbances of the ECG measurement, two electrodes were placed on both sides in the subclavian region and one electrode in the neck [37]. The ECG was recorded while subjects were playing cyberball.
2.5 Questionnaires
The Screening for Somatoform Disorders (SOMS-2) was used to evaluate physical symptoms which are not based on organic causes. It consists of 68 items and includes questions about pain as well as gastrointestinal, cardiovascular, urogenital, and neurological complaints. This questionnaire offers the possibility to compute the somatization index ICD-10 by which a somatoform disorder can be diagnosed [38].
To assess clinical pain the Brief Pain Inventory (BPI) was utilized. It is composed of 11 items that can be assigned to two different domains. The pain severity domain rates pain at its worst, least, average, and the current pain. The pain interference domain quantifies how much pain interferes with seven daily activities (e.g., work, mood, relationships) [39].
The Pain Sensitivity Questionnaire (PSQ) describes 17 daily situations (e.g., burning the tongue on a very hot drink), that need to be rated in terms of painfulness. The PSQ total score can be calculated as the average rating on 14 of the 17 items [40,41].
2.6 Procedure
We implemented a 2 (somatoform pain patients vs healthy controls) × 2 (social inclusion vs social exclusion) mixed-model design. The subjects were told that they were taking part in an experiment on pain perception and that they would play a virtual ball-tossing game with two other players on the internet to bridge the time between the pain measurements. Subjects were familiarized with the pressure pain stimuli and the ECG electrodes were attached. The testing itself started with online questionnaires on demographic data, possible pain medication, the BPI, and the PSQ. Afterward, an induction of social inclusion or social exclusion with the cyberball paradigm took place. Meanwhile, an ECG was recorded. Immediately after that, pressure pain thresholds were measured. Then subjects completed the SOMS-2. Thereafter, a second induction of social inclusion or social exclusion, as well as another ECG and a pressure pain measurement took place. All participants were confronted, respectively, with one round of social inclusion and exclusion, only the experimental order was counterbalanced (inclusion–exclusion or exclusion–inclusion). Finally, a manipulation check was conducted. Subjects rated whether they believed that they had played with a real person during the cyberball paradigm. All participants were informed thoroughly about the actual research objectives after the experiment.
2.7 Data analysis
The HR (average heart rate) and the time domain HRV indices RMSSD (root mean square differences of successive heartbeat intervals) and pNN50 (percentage of successive intervals that differ by >50 ms) were calculated [42,43]. To analyse the ECG data, BrainVision Analyzer, Version 2.2 (Brain Products GmbH, Gilching, Germany) and ARTiiFACT Version 2.13 was utilized [44]. Movement artefacts and ectopic beats were excluded from the data. The provided algorithms for R-peak detection in the QRS complex were used. The results were verified by hand and adjusted where necessary.
Statistical analyses were conducted using SPSS Statistics Version 29.0 for Windows (IBM Corporation, Armonk, USA). Dependent sample t-tests were performed to examine the influence of social exclusion on pressure pain thresholds and HRV. Mixed-model ANOVAs were used to check for potential interaction effects.
3 Results
3.1 Participants characteristics
To analyse the patient and the control group, we conducted between-subjects comparisons. The results are displayed in Table 1. In total, 38 subjects were recruited for this study. There was no significant difference between the control and patient group concerning age and sex. Most of the pain patients fulfilled the diagnostic criteria for F45.41 (94.7%, n = 18) and only 5.3% (n = 1) were diagnosed with F45.40. Because of that, both diagnoses were combined and no subgroup analyses were conducted. In the patient group a wide variety of pain medications like non-opioid analgesics (89.5%, n = 17), opioids (15.8%, n = 3), psychotropic drugs (47.4%, n = 9), and antiepileptic drugs (26.3%, n = 5) were taken, while in healthy controls only non-opioid analgesics as on-demand medication were used (26.3%, n = 5). In somatoform pain patients there were significantly higher levels of somatoform symptoms measured by the somatization index ICD-10 of the SOMS-2. Regarding the specific painful body areas, most pain patients named joints (84.2%, n = 16), head/face (78.9%, n = 15), arms/legs (78.9%, n = 15), and the back (73.7%, n = 14). In the control group considerably fewer participants complained about pain in these body parts (joints: 21.1%, n = 4; head/face: 26.3%, n = 5; arms/legs: 10.5%, n = 2; back: 31.6%, n = 6). Somatoform pain patients also reported significantly higher pain severity and pain interference with daily activities measured by the BPI (e.g., work, mood, relationships), as well as greater pain sensitivity determined by the PSQ.
Participant characteristics
| Healthy controls (n = 19) | Somatoform pain patients (n = 19) | |||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | |
| Age | 40.21 | 17.17 | 44.84 | 11.43 | 0.98 | 0.33 |
| SOMS-2 somatization index | 1.42 | 1.43 | 6.84 | 3.44 | 6.50 | <0.001** |
| BPI pain severity | 0.78 | 0.94 | 4.89 | 2.45 | 6.83 | <0.001** |
| BPI pain interference | 0.32 | 0.59 | 5.57 | 2.45 | 9.08 | <0.001** |
| PSQ total | 3.56 | 1.49 | 5.61 | 2.77 | 2.84 | 0.007** |
| Male | Female | Male | Female | χ² | p | |
| Sex | 5 | 14 | 2 | 17 | 1.58 | 0.21 |
Significant results are displayed in bold and marked as follows: **p < 0.01 (two-tailed).
3.2 Effects on pain and HRV
To investigate the influence of social exclusion on pain measurements and HRV, we carried out within-subjects comparisons. The results are presented in Table 2. In contrast to the inclusion condition, pressure pain thresholds were significantly lower after social exclusion in both healthy controls (t(18) = 3.40, p = 0.003) and somatoform pain patients (t(18) = 2.88, p = 0.010). However, mixed-model ANOVAs showed no substantial interaction between type of subject and the cyberball condition (F(1) = 0.03, p = 0.870; Figure 1), which means that the influence of social exclusion was not greater in somatoform pain patients than in healthy controls. With regard to HRV, RMSSD as a measure of the parasympathetic tone (t(18) = −2.54, p = 0.021; Figure 2) and pNN50 (t(18) = −2.97, p = 0.008; Figure 3) increased significantly in somatoform pain patients after social exclusion, while no effect was found in healthy controls. There was no effect of cyberball on the average heart rate in both groups. No moderating influence of pain medication, pain sensitivity (PSQ), pain severity and interference (BPI), or somatization tendency (SOMS-2) on the effect of social exclusion on pain perception was found.
Effects of social inclusion and exclusion on healthy controls and somatoform pain patients
| Healthy controls (n = 19) | Somatoform pain patients (n = 19) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion | Exclusion | Inclusion | Exclusion | |||||||||
| M | SD | M | SD | t(18) | p | M | SD | M | SD | t(18) | P | |
| Pressure pain threshold (N) | 33.42 | 10.28 | 29.08 | 10.20 | 3.40 | 0.003** | 27.56 | 16.94 | 22.87 | 15.55 | 2.88 | 0.010* |
| Heart rate (bpm) | 76.34 | 9.05 | 75.94 | 8.61 | 0.68 | 0.504 | 81.51 | 14.14 | 81.10 | 13.58 | 0.665 | 0.515 |
| RMSSD (ms) | 25.38 | 12.91 | 25.20 | 15.10 | 0.13 | 0.899 | 20.34 | 8.81 | 22.67 | 10.98 | 2.54 | 0.021* |
| pNN50 (%) | 9.08 | 13.48 | 8.84 | 14.84 | 0.17 | 0.867 | 3.69 | 4.82 | 5.88 | 6.41 | 2.97 | 0.008** |
Differences between groups were calculated by dependent sample t-tests. Significant results are displayed in bold and marked as follows: *p < 0.05 (two-tailed); **p < 0.01 (two-tailed). bpm, beats per minute; ms, milliseconds; N, newton; pNN50, percentage of successive R-R intervals that differ by >50 ms; RMSSD, root mean square differences of successive R-R intervals; SDNN, standard deviation of NN intervals.
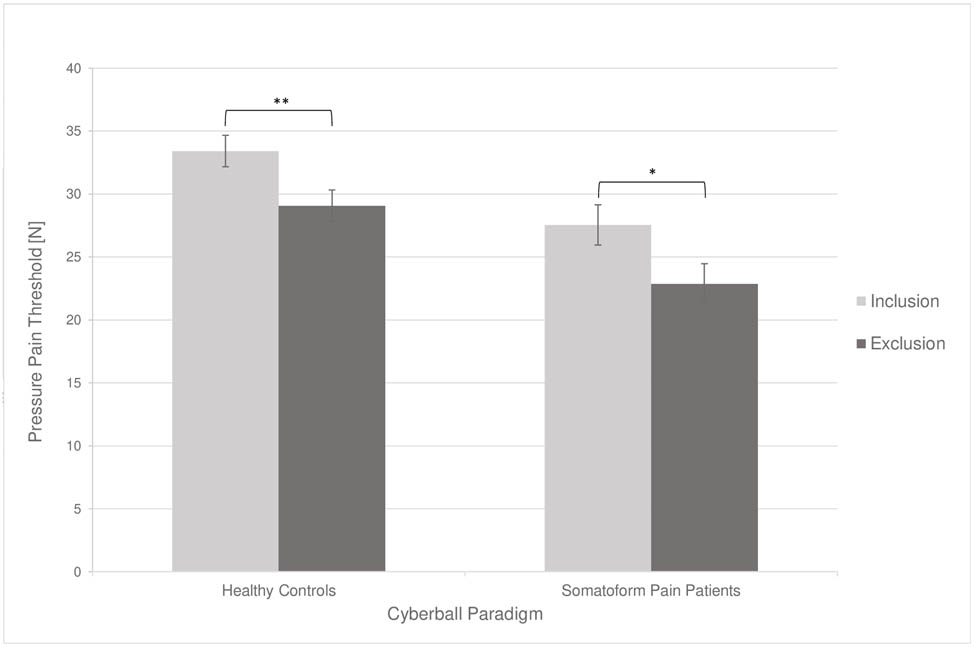
Changes in the subjective pain measurement in dependence of the cyberball condition. Error bars represent 95% confidence intervals. Significant results are marked as follows: *p < 0.05 (two tailed); **p < 0.01 (two-tailed). N, newton.
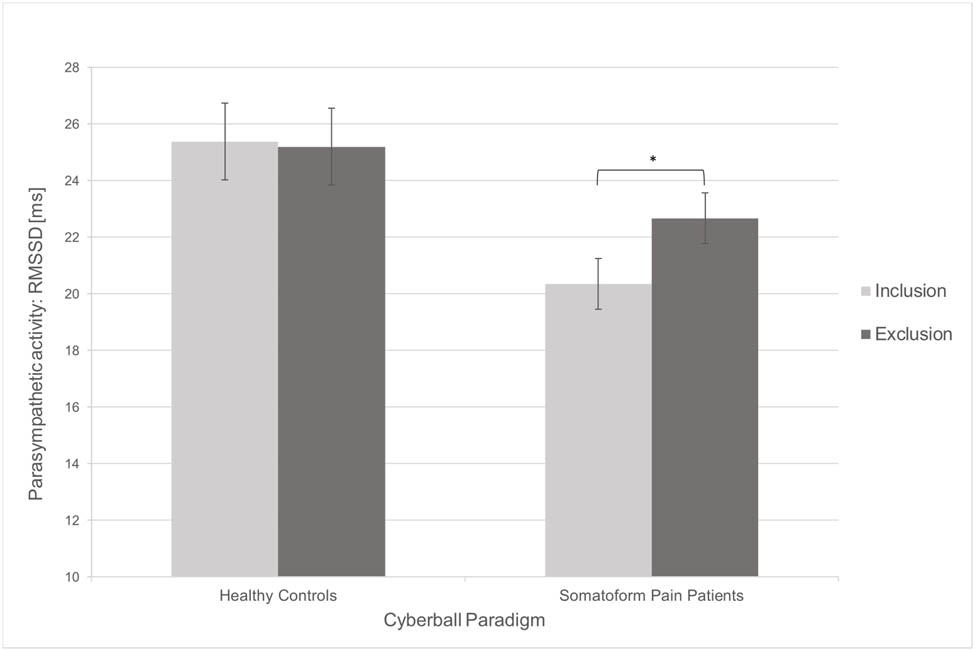
Changes in the objective pain measurement in dependence of the cyberball condition. Error bars represent 95% confidence intervals. Significant results are marked as follows: *p < 0.05 (two-tailed); **p < 0.01 (two-tailed). ms, milliseconds; RMSSD, root mean square differences of successive R-R intervals.
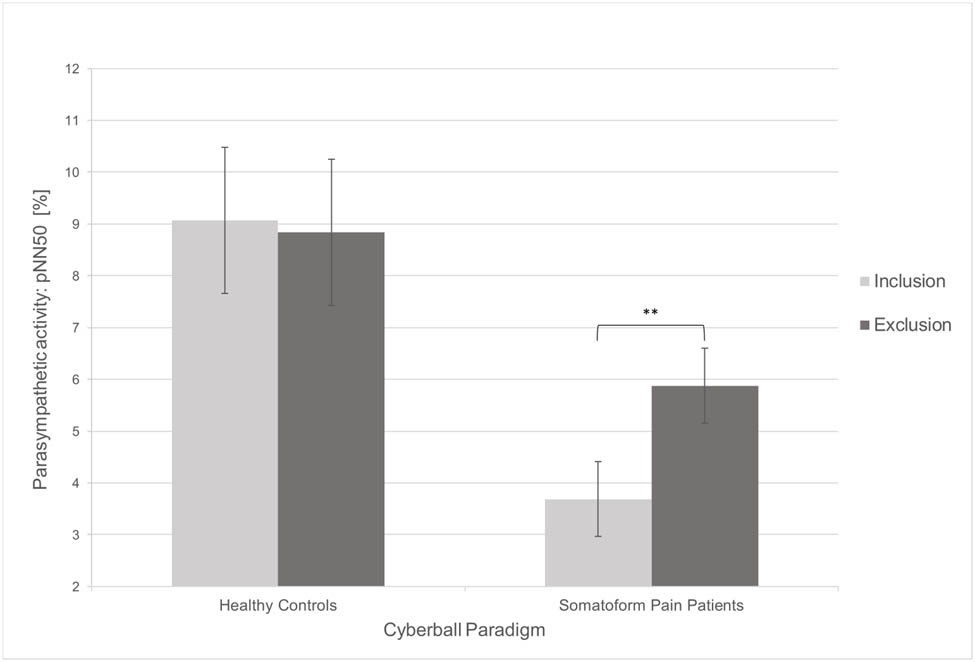
Changes in the objective pain measurement in dependence of the cyberball condition. Error bars represent 95% confidence intervals. Significant results are marked as follows: *p < 0.05 (two-tailed); **p < 0.01 (two-tailed). pNN50, percentage of successive R-R intervals that differ by >50 ms.
4 Discussion
We aimed to compare the effect of social exclusion on pain perception in somatoform pain patients and healthy controls. Regarding pressure pain, social exclusion led to decreased pain thresholds in healthy subjects and somatoform pain patients, without a significant difference between both groups. This result is in line with studies finding greater pain perception after social exclusion in the general population as well as in different clinical samples [10–13]. However, contrary results must be acknowledged. Sometimes, socially excluded individuals experienced less pain, yet another study found greater pain sensitivity after social inclusion, and a recent work reported no effect of social in- or exclusion on pain ratings at all [11,14–16]. This clearly reflects the controversy and the need for an extensive theoretical framework, which integrates these research lines in the pain literature [45–47]. Furthermore, it would be equally helpful to establish a standardized pain measurement procedure in the social pain literature. This would significantly increase the comparability of studies and the informative value of the research area.
Regarding HRV, however, a substantial effect of social exclusion was only detected in somatoform pain patients. RMSSD and pNN50 increased in socially excluded pain patients suggesting a greater activation of parasympathetic nervous system. As already argued in the social pain literature, these findings might indicate an anticipatory regulation process to prevent possible social pain because similar parasympathetic activation patterns have been described for situations that require strong emotional regulation [48–51]. Regarding physical pain, a recent systematic review reported an association between better self-regulation capacities and higher parasympathetic activation [25]. Accordingly, when the parasympathetic element of HRV was high, most studies reported higher pain inhibition capacities (e.g., better management of painful situations) [52–54]. These results are in consonance with the Polyvagal Theory and the Neurovisceral Integration Model which both identify the autonomic nervous system and especially the parasympathetic branch as an important part in the adaptation to various types of stressors including pain [55,56].
Now it is controversial to postulate that somatoform pain patients could have better pain management capacities than the general population. Comparable with the trend of HRV results in this study, a meta-analysis of chronic pain patients showed a significantly reduced RMSSD in comparison to healthy controls, indicating a lower parasympathetic tone in general [57]. Because of the increased pain sensitivity in these patients, pain regulation mechanisms like the parasympathetic component of HRV still might be activated more often and earlier but starts from a lower initial level. This could be an explanation why in this study changes in HRV were only detected in socially excluded somatoform pain patients and not in healthy controls.
To the best of our knowledge, an analysis of social exclusion’s effect on pain perception on patients with somatoform pain disorder was not conducted so far. The current understanding of the functioning of the brain emphasizes the interaction of complex neuronal networks [8,9]. Nevertheless, in view of the neuronal changes in the ACC and the insular cortex, a considerable mutual impact of social and physical pain appears probable, especially in somatoform pain patients. Therefore, a strong influence of social pain on physical pain and vice versa seems very likely. Furthermore, the urgency of a better understanding of pain patients has become more and more clear as one in five US adults is suffering from chronic pain producing enormous healthcare costs and productivity losses [58]. Hopefully, this study provides a first step to examine the effect of social exclusion on pain perception in somatoform pain patients.
Possible limitations of this work should be considered in further research. This study lacks baseline and recovery data for pressure pain and HRV at the beginning and the end of the study. These measurements would have provided useful information and should be included in future studies. Furthermore, different pain modalities like mechanical, thermal, and electrical stimuli could be combined to achieve a broader understanding of the influence of social exclusion on pain perception. However, as described above, the establishment of a standardized pain measurement procedure in social pain studies would be preferable. A larger sample size and comparisons with other pain disorders, such as fibromyalgia, could increase the robustness and generalizability of the results in future studies. Lastly, the influence of different moderating variables like the mood state, social distress caused by the cyberball paradigm or comorbidities could be integrated in the statistical analyses.
From our point of view, it might be interesting to illuminate two research areas in the context of social pain and somatoform pain patients. First, a lot of chronic pain patients report stigmatization and bullying. This is of particular interest because in the framework of the physical–social pain overlap devaluations might provide an explanation for the maintenance of chronic pain. Future studies should address this issue. Second, the investigation of interventions with a positive effect on the parasympathetic component of HRV like breathing techniques or meditation could offer a possible therapeutical approach to achieve better pain management skills [59,60].
Acknowledgements
The authors would like to thank Heike Althen, Maren Schmidt-Kassow, and Maurice Christian for their extensive technical support. They also thank all the participants for their time and effort in participating.
-
Research ethics: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as amended in 2013) and has been approved by the ethics committee of the Medical School, Goethe University Frankfurt (No. 20-842).
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission. F.K. and M.d.G. researched the literature and conceived the study. All authors were involved in protocol development, gaining ethical approval, patient recruitment, and data analysis. F.K. wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
-
Competing interests: The authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Research funding: None declared.
-
Supplementary materials: Data set. Social exclusion in somatoform pain patients. (xlsx)
-
Data availability: The raw data can be obtained on request from the corresponding author.
-
Artificial intelligence/machine learning tools: Not applicable.
References
[1] Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–82. 10.1097/j.pain.0000000000001939.Suche in Google Scholar PubMed PubMed Central
[2] Eisenberger NI. The neural bases of social pain: evidence for shared representations with physical pain. Psychosom Med. 2012;74(2):126–35. 10.1097/PSY.0b013e3182464dd1.Suche in Google Scholar PubMed PubMed Central
[3] Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131(2):202–23. 10.1037/0033-2909.131.2.202.Suche in Google Scholar PubMed
[4] Vervoort T, Karos K, Trost Z, Prkachin KM, eds. Social and interpersonal dynamics in pain: we don’t suffer alone. Cham: Springer International Publishing; 2018.10.1007/978-3-319-78340-6Suche in Google Scholar
[5] Eisenberger NI, Lieberman M. Why it hurts to be left out: the neurocognitive overlap between physical and social pain. In: Williams KD, Forgas JP, von Hippel W, editors. The social outcast: ostracism, social exclusion, rejection, and bullying. New York: Psychology Press; 2005. 109–27.Suche in Google Scholar
[6] Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–2. 10.1126/science.1089134.Suche in Google Scholar PubMed
[7] Rotge J-Y, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, et al. A meta-analysis of the anterior cingulate contribution to social pain. Soc Cogn Affect Neurosci. 2015;10(1):19–27. 10.1093/scan/nsu110.Suche in Google Scholar PubMed PubMed Central
[8] Pessoa L. The entangled brain: how perception, cognition, and emotion are woven together. Cambridge: The MIT Press; 2022.10.7551/mitpress/14636.001.0001Suche in Google Scholar
[9] Pessoa L. The cognitive-emotional brain: from interactions to integration. Cambridge: MIT Press; 2013 (The MIT Press Ser). Available from. https://ebookcentral.proquest.com/lib/kxp/detail.action?docID=3339689.10.7551/mitpress/9780262019569.001.0001Suche in Google Scholar
[10] Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126(1–3):132–8. 10.1016/j.pain.2006.06.024.Suche in Google Scholar PubMed
[11] Bernstein MJ, Claypool HM. Social exclusion and pain sensitivity: why exclusion sometimes hurts and sometimes numbs. Pers Soc Psychol Bull. 2012;38(2):185–96. 10.1177/0146167211422449.Suche in Google Scholar PubMed
[12] Bungert M, Koppe G, Niedtfeld I, Vollstädt-Klein S, Schmahl C, Lis S, et al. Pain processing after social exclusion and its relation to rejection sensitivity in borderline personality disorder. PLoS One. 2015;10(8):e0133693. 10.1371/journal.pone.0133693.Suche in Google Scholar PubMed PubMed Central
[13] Bach P, Frischknecht U, Bungert M, Karl D, Vollmert C, Vollstädt-Klein S, et al. Effects of social exclusion and physical pain in chronic opioid maintenance treatment: fMRI correlates. Eur Neuropsychopharmacol. 2019;29(2):291–305. 10.1016/j.euroneuro.2018.11.1109.Suche in Google Scholar PubMed
[14] DeWall CN, Baumeister RF. Alone but feeling no pain: effects of social exclusion on physical pain tolerance and pain threshold, affective forecasting, and interpersonal empathy. J Pers Soc Psychol. 2006;91(1):1–15. 10.1037/0022-3514.91.1.1.Suche in Google Scholar PubMed
[15] Canaipa R, Castro-Caldas A, Moreira JM, Pimentel-Santos F, Branco JC, Treister R. Impaired pain modulation in fibromyalgia patients in response to social distress manipulation. Clin J Pain. 2017;33(7):611–9. 10.1097/AJP.0000000000000447.Suche in Google Scholar PubMed
[16] Fales JL, Noel M. The effects of brief social exclusion on pain perception and pain memory in adolescents. J Adolesc Health. 2020;66(5):623–5. 10.1016/j.jadohealth.2020.01.018.Suche in Google Scholar PubMed
[17] Iffland B, Sansen LM, Catani C, Neuner F. The trauma of peer abuse: effects of relational peer victimization and social anxiety disorder on physiological and affective reactions to social exclusion. Front Psychiatry. 2014;5:26. 10.3389/fpsyt.2014.00026.Suche in Google Scholar PubMed PubMed Central
[18] Jobst A, Albert A, Bauriedl-Schmidt C, Mauer MC, Renneberg B, Buchheim A, et al. Social exclusion leads to divergent changes of oxytocin levels in borderline patients and healthy subjects. Psychother Psychosom. 2014;83(4):252–4. 10.1159/000358526.Suche in Google Scholar PubMed
[19] Rubeis J, de, Sütterlin S, Lange D, Pawelzik M, van Randenborgh A, Victor D, et al. Attachment status affects heart rate responses to experimental ostracism in inpatients with depression. PLoS One. 2016;11(3):e0150375. 10.1371/journal.pone.0150375.Suche in Google Scholar PubMed PubMed Central
[20] Möller H-J, Laux G, Deister A. Psychiatrie, Psychosomatik und Psychotherapie. Stuttgart, Germany: Georg Thieme Verlag; 2015.10.1055/b-003-120842Suche in Google Scholar
[21] Perez DL, Barsky AJ, Vago DR, Baslet G, Silbersweig DA. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40–50. 10.1176/appi.neuropsych.13070170.Suche in Google Scholar PubMed
[22] Cohen M, Quintner J, Buchanan D, Nielsen M, Guy L. Stigmatization of patients with chronic pain: the extinction of empathy. Pain Med. 2011;12(11):1637–43.10.1111/j.1526-4637.2011.01264.xSuche in Google Scholar PubMed
[23] Waugh OC, Byrne DG, Nicholas MK. Internalized stigma in people living with chronic pain. J Pain. 2014;15(5):550.e1-10. 10.1016/j.jpain.2014.02.001.Suche in Google Scholar PubMed
[24] de Ruddere L, Bosmans M, Crombez G, Goubert L. Patients are socially excluded when their pain has no medical explanation. J Pain. 2016;17(9):1028–35. 10.1016/j.jpain.2016.06.005.Suche in Google Scholar PubMed
[25] Forte G, Troisi G, Pazzaglia M, Pascalis V, de, Casagrande M. Heart rate variability and pain: a systematic review. Brain Sci. 2022;12(2). 10.3390/brainsci12020153.Suche in Google Scholar PubMed PubMed Central
[26] Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 2017;8:213. 10.3389/fpsyg.2017.00213.Suche in Google Scholar PubMed PubMed Central
[27] Benarroch EE. Pain-autonomic interactions: a selective review. Clin Auton Res. 2001;11(6):343–9. 10.1007/BF02292765.Suche in Google Scholar PubMed
[28] Tracy LM, Koenig J, Georgiou-Karistianis N, Gibson SJ, Giummarra MJ. Heart rate variability is associated with thermal heat pain threshold in males, but not females. Int J Psychophysiol. 2018;131:37–43. 10.1016/j.ijpsycho.2018.02.017.Suche in Google Scholar PubMed
[29] Pieritz K, Schäfer SJ, Strahler J, Rief W, Euteneuer F. Chronic stress moderates the impact of social exclusion on pain tolerance: an experimental investigation. J Pain Res. 2017;10:1155–62. 10.2147/JPR.S129872.Suche in Google Scholar PubMed PubMed Central
[30] Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60. 10.3758/BRM.41.4.1149.Suche in Google Scholar PubMed
[31] Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) im Auftrag des Bundesministeriums für Gesundheit (BMG) unter Beteiligung der Arbeitsgruppe ICD des Kuratoriums für Fragen der Klassifikation im Gesundheitswesen, editor. ICD-10-GM Version 2021, Systematisches Verzeichnis, Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme, 10. Revision, Stand: 18. September 2020. Köln; 2020.Suche in Google Scholar
[32] World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. 10.1001/jama.2013.281053.Suche in Google Scholar PubMed
[33] Williams KD, Cheung CKT, Choi W. Cyberostracism: effects of being ignored over the Internet. J Pers Soc Psychol. 2000;79(5):748–62. 10.1037//0022-3514.79.5.748.Suche in Google Scholar PubMed
[34] Williams KD. Hompage of Kipling D. Williams; 2018 [cited 2024 Mar 29]. https://www3.psych.purdue.edu/∼willia55/Announce/cyberball.htm.Suche in Google Scholar
[35] Zhang Q, Li X, Wang K, Zhou X, Dong Y, Zhang L, et al. Dull to social acceptance rather than sensitivity to social ostracism in interpersonal interaction for depression: behavioral and electrophysiological evidence from Cyberball tasks. Front Hum Neurosci. 2017;11:162. 10.3389/fnhum.2017.00162.Suche in Google Scholar PubMed PubMed Central
[36] Pauli P, Wiedemann G, Nickola M. Pain sensitivity, cerebral laterality, and negative affect. Pain. 1999;80(1–2):359–64. 10.1016/s0304-3959(98)00231-0.Suche in Google Scholar PubMed
[37] Liddell BJ, Courtney BS. Attachment buffers the physiological impact of social exclusion. PLoS One. 2018;13(9):e0203287. 10.1371/journal.pone.0203287.Suche in Google Scholar PubMed PubMed Central
[38] Rief W, Hiller W. Screening für Somatoforme Störungen: SOMS. 2. Aufl. Bern, Göttingen, Toronto, Seattle: Huber; 2008.10.1016/B978-343722481-2.50021-0Suche in Google Scholar
[39] Radbruch L, Loick G, Kiencke P, Lindena G, Sabatowski R, Grond S, et al. Validation of the German version of the Brief Pain Inventory. J Pain Symptom Manag. 1999;18(3):180–7. 10.1016/S0885-3924(99)00064-0.Suche in Google Scholar PubMed
[40] Ruscheweyh R, Marziniak M, Stumpenhorst F, Reinholz J, Knecht S. Pain sensitivity can be assessed by self-rating: development and validation of the Pain Sensitivity Questionnaire. Pain. 2009;146(1–2):65–74.10.1016/j.pain.2009.06.020Suche in Google Scholar PubMed
[41] Ruscheweyh R, Verneuer B, Dany K, Marziniak M, Wolowski A, Çolak-Ekici R, et al. Validation of the pain sensitivity questionnaire in chronic pain patients. Pain. 2012;153(6):1210–8. 10.1016/j.pain.2012.02.025.Suche in Google Scholar PubMed
[42] Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. 10.3389/fpubh.2017.00258.Suche in Google Scholar PubMed PubMed Central
[43] Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, et al. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–81. 10.1093/oxfordjournals.eurheartj.a014868.Suche in Google Scholar
[44] Kaufmann T, Sütterlin S, Schulz SM, Vögele C. ARTiiFACT: a tool for heart rate artifact processing and heart rate variability analysis. Behav Res. 2011;43(4):1161–70. 10.3758/s13428-011-0107-7.Suche in Google Scholar PubMed
[45] Eisenberger NI. Social pain and the brain: controversies, questions, and where to go from here. Annu Rev Psychol. 2015;66:601–29. 10.1146/annurev-psych-010213-115146.Suche in Google Scholar PubMed
[46] Karos K. On the overlap between physical and social pain. In: Vervoort T, Karos K, Trost Z, Prkachin KM, editors. Social and interpersonal dynamics in pain: We don’t suffer alone. Cham: Springer International Publishing; 2018. p. 173–95. 10.1007/978-3-319-78340-6_9.Suche in Google Scholar
[47] Karos K. The enduring mystery of pain in a social context. J Adolesc Health. 2020;66(5):524–5. 10.1016/j.jadohealth.2020.02.005.Suche in Google Scholar PubMed
[48] Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–56. 10.1016/s0165-0327(00)00338-4.Suche in Google Scholar PubMed
[49] Smith TW, Cribbet MR, Nealey-Moore JB, Uchino BN, Williams PG, Mackenzie J, et al. Matters of the variable heart: respiratory sinus arrhythmia response to marital interaction and associations with marital quality. J Pers Soc Psychol. 2011;100(1):103–19. 10.1037/a0021136.Suche in Google Scholar PubMed
[50] Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiol. 2006;43(6):612–22. 10.1111/j.1469-8986.2006.00467.x.Suche in Google Scholar PubMed
[51] Gulewitsch MD, Jusyte A, Mazurak N, Weimer K, Schönenberg M. Preliminary evidence for increased parasympathetic activity during social inclusion and exclusion in adolescents with functional abdominal pain. J Psychosom Res. 2017;98:106–12. 10.1016/j.jpsychores.2017.05.008.Suche in Google Scholar PubMed
[52] Evans DR, Eisenlohr-Moul TA, Button DF, Baer RA, Segerstrom SC. Self-regulatory deficits associated with unpracticed mindfulness strategies for coping with acute pain. J Appl Soc Psychol. 2014;44(1):23–30. 10.1111/jasp.12196.Suche in Google Scholar PubMed PubMed Central
[53] Luo X, Liu J, Che X. Investigating the influence and a potential mechanism of self-compassion on experimental pain: evidence from a compassionate self-talk protocol and heart rate variability. J Pain. 2020;21(7–8):790–7. 10.1016/j.jpain.2019.11.006.Suche in Google Scholar PubMed
[54] Pascalis V, de, Scacchia P. The influence of reward sensitivity, heart rate dynamics and EEG-delta activity on placebo analgesia. Behav Brain Res. 2019;359:320–32. 10.1016/j.bbr.2018.11.014.Suche in Google Scholar PubMed
[55] Porges Stephen W. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med. 2009;76(Suppl 2):S86–90. 10.3949/ccjm.76.s2.17.Suche in Google Scholar PubMed PubMed Central
[56] Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3). 10.1016/s0165-0327(00)00338-4.Suche in Google Scholar PubMed
[57] Koenig J, Falvay D, Clamor A, Wagner J, Jarczok MN, Ellis RJ, et al. Pneumogastric (Vagus) nerve activity indexed by heart rate variability in chronic pain patients compared to healthy controls: a systematic review and meta-analysis. Pain Physician. 2016;19(1):E55–78. 10.36076/ppj/2016.19.E55.Suche in Google Scholar
[58] Kuehn BM. News from the centers for disease control and prevention. J Am Med Assoc. 2018;319:2471.10.1001/jama.2018.7441Suche in Google Scholar PubMed
[59] Courtois I, Gholamrezaei A, Jafari H, Lautenbacher S, van Diest I, van Oudenhove L, et al. Respiratory hypoalgesia? The effect of slow deep breathing on electrocutaneous, thermal, and mechanical pain. J Pain. 2020;21(5–6):616–32. 10.1016/j.jpain.2019.10.002.Suche in Google Scholar PubMed
[60] Adler-Neal AL, Waugh CE, Garland EL, Shaltout HA, Diz DI, Zeidan F. The role of heart rate variability in mindfulness-based pain relief. J Pain. 2020;21(3–4):306–23. 10.1016/j.jpain.2019.07.003.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)
Artikel in diesem Heft
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)


