Associations between cognitive test scores and pain tolerance: The Tromsø study
-
Tonje Anita Melum
, Ólöf A. Steingrímsdóttir
Abstract
Objectives
Previous studies have suggested that experimental pain sensitivity is associated with cognitive function. The aim of this study is to assess this relationship in a large population-based sample.
Methods
We included 5,753 participants (aged 40–84 years) from the seventh wave of the population-based Tromsø Study who had been examined with cognitive tests and experimental pain assessments, and for whom information on covariates were available. Cox regression models were fitted using standardized scores on cognitive tests (12-word immediate recall test, digit symbol coding test, and Mini-Mental State Examination [MMS-E]) as the independent variable and cold pressor or cuff pressure pain tolerance as the dependent variables. Statistical adjustment was made for putative confounders, namely, age, sex, education, smoking, exercise, systolic blood pressure, body mass index, symptoms indicating anxiety or depression, analgesic use, and chronic pain.
Results
In multivariate analysis, cold pressor tolerance time was significantly associated with test scores on the 12-word immediate recall test (hazard ratio [HR] 0.93, 95% confidence interval [CI] 0.90–0.97, p < 0.001), the digit symbol coding test (HR 0.94, 95% CI 0.89–0.98, p = 0.004), and the MMS-E (HR 0.93, 95% CI 0.90–0.96 p < 0.001). Tolerance to cuff pressure algometry was significantly associated with 12-word immediate recall (HR 0.94–0.97, p < 0.001) and Digit Symbol Coding test scores (HR 0.93, 95% CI 0.89–0.96, p < 0.001) while there was no significant association with Mini Mental State Examination test score (HR 0.98, 95% CI 0.95–1.00, p = 0.082).
Conclusion
Lower pain tolerance was associated with poorer performance on cognitive tests.
1 Introduction
Pain and cognition are intertwined. They are both processed by wide networks of brain regions, which overlap considerably, and several neurotransmitters and receptor systems are involved in the processing of both pain and cognition [1]. People with chronic pain have lower performance on cognitive tests, both in specific domains such as attention, memory, and psychomotor speed and in screening tests of general cognitive function such as Mini-Mental State Examination (MMS-E) [1,2,3]. Proposed explanations of this association include that pain occupies resources in brain regions important for cognitive processing [4], or induces unfavorable neuroplastic changes or release of neurochemical mediators [2] that have adverse consequences for cognitive processing. However, a bidirectional relationship must be considered. Given the shared neuroanatomical and neurochemical underpinnings [1] and the role of cognition in the evaluative component of pain, it is reasonable to hypothesize that variation in brain health and cognitive performance could affect pain perception and modulation. While clinical pain varies depending on the type and severity of the causal pathology, experimental pain assessments provide a unique opportunity for studying the relationship with a controlled nociceptive stimulus, providing a critical test of this hypothesis.
A relationship between experimental pain assessments and score on tests of specific cognitive domains has been found by several studies. Higher tolerance to the cold pressor test (CPT) was associated with better performance on measurements of cognitive inhibitory control, namely, stop-signal [5] and Stroop [6,7,8] tasks. However, no association was found between CPT tolerance and other tests of executive function [6,7,8]. A study on pain sensitivity assessed by threshold and tolerance to cuff pressure algometry (CPA) and thresholds to manual pressure pain found no significant correlation between these measures and stop-signal or Stroop tasks [9]. This suggests that the relationship might depend both on type of experimental pain assessment and type of cognitive test. Importantly, these studies have mainly included relatively small convenience samples of healthy volunteers and hence one cannot infer whether variation in cognitive function is related to variation in pain sensitivity in the general population. In a previous study by our group, it was shown that longer pain tolerance, as measured by CPT, was associated with higher performance on immediate recall and digit symbol coding task [10] in a sample of 4,623 participants from a general population. To our knowledge, this is the only study on a large, population-based sample, and no studies have examined the relationships between experimental pain assessments and screening tests of general cognitive function.
In the present study, we aimed to expand the tests used in our earlier work to include CPA tolerance as an experimental pain method and MMS-E as an additional cognitive test. This allowed for the assessment of whether the association is consistent across different pain stimuli (applied to different body parts) and another cognitive test, in addition to test whether our previous findings could be replicated in a new sample.
2 Methods
2.1 Study design and participants
We included all 5,753 participants of the seventh survey of the population-based Tromsø Study, who had completed cognitive testing and experimental pain assessment with CPT and/or CPA tolerance test, and for whom information on covariates were available (Figure 1). Details on design of the seventh wave of the Tromsø study have been published previously [11].
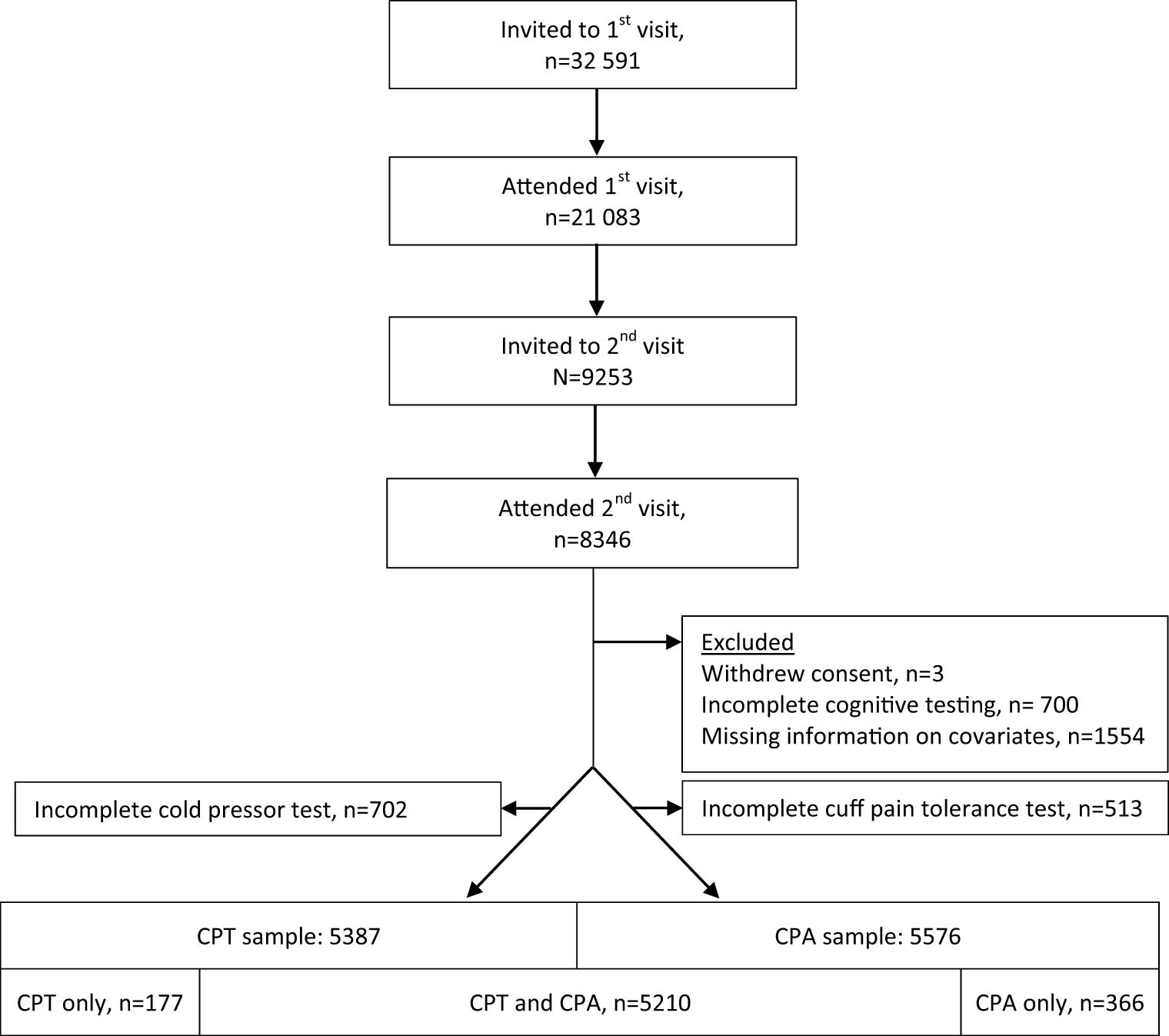
Flow chart of participants of the seventh wave of the Tromsø Study and the present study. All inhabitants in the municipality of Tromsø aged 40 years or older were invited by postal letter and 64.7% participated. Among those invited, 13,028 were pre-marked for invitation to a second visit, if they attended the first visit. At the first visit, participants completed questionnaires, and underwent blood sampling and clinical examinations, including the CPT and CPA. At the second visit, extended examinations were performed, including cognitive testing. Participants were included in the present study if they had completed cognitive testing (12-word immediate recall test, digit symbol coding test, and MMS-E), CPT, and/or CPA, and had available information on covariates (age, sex, education, smoking, exercise, systolic blood pressure, body mass index, symptoms indicating anxiety or depression, analgesic use, and chronic pain). Abbreviations: CPT, cold pressor test; CPA, cuff pressure algometry.
2.2 Cognitive tests
The cognitive assessments included three tests: (a) Immediate 12-word recall, a test of short-term verbal memory [12,13]. Twelve nouns were shown written on a board and read out loud with 5 s intervals, before the participant was asked to recall as many as possible within 2 min (score 0–12 according to the number of words recalled), (b) the digit symbol coding test also used in the Wechsler Adult Intelligence Scale, which measures motor speed, attention, and visuoperceptual functions [14]. Nine numbers were paired with nine symbols, and participants were asked to fill in symbols in blank numbered squares using this key within 90 s (maximum score 96), and (c) MMS-E, commonly used as a screening tool for dementia, covers several domains on cognitive function including orientation, memory, attention, and language (maximum score 30 points) [15,16].
2.3 Experimental pain assessment
In the CPT, the participants were asked to keep their hand and wrist submerged in cold water (3°C) for as long as they were able to or until the maximum time (120 s). Constant temperature was ensured by continuous exchange between the 13 L cold water bath and a circulating cooler (FP40-HE, Julabo GmbH Germany). Time with hand in water bath was used as a measure of CPT tolerance. CPA tolerance was assessed by inflating a blood pressure cuff around one leg at a time, by 1 kPa/s up to a maximum limit of 100 kPa. Inflation and pressure were controlled by a CPA device (NociTech, Aalborg, Denmark).
The participant was instructed to press a button to stop the test if the pain became unbearable. Pain tolerance was recorded as kPa at button press (equal to endurance time as the pressure increased by 1 kPa per second) or at the maximum limit, whichever came first. For this study, result from CPA on the non-dominant leg was used as there were fewer missing on this variable than CPA on the dominant leg. Reasons for exclusion included participants’ decline, inability to comprehend instructions, or medical issues that were considered to interfere or put the participant at risk if exposed to cold or pressure to the calf.
2.4 Covariates
Information on covariates were obtained from on-site measurements (systolic blood pressure and body mass index [BMI]) or questionnaire (education level, smoking [current, previous, or never daily smoking], exercise frequency, symptoms of anxiety or depression measured with the 10-item version of Hopkins Symptom Checklist [HSCL-10] [17], frequency of analgesic use [prescription or non-prescription], and presence of chronic pain [yes or no]) [11].
2.5 Statistical analyses
For descriptive purposes, participants were categorized as pain tolerant or pain sensitive according to whether or not they endured the full 120 s of CPT. Group differences were evaluated with t-test or Wilcoxon rank-sum test for continuous variables and with Pearson chi-square for categorical variables. Kaplan–Meier curves were created for visualization of CPT and CPA tolerance according to cognitive test scores (above or below mean for immediate recall and coding test, for MMS-E according to whether score indicated normal [score of 28–30 points], possible cognitive impairment (25–27) or cognitive impairment [≤24]) [16]. In order to avoid the disadvantages related to dichotomization, CPT and CPA tolerance and cognitive test scores were used as continuous variables in the analyses. As CPT and CPA tolerance times are right-censored due to the maximum time, Cox proportional hazard models were fitted for analysis with pain tolerance time to CPT and CPA as time variables (censored at the 120 and 100 s maximum times) and test abortion as event. Cognitive test scores, standardized by z-transformation, were used as the independent variable. Variables that were based on previous evidence could be associated with both pain and cognition were treated as potential confounders and added as covariates in three steps: first age, sex, and education (Model 1), then additional adjustment for smoking, exercise, BMI, blood pressure, and depression (Model 2), and last additional adjustment for chronic pain and analgesic use (Model 3). Interaction terms were tested for age, sex, and chronic pain by adding the respective variable multiplied with cognitive test score. Since a substantial proportion of the seventh survey participants also had participated in the previous sixth survey of the Tromsø Study, we performed a sensitivity analysis by excluding those who had attended cognitive testing and CPT in the previous sixth survey in order to assess the association in an independent sample.
Analyses were performed in STATA (version 17 for Windows; StataCorp LLC, Texas, USA).
3 Results
A sample of 5,387 participants were included for analysis on CPT tolerance time (Figure 1). Median age was 63 years (range 40–84) and 51.9% were women. In this sample, 1,994 (37%) kept their hand in the water until the maximum time and hence categorized as pain tolerant, while 3,393 (63%) withdrew it earlier and were categorized as pain sensitive (categorization for descriptive purposes only). Pain-tolerant participants had a lower proportion of women, fewer current smokers, and lower mean BMI, while their education level, exercise frequency, and mean systolic blood pressure were higher (Table 1). A higher proportion of the pain-sensitive participants had an HSCL-10 score indicative of anxiety or depression. Pain-sensitive participants reported more frequent use of analgesic medication, while the proportions who reported chronic pain were similar in the two groups. The pain-tolerant participants had higher mean scores on immediate recall and coding test and for MMS-E, the upper limit of the interquartile range was higher in this group indicating a distribution with more participants with higher scores. There was no significant difference in the proportions with possible or definite cognitive impairment according to the levels of MMS-E scores.
Descriptive characteristics of all participants in CPT sample and according to CPT tolerance*
| All participants n = 5,387 | Pain tolerant n = 1,994 (37%) | Pain sensitive n = 3,393 (63%) | P value** | |
|---|---|---|---|---|
| Age in years, median (interquartile range) | 63 (54–69) | 63 (53–69) | 63 (55–69) | 0.457 |
| Women, n (%) | 2,793 (51.9) | 904 (45.3) | 1,889 (55.7) | <0.001 |
| Education, n (%) | 0.003 | |||
| Primary/secondary school, up to 10 years | 1,278 (23.7) | 442 (22.2) | 836 (24.6) | |
| Upper secondary, 3 years | 1,538 (28.6) | 535 (26.8) | 1,003 (29.6) | |
| College or university, 1–3 years | 1,098 (20.4) | 425 (21.3) | 673 (19.8) | |
| College or university, 4 years or more | 1,473 (27.3) | 592 (29.7) | 881 (26.0) | |
| Exercise | <0.001 | |||
| Never | 176 (3.3) | 46 (2.3) | 130 (3.8) | |
| Less than once per week | 591 (11.0) | 197 (9.9) | 394 (11.6) | |
| Once a week | 748 (13.9) | 246 (12.3) | 502 (14.8) | |
| 2–3 times a week | 2,316 (43.0) | 887 (44.5) | 1,429 (72.4) | |
| Approximately every day | 1,556 (28.9) | 618 (31.0) | 938 (27.7) | |
| Smoking, n (%) | <0.001 | |||
| Never | 2,169 (40.3) | 910 (45.6) | 1,259 (37.1) | |
| Previous | 2,595 (48.2) | 887 (44.5) | 1,708 (50.3) | |
| Current | 623 (11.6) | 197 (9.9) | 426 (12.6) | |
| BMI in kg/cm2, mean value ± SD | 27.3 ± 4.4 | 27.1 ± 4.2 | 27.4 ± 4.5 | 0.012 |
| Systolic BP mean value ± SD | 131.8 ± 19.3 | 132.7 ± 18.9 | 131.3 ± 19.6 | 0.010 |
| Anxiety or depression (HCSL-10 ≥ 1.85), n (%) | 339 (6.3) | 106 (5.3) | 233 (6.9) | 0.024 |
| Chronic pain yes, n (%) | 1,854 (34.4) | 668 (33.5) | 1,186 (35.0) | 0.278 |
| Analgesics last four weeks | <0.001 | |||
| Not used | 2,907 (54.0) | 1,156 (58.0) | 1,751 (51.6) | |
| Less than weekly | 1,663 (30.9) | 568 (28.5) | 1,095 (32.3) | |
| Weekly | 597 (11.1) | 199 (10.0) | 398 (11.7) | |
| Daily | 220 (4.08) | 71 (3.6) | 149 (4.4) | |
| Immediate recall test score, mean value ± SD | 7.5 ± 1.9 | 7.6 ± 1.9 | 7.4 ± 1.9 | <0.001 |
| Coding test score, mean value ± SD | 44.8 ± 11.8 | 45.3 ± 11.7 | 44.5 ± 11.9 | 0.0145 |
| MMS-E test score, median (interquartile range) | 29 (27–29) | 29 (27–30) | 29 (27–29) | 0.001 |
| MMS-E deficit, n (%) | ||||
| Normal 28–30 | 3,914 (72.7) | 1,477 (74.1) | 2,437 (71.8) | 0.156 |
| Possible impairment 25–27 | 1,263 (23.5) | 448 (22.5) | 815 (24.0) | |
| Cognitive impairment 24 or lower | 210 (3.9) | 69 (3.5) | 141 (4.2) |
*Participants were categorized as pain tolerant if they endured the whole 120 s of the CPT, and pain sensitive if they withdrew their hand at an earlier time. **P-value is for difference between pain-sensitive and pain-tolerant group, assessed with t-test for continuous variables and with Pearson chi-square for categorical variables. As age and MMS were not normally distributed, Wilcoxon rank-sum test was used for difference between groups for these variables. Abbreviations: CPT, cold pressor test; BMI, body mass index; HSCL-10: Hopkins symptom check list (10-item version); MMS-E: Mini Mental State Examination.
Kaplan–Meier curves showing raw data of CPT tolerance time stratified by immediate recall and coding test scores indicated that participants with a score below the mean value tended to withdraw their hand from the water at an earlier time (Figure 2). Kaplan–Meier curves of CPT tolerance time, according to MMS-E score group, indicated that participants in lower categories of cognitive function showed increased likelihood of hand withdrawal (Figure 2).
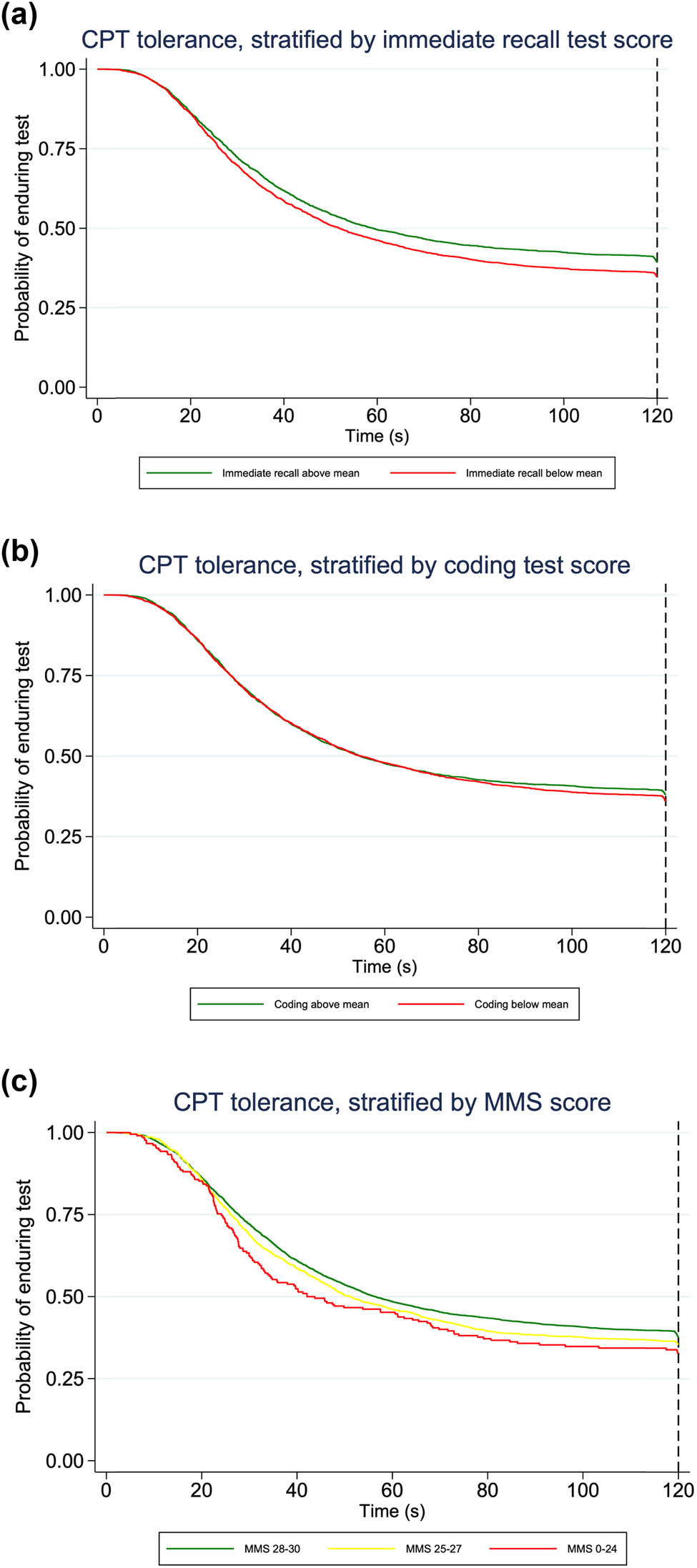
Kaplan–Meier curves of CPT tolerance time by score on cognitive test. Probability of keeping the hand in the water bath in participants stratified by scores on cognitive test. The maximum time was 120 s, indicated by the dotted reference line. (a and b) For immediate recall and coding test, participants are grouped according to test score above or below mean. (c) For MMS-E, participants are grouped according to whether the score is considered normal (score of 28–30 points), possible cognitive impairment (25–27) or cognitive impairment (≤24). CPT: Cold pressor test; MMS-E: Mini Mental State Examination.
Multivariable adjusted analysis on the relationship between cognitive test score and CPT tolerance time showed a significant association between pain tolerance time and cognitive test scores for all three tests (Table 2). Adding covariates to the models had minimal impact on the relationships. For immediate recall, the hazard ratio (HR) was 0.93 (95% confidence interval [CI] 0.90–0.97, p < 0.001) when adjusting for all covariates. The results for coding test and MMS-E were similar (HR 0.94, 95% CI 0.89–0.98, p = 0.004, and HR 0.93, 95% CI 0.90–0.96, p < 0.001, respectively). Sensitivity analysis showed that results were similar when 1,786 participants who had attended cognitive testing and CPT in the previous sixth survey [10] were excluded (Table S1), indicating the presence of association across independent samples.
Cox regression analyses of the association between cognitive test scores and pain tolerance
| Immediate recall test score (standardized by z-transformation) | Coding test score (standardized by z-transformation) | MMS-E test score (standardized by z-transformation) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cold pressor test ( n = 5,387) | |||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Model 1: | 0.92 | 0.89–0.96 | <0.001 | 0.93 | 0.89–0.97 | 0.002 | 0.93 | 0.90–0.96 | <0.001 |
| Model 2: | 0.93 | 0.90–0.97 | <0.001 | 0.94 | 0.89–0.98 | 0.004 | 0.93 | 0.90–0.96 | <0.001 |
| Model 3: | 0.93 | 0.90–0.97 | <0.001 | 0.94 | 0.89–0.98 | 0.004 | 0.93 | 0.90–0.96 | <0.001 |
| Cuff pain tolerance test ( n = 5,576) | |||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Model 1: | 0.94 | 0.91–0.96 | <0.001 | 0.92 | 0.89–0.95 | <0.001 | 0.97 | 0.94–1.00 | 0.036 |
| Model 2: | 0.94 | 0.91–0.97 | <0.001 | 0.93 | 0.89–0.96 | <0.001 | 0.98 | 0.95–1.00 | 0.090 |
| Model 3: | 0.94 | 0.91–0.97 | <0.001 | 0.93 | 0.89–0.96 | <0.001 | 0.98 | 0.95–1.00 | 0.082 |
Analyses are Cox regression with cognitive test score, standardized by z-transformation, as independent variable and endurance time of CPT or cuff pain algometry as outcome, adjusted for covariates as specified by model: Model 1: adjusted for sex, age, and education. Model 2: adjusted for age, sex, education, smoking, exercise, body mass index, blood pressure, and depression. Model 3: adjusted for age, sex, education, smoking, exercise, body mass index, blood pressure, depression, chronic pain, and analgesic use. Abbreviation: MMS-E: Mini Mental State Examination.
For analysis of CPA tolerance, 5,576 participants were included (Figure 1), of whom 383 (6.9%) were CPA tolerant and endured the full time of the test, while the majority (n = 5,193, 93.1%) stopped the test at an earlier time. Kaplan–Meier curves of CPA tolerance showed similar patterns as for CPT for immediate recall and coding test, while for MMS-E, the pattern was less clear (Figure 3). Analysis of CPA tolerance showed similar findings as for CPT, with scores of immediate recall and coding test significantly associated with hazard of aborting the CPA test: when adjusting for all covariates, HR was 0.94 (95% CI 0.91–0.97, p < 0.001) for immediate recall and 0.93 (95% CI 0.89–0.96, p < 0.001) for coding test. The association with MMS-E was weaker and was not significant in the fully adjusted model (HR 0.98, 95% CI 0.95–1.00, p = 0.082) (Table 2).
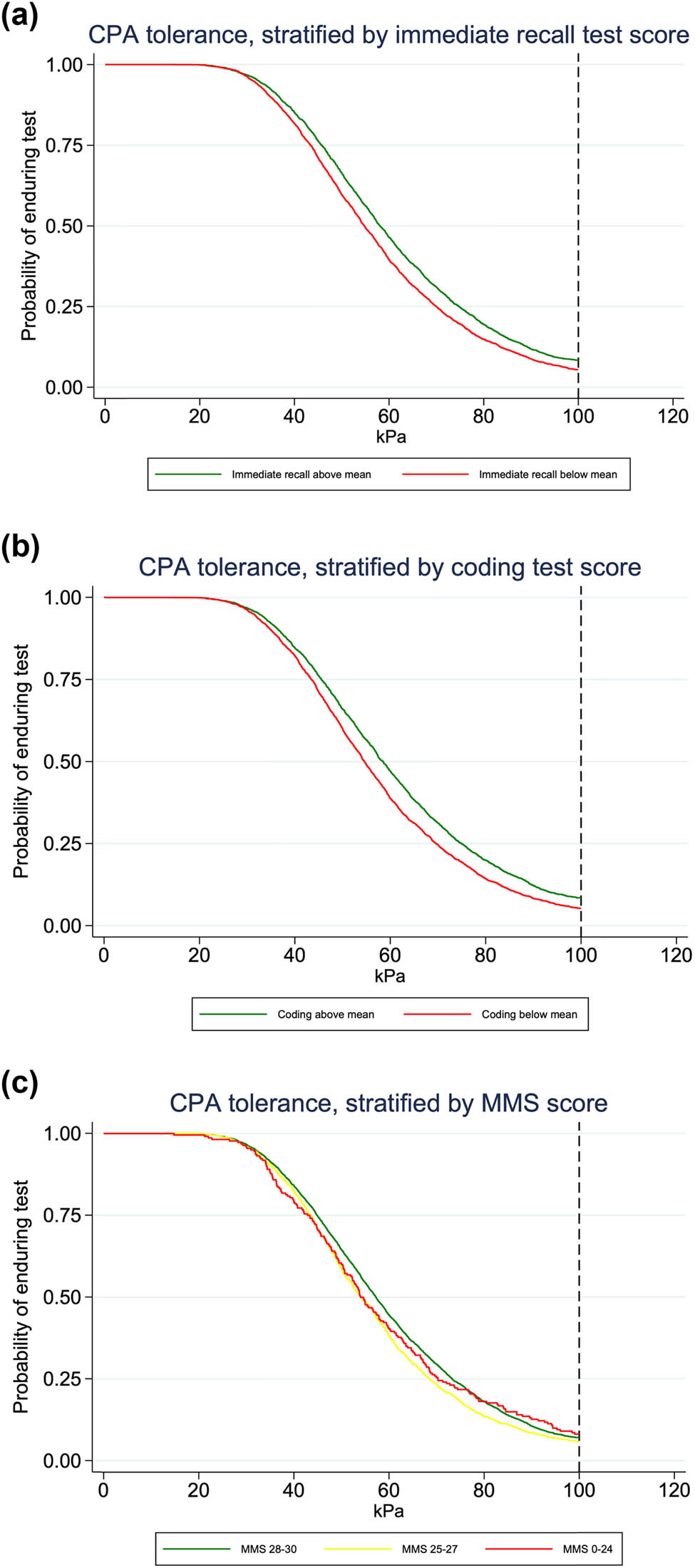
Kaplan–Meier curves of CPA tolerance by score on cognitive test. Probability of enduring CPA in participants stratified by scores on cognitive test. The pressure increased by 1 kPa/s. The maximum pressure was 100 kPa, indicated by the dotted reference line. (a and b) CPA endurance by scores on immediate recall and coding test. Participants are grouped according to test score above or below mean value. (c) CPA endurance by MMS-E score. Participants are grouped according to whether the score is considered normal (score of 28–30 points), possible cognitive impairment (25–27) or cognitive impairment (≤24). CPA: Cuff pressure algometry; MMS-E: Mini Mental State Examination.
We found a borderline significant interaction effect for age on the relationship between immediate recall test and CPT and a significant interaction effect on the relationship between all three cognitive tests and CPA tolerance (Table S2). Subgroup analysis indicated that the association between immediate recall test and CPT was stronger in the youngest age group (Table 3). For CPA tolerance, subgroup analysis suggested stronger effect in the younger participants for immediate recall and MMS-E, while stronger effect in the oldest age group for coding test (Table 3). We found significant interaction effect of sex on the association between MMS-E and CPT. Analyses on this relationship stratified by sex suggested somewhat stronger effect in men (Table 4). While subgroup analysis suggests a somewhat stronger relationship with immediate recall for women, the p-value of this interaction term was not significant (p = 0.683). There was no significant interaction effect of chronic pain on the relationship between any of the cognitive test scores and CPT or CPA tolerance time (Table S2).
Subgroup analysis according to age groups
| Immediate recall test score (standardized by z-transformation) | Coding test score (standardized by z-transformation) | MMS-E test score (standardized by z-transformation) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CPT (n = 5,387) | |||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Age 40–59 (n = 1,959) | 0.88 | 0.83–0.94 | <0.001 | 0.96 | 0.89–1.03 | 0.289 | 0.88 | 0.83–0.94 | <0.001 |
| Age 60–69 (n = 2,233) | 0.97 | 0.92–1.03 | 0.390 | 0.93 | 0.86–0.99 | 0.031 | 0.95 | 0.89–1.01 | 0.076 |
| Age 70–84 (n = 1,195) | 0.93 | 0.86–1.01 | 0.067 | 0.89 | 0.81–0.99 | 0.031 | 0.93 | 0.88–0.98 | 0.005 |
| Cuff pain tolerance test (n = 5,576) | |||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Age 40–59 (n = 2,021) | 0.91 | 0.87–0.96 | <0.001 | 0.94 | 0.89–1.00 | 0.052 | 0.95 | 0.90–0.99 | 0.025 |
| Age 60–69 (n = 2,334) | 0.94 | 0.90–0.99 | 0.022 | 0.95 | 0.89–1.00 | 0.064 | 0.97 | 0.92–1.02 | 0.195 |
| Age 70–84 (n = 1,221) | 0.96 | 0.90–1.02 | 0.193 | 0.86 | 0.80–0.93 | <0.001 | 0.99 | 0.95–1.03 | 0.634 |
Analyses are Cox regression with cognitive test score, standardized by z-transformation, as independent variable and endurance of CPT or cuff pain algometry as outcome, adjusted for age, sex, education, smoking, exercise, body mass index, blood pressure, depression, chronic pain, and analgesic use.
Subgroup analyses of the association between cognitive test scores and CPT tolerance time according to sex
| Immediate recall test score (standardized by z-transformation) | Coding test score (standardized by z-transformation) | MMS-E test score (standardized by z-transformation) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CPT (n = 5,387) | ||||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95%CI | p | ||
| Men (n = 2,954) | 0.95 | 0.90–1.01 | 0.075 | 0.94 | 0.88–1.01 | 0.089 | 0.91 | 0.86–0.96 | <0.001 | |
| Women (n = 2,793) | 0.92 | 0.88–0.97 | 0.001 | 0.94 | 0.88–0.99 | 0.029 | 0.95 | 0.91–0.99 | 0.016 | |
Analyses are Cox regression with cognitive test score, standardized by z-transformation, as independent variable and time with hand in cold-water bath as outcome, adjusted for age, education, smoking, exercise, body mass index, blood pressure, depression, chronic pain, and analgesic use. Abbreviation: MMS-E: Mini Mental State Examination.
4 Discussion
Our main finding was that pain tolerance for two different experimental pain modalities, applied to hand and leg, was associated with cognitive function across all three cognitive tests. The results are similar to observations from the sixth survey of Tromsø Study on the association between immediate recall test and coding test and CPT [10], indicating consistency across time and samples. While the addition of CPA and MMS-E provides new information on the association with these tests as well as consistency across pain and cognitive assessments, replication of previous findings is in itself an important contribution: while reproducibility is a cornerstone in science, it has been shown that less than half of original effects were reproduced in a large study on replication of results from cognitive and social psychology studies [18].
The main pattern emerging from our study is a consistent association between cognitive test scores and pain tolerance in a general population. The design of our study does however not allow for causal inference. Possible explanations of a cross-sectional association are a causal relationship where one variable is caused by the other or vice versa, or both being caused by other variables (confounding). It seems reasonable that variation in cognitive function could cause variation in pain tolerance. Plausible interpretations of our findings are that cognitive function, and/or brain conditions affecting cognitive function, affects pain processing. This is supported by results from other studies that have shown that poorer cognitive performance predicts chronic pain in population-based and surgical cohorts [19,20]. Reduced cognitive function in chronic pain populations may be a cause or risk factor for, rather than or in addition to, a consequence of chronic pain.
A reverse association is possible, but does not seem as plausible. Adjustment for possible confounders previously known to be associated with both cognition and pain sensitivity had little impact on the results. Adjustment for chronic pain had minimal impact on the effect estimates, and there was no interaction effect of chronic pain. This may partly be due to the questionnaire-based definition of chronic pain, where approximately one third of our sample fulfilled this criterion (Table 1). However, our findings indicate that there is a relationship between cognitive function and pain tolerance that does not depend on chronic pain.
The pattern of age group differences in previous analysis on data from the sixth Tromsø Study [10] was not supported in the present study. Significant interaction effect of sex was seen for the relationship between MMS-E and CPT tolerance, with small difference between sexes, but not for the other cognitive test or with CPA as the outcome. Inconsistent findings regarding the presence of interaction effects and distribution of effects across strata may reflect Type I error. The weaker association between MMS-E and CPA tolerance might also be related to the ceiling effect in MMS-E [21], which can make it less sensitive for variation within a population in which most participants are cognitively healthy.
Clinical relevance of experimental pain sensitivity is suggested by such studies finding higher pain sensitivity to be associated with chronic pain [22,23,24] and with subsequent postoperative pain [25] and non-recovery after acute whiplash [26]. Though large prospective studies are lacking, these findings suggest that experimental pain sensitivity could be a risk factor for the development and severity of chronic pain.
Our findings suggest that people with lower cognitive performance are less tolerant to pain. Individuals with cognitive impairments are likely underrepresented in our study, due to lower probability of attendance to the Tromsø Study and exclusion from experimental pain assessment if understanding of instructions was insufficient. Consequently, smaller variance within our sample could be expected to weaken our results. Meanwhile, it seems likely that the association we find in our sample is also present in these groups. This implies that particular care should be taken by health professionals in treatment of these groups, as pain might be experienced as more intense – or harder to deal with – by these persons.
4.1 Limitations and strengths
The major strengths of our study are the large sample recruited from a general population, consistency with findings from the previous study, and consistency across pain modalities and cognitive tests. While there are some differences in CPT tolerance across the sixth and seventh wave of the Tromsø study, these do not affect the relationship with cognitive test scores. The cognitive tests in the Tromsø study were selected based on their suitability to detect early cognitive decline and to be used as screening tests in a large number of participants, and are less suited for identifying more specific cognitive deficits. Hence, we are unable to distinguish associations with specific cognitive functions from a more general deficit in cognitive ability, which is a limitation to our study.
5 Conclusion
Cognitive function assessed by immediate recall, coding test, and MMS-E is associated with pain tolerance and these associations are independent of the presence of chronic pain. These findings replicate and extend previous findings, and as such appear to be robust. In summary, there is a consistent association between cognitive test scores and pain tolerance, suggesting that people with poorer performance on cognitive tests are more sensitive to pain.
-
Research ethics: The study was conducted in accordance with the Declaration of Helsinki. The Tromsø study and the present study were approved by the Regional Committee for Medical and Health Research Ethics (2014/940/REK Nord and 2017/1951/REK Nord, respectively).
-
Informed consent: All participants signed the written informed consent.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Henrik Schirmer: Received a research grant to institution as part of a joint venture research collaboration on heart failure with Novartis, consultant fees from Norvartis and lecture fees from Amgen, Astra Zeneca, Boehringer, and Novartis in person. None of the other authors have declared any competing interests. Audun Stubhaug is a Honorary Editor of Scandinavian Journal of Pain, Christopher S. Nielsen is a Section Editor of Scandinavian Journal of Pain.
-
Research funding: This project was funded by a PhD grant from Northern Norway Regional Health Authority (Grant number HNF1460-19).
-
Data availability: Data availability is restricted due to their sensitive nature. De-identified data can be obtained by application to the Tromsø study. Contact tromsous@uit.no for details.
References
[1] Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93(3):385–404.10.1016/j.pneurobio.2011.01.002Search in Google Scholar PubMed
[2] Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev. 2000;10(3):131–49.10.1023/A:1009020914358Search in Google Scholar
[3] Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, Lorimer Moseley G. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain. 2013;154(8):1181–96.10.1016/j.pain.2013.03.002Search in Google Scholar PubMed
[4] Eccleston C, Crombez G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125(3):356–66.10.1037//0033-2909.125.3.356Search in Google Scholar
[5] Karsdorp PA, Geenen R, Vlaeyen JW. Response inhibition predicts painful task duration and performance in healthy individuals performing a cold pressor task in a motivational context. Eur J Pain. 2014;18(1):92–100.10.1002/j.1532-2149.2013.00348.xSearch in Google Scholar PubMed
[6] Zhou S, Kemp J, Després O, Pebayle T, Dufour A. The association between inhibition and pain tolerance in the elderly: evidence from event-related potentials. Eur J Pain. 2015;19(5):669–76.10.1002/ejp.588Search in Google Scholar PubMed
[7] Bjekić J, Živanović M, Purić D, Oosterman JM, Filipović SR. Pain and executive functions: A unique relationship between Stroop task and experimentally induced pain. Psychol Res. 2018;82(3):580–9.10.1007/s00426-016-0838-2Search in Google Scholar PubMed
[8] Oosterman JM, Dijkerman HC, Kessels RP, Scherder EJ. A unique association between cognitive inhibition and pain sensitivity in healthy participants. Eur J Pain. 2010;14(10):1046–50.10.1016/j.ejpain.2010.04.004Search in Google Scholar PubMed
[9] Gajsar H, Meyer M, Hasenbring MI, Vaegter HB. Pain and executive function: no association between remote exercise-induced hypoalgesia and cognitive inhibition in pain-free participants. Scand J Pain. 2022;22(1):173–85.10.1515/sjpain-2021-0071Search in Google Scholar PubMed
[10] Jacobsen HB, Stubhaug A, Schirmer H, Inge Landro N, Wilsgaard T, Mathiesen EB, et al. Neuropsychological functions of verbal recall and psychomotor speed significantly affect pain tolerance. Eur J Pain. 2019;23(9):1608–18.10.1002/ejp.1437Search in Google Scholar PubMed PubMed Central
[11] Hopstock LA, Grimsgaard S, Johansen H, Kanstad K, Wilsgaard T, Eggen AE. The seventh survey of the Tromsø Study (Tromsø7) 2015–2016: Study design, data collection, attendance, and prevalence of risk factors and disease in a multipurpose population-based health survey. Scand J Public Health. 2022:14034948221092294.10.1177/14034948221092294Search in Google Scholar PubMed PubMed Central
[12] Johnsen B, Strand BH, Martinaityte I, Lorem GF, Schirmer H. Leisure time physical activities’ association with cognition and dementia: A 19 years’ life course study. Front Aging Neurosci. 2022;14:906678.10.3389/fnagi.2022.906678Search in Google Scholar PubMed PubMed Central
[13] Bäckman L, Forsell Y. Episodic memory functioning in a community-based sample of old adults with major depression: Utilization of cognitive support. J Abnorm Psychol. 1994;103(2):361–70.10.1037//0021-843X.103.2.361Search in Google Scholar PubMed
[14] Jaeger J. Digit symbol substitution test: The case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513–9.10.1097/JCP.0000000000000941Search in Google Scholar PubMed PubMed Central
[15] Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.10.1016/0022-3956(75)90026-6Search in Google Scholar PubMed
[16] The Norwegian National Centre for Ageing and Health. Manual norsk revidert mini mental status evaluering (MMSE-NR-3); 2021.Search in Google Scholar
[17] Strand BH, Dalgard OS, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: A comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry. 2003;57(2):113–8.10.1080/08039480310000932Search in Google Scholar PubMed
[18] Open Science Collaboration. Psychology. Estimating the reproducibility of psychological science. Science. 2015;349(6251):aac4716.10.1126/science.aac4716Search in Google Scholar PubMed
[19] Rouch I, Dorey JM, Strippoli MF, Gholam M, Marques-Vidal P, Laurent B, et al. Does cognitive functioning predict chronic pain in older adult? Results from the CoLaus|PsyCoLaus longitudinal study. J Pain. 2021;22(8):905–13.10.1016/j.jpain.2021.01.007Search in Google Scholar PubMed
[20] Attal N, Masselin-Dubois A, Martinez V, Jayr C, Albi A, Fermanian J, et al. Does cognitive functioning predict chronic pain? Results from a prospective surgical cohort. Brain. 2014;137(Pt 3):904–17.10.1093/brain/awt354Search in Google Scholar PubMed
[21] Philipps V, Amieva H, Andrieu S, Dufouil C, Berr C, Dartigues JF, et al. Normalized mini-mental state examination for assessing cognitive change in population-based brain aging studies. Neuroepidemiology. 2014;43(1):15–25.10.1159/000365637Search in Google Scholar PubMed
[22] Stabell N, Stubhaug A, Flægstad T, Nielsen CS. Increased pain sensitivity among adults reporting irritable bowel syndrome symptoms in a large population-based study. Pain. 2013;154(3):385–92.10.1016/j.pain.2012.11.012Search in Google Scholar PubMed
[23] Staud R, Weyl EE, Price DD, Robinson ME. Mechanical and heat hyperalgesia highly predict clinical pain intensity in patients with chronic musculoskeletal pain syndromes. J Pain. 2012;13(8):725–35.10.1016/j.jpain.2012.04.006Search in Google Scholar PubMed PubMed Central
[24] Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. 2012;20(10):1075–85.10.1016/j.joca.2012.06.009Search in Google Scholar PubMed
[25] Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90(3):261–9.10.1016/S0304-3959(00)00406-1Search in Google Scholar PubMed
[26] Kasch H, Qerama E, Bach FW, Jensen TS. Reduced cold pressor pain tolerance in non-recovered whiplash patients: A 1-year prospective study. Eur J Pain. 2005;9(5):561–9.10.1016/j.ejpain.2004.11.011Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)
Articles in the same Issue
- Editorial Comment
- From pain to relief: Exploring the consistency of exercise-induced hypoalgesia
- Christmas greetings 2024 from the Editor-in-Chief
- Original Articles
- The Scandinavian Society for the Study of Pain 2022 Postgraduate Course and Annual Scientific (SASP 2022) Meeting 12th to 14th October at Rigshospitalet, Copenhagen
- Comparison of ultrasound-guided continuous erector spinae plane block versus continuous paravertebral block for postoperative analgesia in patients undergoing proximal femur surgeries
- Clinical Pain Researches
- The effect of tourniquet use on postoperative opioid consumption after ankle fracture surgery – a retrospective cohort study
- Changes in pain, daily occupations, lifestyle, and health following an occupational therapy lifestyle intervention: a secondary analysis from a feasibility study in patients with chronic high-impact pain
- Tonic cuff pressure pain sensitivity in chronic pain patients and its relation to self-reported physical activity
- Reliability, construct validity, and factorial structure of a Swedish version of the medical outcomes study social support survey (MOS-SSS) in patients with chronic pain
- Hurdles and potentials when implementing internet-delivered Acceptance and commitment therapy for chronic pain: a retrospective appraisal using the Quality implementation framework
- Exploring the outcome “days with bothersome pain” and its association with pain intensity, disability, and quality of life
- Fatigue and cognitive fatigability in patients with chronic pain
- The Swedish version of the pain self-efficacy questionnaire short form, PSEQ-2SV: Cultural adaptation and psychometric evaluation in a population of patients with musculoskeletal disorders
- Pain coping and catastrophizing in youth with and without cerebral palsy
- Neuropathic pain after surgery – A clinical validation study and assessment of accuracy measures of the 5-item NeuPPS scale
- Translation, contextual adaptation, and reliability of the Danish Concept of Pain Inventory (COPI-Adult (DK)) – A self-reported outcome measure
- Cosmetic surgery and associated chronic postsurgical pain: A cross-sectional study from Norway
- The association of hemodynamic parameters and clinical demographic variables with acute postoperative pain in female oncological breast surgery patients: A retrospective cohort study
- Healthcare professionals’ experiences of interdisciplinary collaboration in pain centres – A qualitative study
- Effects of deep brain stimulation and verbal suggestions on pain in Parkinson’s disease
- Painful differences between different pain scale assessments: The outcome of assessed pain is a matter of the choices of scale and statistics
- Prevalence and characteristics of fibromyalgia according to three fibromyalgia diagnostic criteria: A secondary analysis study
- Sex moderates the association between quantitative sensory testing and acute and chronic pain after total knee/hip arthroplasty
- Tramadol-paracetamol for postoperative pain after spine surgery – A randomized, double-blind, placebo-controlled study
- Cancer-related pain experienced in daily life is difficult to communicate and to manage – for patients and for professionals
- Making sense of pain in inflammatory bowel disease (IBD): A qualitative study
- Patient-reported pain, satisfaction, adverse effects, and deviations from ambulatory surgery pain medication
- Does pain influence cognitive performance in patients with mild traumatic brain injury?
- Hypocapnia in women with fibromyalgia
- Application of ultrasound-guided thoracic paravertebral block or intercostal nerve block for acute herpes zoster and prevention of post-herpetic neuralgia: A case–control retrospective trial
- Translation and examination of construct validity of the Danish version of the Tampa Scale for Kinesiophobia
- A positive scratch collapse test in anterior cutaneous nerve entrapment syndrome indicates its neuropathic character
- ADHD-pain: Characteristics of chronic pain and association with muscular dysregulation in adults with ADHD
- The relationship between changes in pain intensity and functional disability in persistent disabling low back pain during a course of cognitive functional therapy
- Intrathecal pain treatment for severe pain in patients with terminal cancer: A retrospective analysis of treatment-related complications and side effects
- Psychometric evaluation of the Danish version of the Pain Self-Efficacy Questionnaire in patients with subacute and chronic low back pain
- Dimensionality, reliability, and validity of the Finnish version of the pain catastrophizing scale in chronic low back pain
- To speak or not to speak? A secondary data analysis to further explore the context-insensitive avoidance scale
- Pain catastrophizing levels differentiate between common diseases with pain: HIV, fibromyalgia, complex regional pain syndrome, and breast cancer survivors
- Prevalence of substance use disorder diagnoses in patients with chronic pain receiving reimbursed opioids: An epidemiological study of four Norwegian health registries
- Pain perception while listening to thrash heavy metal vs relaxing music at a heavy metal festival – the CoPainHell study – a factorial randomized non-blinded crossover trial
- Observational Studies
- Cutaneous nerve biopsy in patients with symptoms of small fiber neuropathy: a retrospective study
- The incidence of post cholecystectomy pain (PCP) syndrome at 12 months following laparoscopic cholecystectomy: a prospective evaluation in 200 patients
- Associations between psychological flexibility and daily functioning in endometriosis-related pain
- Relationship between perfectionism, overactivity, pain severity, and pain interference in individuals with chronic pain: A cross-lagged panel model analysis
- Access to psychological treatment for chronic cancer-related pain in Sweden
- Validation of the Danish version of the knowledge and attitudes survey regarding pain
- Associations between cognitive test scores and pain tolerance: The Tromsø study
- Healthcare experiences of fibromyalgia patients and their associations with satisfaction and pain relief. A patient survey
- Video interpretation in a medical spine clinic: A descriptive study of a diverse population and intervention
- Role of history of traumatic life experiences in current psychosomatic manifestations
- Social determinants of health in adults with whiplash associated disorders
- Which patients with chronic low back pain respond favorably to multidisciplinary rehabilitation? A secondary analysis of a randomized controlled trial
- A preliminary examination of the effects of childhood abuse and resilience on pain and physical functioning in patients with knee osteoarthritis
- Differences in risk factors for flare-ups in patients with lumbar radicular pain may depend on the definition of flare
- Real-world evidence evaluation on consumer experience and prescription journey of diclofenac gel in Sweden
- Patient characteristics in relation to opioid exposure in a chronic non-cancer pain population
- Topical Reviews
- Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans
- What do we know about Indigenous Peoples with low back pain around the world? A topical review
- The “future” pain clinician: Competencies needed to provide psychologically informed care
- Systematic Reviews
- Pain management for persistent pain post radiotherapy in head and neck cancers: systematic review
- High-frequency, high-intensity transcutaneous electrical nerve stimulation compared with opioids for pain relief after gynecological surgery: a systematic review and meta-analysis
- Reliability and measurement error of exercise-induced hypoalgesia in pain-free adults and adults with musculoskeletal pain: A systematic review
- Noninvasive transcranial brain stimulation in central post-stroke pain: A systematic review
- Short Communications
- Are we missing the opioid consumption in low- and middle-income countries?
- Association between self-reported pain severity and characteristics of United States adults (age ≥50 years) who used opioids
- Could generative artificial intelligence replace fieldwork in pain research?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increases
- Original Experimental
- Confirmatory study of the usefulness of quantum molecular resonance and microdissectomy for the treatment of lumbar radiculopathy in a prospective cohort at 6 months follow-up
- Pain catastrophizing in the elderly: An experimental pain study
- Improving general practice management of patients with chronic musculoskeletal pain: Interdisciplinarity, coherence, and concerns
- Concurrent validity of dynamic bedside quantitative sensory testing paradigms in breast cancer survivors with persistent pain
- Transcranial direct current stimulation is more effective than pregabalin in controlling nociceptive and anxiety-like behaviors in a rat fibromyalgia-like model
- Paradox pain sensitivity using cuff pressure or algometer testing in patients with hemophilia
- Physical activity with person-centered guidance supported by a digital platform or with telephone follow-up for persons with chronic widespread pain: Health economic considerations along a randomized controlled trial
- Measuring pain intensity through physical interaction in an experimental model of cold-induced pain: A method comparison study
- Pharmacological treatment of pain in Swedish nursing homes: Prevalence and associations with cognitive impairment and depressive mood
- Neck and shoulder pain and inflammatory biomarkers in plasma among forklift truck operators – A case–control study
- The effect of social exclusion on pain perception and heart rate variability in healthy controls and somatoform pain patients
- Revisiting opioid toxicity: Cellular effects of six commonly used opioids
- Letter to the Editor
- Post cholecystectomy pain syndrome: Letter to Editor
- Response to the Letter by Prof Bordoni
- Response – Reliability and measurement error of exercise-induced hypoalgesia
- Is the skin conductance algesimeter index influenced by temperature?
- Skin conductance algesimeter is unreliable during sudden perioperative temperature increase
- Corrigendum
- Corrigendum to “Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors”
- Obituary
- A Significant Voice in Pain Research Björn Gerdle in Memoriam (1953–2024)

