Abstract
Nano-based systems can be used to transport active medicinal products to specific parts of the body. Most challenges with drug delivery, such as low water solubility and poor bioavailability, can be solved using nanotechnology. In addition, nanoparticles can overcome various physiological obstacles to increase load distribution to desired sites. Nanoparticles can carry a load of medication or therapeutic agent, such as a DNA-related substance, to enhance distribution time and deliver the drug to the target site in either a nonspecific (through enhanced permeability and retention (EPR)) or specific (through binding specific target receptors) manner. Moreover, nanoparticle drug delivery systems have been employed in the clinic since the early 1990s. Since then, the field of nanomedicine has developed with growing technical needs to improve the delivery of various medications. Over these past decades, newer generations of nanoparticles have emerged that are capable of conducting new delivery activities that could enable therapy via innovative therapeutic modalities. This review highlights different types of approved and currently marketed nanoparticles, such as nanocrystals, liposomes, lipid nanoparticles, PEGylated polymeric nanoparticles, protein-based nanoparticles, and metal-based nanoparticles. Furthermore, it explores the use of vaccine-loaded nanoparticles for COVID-19 prophylaxis.
1 Introduction
In pharmaceutical research and clinical settings, nanoparticulate pharmaceutical drug delivery systems (NDDSs) are commonly used to increase the efficacy of medicines [1]. Liposomes, polymers, micelles, metal nanoparticles, carbon nanotubes, solid lipid nanoparticles, noisomes, and dendrimers are various types of nanoparticles that can be used to deliver drugs and target diseases. Drug-related problems such as low water solubility, poor bioavailability, and off-target delivery can be solved using NDDSs. NDDSs have been used to improve drug stability, to improve distribution time, and to target specific sites in the body [2,3,4].
NDDSs have been developed to overcome various physiological obstacles and distribute loads to specific target sites [5,6,7,8]. Characteristics of multifunctional NDDSs include carrying an adequate load of therapeutic substance, enhancing distribution time, and reaching specific target receptors [4]. In addition, multifunctional NDDSs may be developed to react to various stimuli that are characteristic of the pathological location, by adding elements that respond to irregular pH, temperature, oxidation, and overexpression of some biological molecules. Multifunctional NDDSs can also react to external stimuli, such as ultrasound or magnetic fields. The addition of an imaging contrast moiety can allow the monitoring of distribution, target accumulation, or therapy effectiveness [1,4].
In the last few years, the number of newly developed drugs with considerable lipophilicity, high molecular weight, and low water solubility has increased. Approximately 40% of the market-approved drugs and almost 90% of discovery pipeline molecules have poor water solubility [4]. Much of the failure of newly developed medicines is due to the poor solubility of the drugs in water. It is known that low solubility and poor dissolution can lead to lower bioavailability and sub-optimal drug delivery. Nanoparticle-based formulations, lipid-based drug delivery systems, prodrugs, amorphous solid dispersions, salt formation, co-crystals, and cyclodextrin complexes are widely used to increase the dissolution rate of these molecules [4,9,10,11,12].
The preparation of drugs in the nanoparticle form improves the dissolution rate of many medications. Drug-loaded nanoparticles have a greater surface area and a higher overall coefficient of transfer of solution than their micron-scale counterparts. In addition, ultrafine particles tend to display excellent saturation solubility, particularly those less than 100 nm. This phenomenon is described by the Ostwald–Freundlich equation [13]. According to the Noyes–Whiney equation [14], the dissolution rates are also improved. This enhances the bioavailability of the drugs. Other benefits of drug-loaded nanoparticles include improved safety of dosage escalation and increased efficacy and tolerability profiles. In addition, they can improve solubility and dissolution rates, leading to improved bioavailability [15].
Nanoparticle-loaded medications may be formed by three different methods (top-down, bottom-up, and combinative techniques). Top-down methods include high-pressure homogenization (HPH), stirred media milling, and ball milling. This technique involves high shear-impact forces to decrease coarse particle drug crystals to the nanometer scale [16]. Bottom-up methods include particle build-up liquid antisolvent precipitation (LASP), dissolved molecule precipitation, and supercritical fluid precipitation. Melt emulsification is another example of a bottom-up methodology and is used for medicines with low melting points [17]. In this method, the drug is distributed and heated to melt the crystals in an aqueous stabilizer solvent, followed by flash cooling to generate nanoparticulate medications. Combinative methods include a combination of bottom-up and top-down approaches [15]. This review classifies nanoparticles and highlights their applications, with a focus on marketed and approved nanoparticles. Furthermore, we discuss the use of vaccine-loaded nanoparticles in the prevention of COVID-19.
2 Types of therapeutic nanoparticles
Nanoparticles can be classified into four major categories: nanocrystalline, polymeric, nonpolymeric, and lipid-based nanoparticles (Figure 1). Micelles, drug conjugates, gels, protein nanoparticles, and dendrimers are polymer-based nanoparticles. Nanodiamonds, silica, quantum dots, carbon nanotubes, and metallic nanoparticles are nonpolymeric nanoparticles. Lipid-based nanoparticles include liposomes and solid lipid nanoparticles (SLNs) [18]. Crystalline nanoparticles are formulated by mixing therapeutic agents in the crystal state. In this section, we briefly summarize various kinds of therapeutic nanoparticles and their medical applications, as well as their delivery techniques, in difficult pathophysiological situations [19,20] (Figure 1).
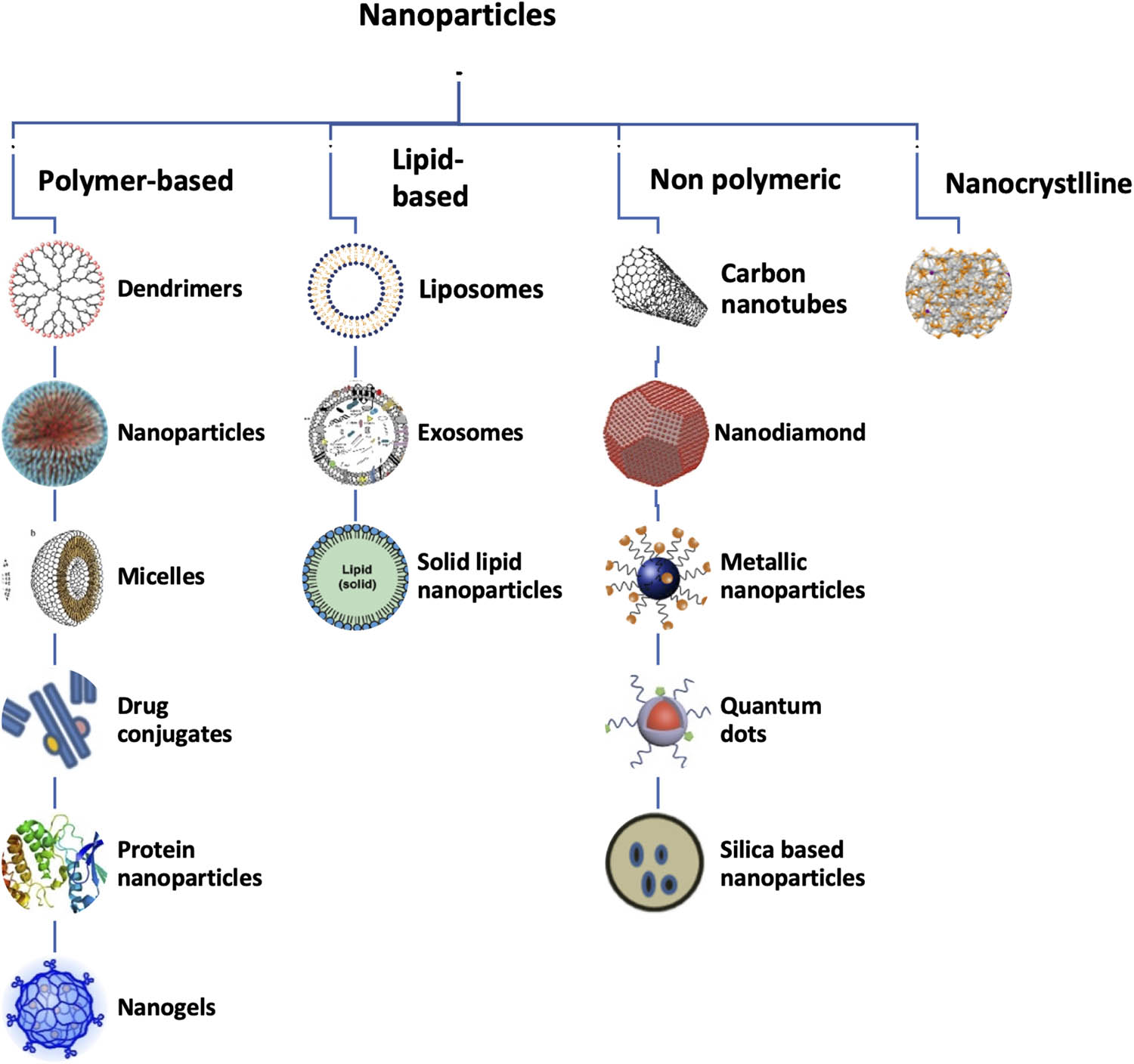
Schematic diagram representing the different types of nanoparticles due to varying types of formulations.
2.1 Polymeric-based nanoparticles
2.1.1 Dendrimers
Owing to their hyperbranched, compartmentalized design and large monodispersity, dendrimers are widely used in medical practice. By tweaking the number of branches, the dimensions of the particles can be reduced to a minimum (1–5 nm). They can be made by spherically shaped polymerization, which results in the production of cavities within the dendrimer particle [21,22]. High entrapment efficiency was found with high-generation dendrimers. For example, dendrimers that contain over 64 surface groups were compared with relatively small dendrimers. These can be used for the delivery of medications. Dendrimers have free end groups that can be easily swapped with biocompatible conjugate molecules to improve their limited cytotoxicity and permeability. Moreover, surface improvements can enhance the delivery of therapeutic agents to specific target sites. Designing dendrimers through encapsulation or complexation makes them attractive vehicles for simultaneous delivery to target sites. Biological molecules such as genes, drugs, vaccines, and mono-polymers or co-polymers such as chitin, polyethyleneimine, polyamidoamine, and poly(propyleneimine) are currently used to form dendrimers [22,23].
2.1.2 Polymer-based nanoparticles
Polymer-based nanoparticles are either synthetic or natural. They offer an alternative approach for medicinal applications such as biocompatibility, nonimmunogenicity, nontoxicity, and biodegradability [24]. Polyester formulations are used to reduce the immunogenicity and toxicity of synthetic polymers. Natural polymer-based nanoparticles such as chitosan, gelatin, albumin, and alginate appear to solve toxicity problems and offer a significant increase in efficacy compared to traditional approaches. On the basis of their structure, they may be classified as nanocapsules or nanospheres [25]. A multitude of polymeric nanoparticle preparation techniques can be used to tweak the release characteristics of integrated therapeutic agents, allowing the concentration of agents at the target site. Specific recognition ligands that enhance the drug specificity in targeted tissues may easily alter and functionalize the surface of polymeric nanoparticles [26,27,28,29,30,31].
2.1.3 Micelles
Micellar solutions are most commonly used to carry low-solubility therapeutic agents. Micelles are approximately 100 µm in diameter and form aggregates in the solvent. The constituent molecules of polymeric micelles are structured in a spherical configuration, where a mantle of hydrophilic groups surrounds the hydrophobic centers. The hydrophilic surface offers protection against nonspecific absorption by the reticuloendothelial system, maintaining its high stability within physiological systems [32]. Conversely, water-insoluble hydrophobic therapeutic agents can be mechanically trapped by the hydrophobic centers of polymeric micelles. It is also possible to covalently bind product molecules to this hydrophobic center. The dynamic design of polymeric micelles offers a prominent therapeutic agent delivery mechanism that enables flexible loading, targeted ligand conjugation, and a lower dissolution rate [32,33,34].
2.1.4 Drug conjugates
For low-molecular-weight agents, conjugation of polymers with drug molecules is common, especially in cancer treatments. The total molecular weight of medications is increased by conjugation, which affects the pharmacokinetic disposition of the cells. Polymer–drug conjugates are highly soluble, stable carriers and improve permeability and retention in cancer cells [35]. For continuous drug release and improved drug capacity, covalently conjugated polymer drugs are highly stable [36]. pH-Sensitive polymeric drug conjugates are formed by the use of pH-responsive polymer–drug chemical bonds. Thus, regardless of the medium acidity, pH-responsive nanoparticles are used for the controlled release of drugs at the tumor site [37,38]. For instance, polymeric conjugation of paclitaxel and doxorubicin, in combination, improved the bioavailability of the drugs [36,39,40].
2.1.5 Protein nanoparticles
Protein nanoparticles, including viruses, enzymes, and virus-like particles, are formed by recombinant activity. The synthesis of materials, drug and gene administration, imaging, and vaccine development benefit from this technology. Viruses are natural carrier systems for transporting genetic material encapsulated by capsid proteins. Virus-like particles (VLPs), a type of protein nanoparticle, are classified as nanocarrier systems with a morphologically similar, virus-isolated structure but do not contain viral genetic material [41,42]. Caged proteins (CPs) are nano-formulation systems of self-assembled proteins that are morphologically similar to viruses but are not derived from them. VLPs and CPs are enticing nanocarrier structures that can cause antigen-specific immune responses against cancer cells to develop cancer vaccines [43]. Protein nanoparticles that are isolated proteins of animal or plant origin, such as gelatin, silk, albumin, elastin, collagen, and soy, are formed by self-assembling protein polymers. Protein polymers are self-assembled into usable drug delivery carriers by genetic modification, with polymer-based nanoparticle benefits [44,45]. Abraxane is a protein nanoparticle drug approved by the Food and Drug Administration (FDA) that enables the delivery of paclitaxel by albumin. Another example, an HIV vaccine made from VLPs, has contributed to significant development, accelerating research into protein nanoparticles for clinical use [46,47].
2.1.6 Nanogels
Gels are colloidal or polymeric nonfluid networks that swell when in contact with a fluid. Nanogels are defined by the International Union for Pure and Applied Chemistry (IUPAC) as gel particles with these properties but with a diameter of less than 100 nm [48]. The swelling properties of flexible scale and elevated water concentration of nanogels are the outcomes of physically or chemically cross-linked natural or synthetic polymers [49,50]. Physically cross-linked amphiphilic polysaccharides have been physically cross-linked to prepare the first recorded nanogel, where cholesterol-bearing pullulans were self-assembled (by hydrophobic interactions) into water nanogels [51,52]. Compared to other nanocarrier structures, nanogels provide many benefits, such as reduced untimely medication leakage, encapsulation of multiple therapeutic molecules in the same formulation, and simple administration by parental or mucosal routes. Nanogels have been used in various applications, including biochemical separation, cell culture, bio-catalysis, drug delivery, antitumor therapy, and biosensors. Among these, the most commonly researched uses of nanogels are the delivery of therapeutics such as nucleic acids, antibiotics, cytokines, and nasal vaccines [49,50,51].
2.2 Nonpolymeric nanoparticles
2.2.1 Carbon nanotubes
Carbon nanotubes are tubular structures based on carbon that are 1 nm in diameter and 1–100 nm in thickness [53]. Such structures are obtained by wrapping a single layer of graphite, called graphene, into a smooth cylinder. Single-walled nanotubes (SWNTs), multiwalled nanotubes (MWNTs), and C60 fullerenes have been used in carbon nanotube configurations. Carbon nanotubes are an appealing nonpolymeric transporter for therapeutic agents because of their size and stable geometric form. In addition, the internal diameters of SWNTs and C60 fullerenes are 1–2 nm, which is approximately half the average diameter of the DNA helix [54]. Through endocytosis or direct entry via the cell membrane, SWNTs and MWNTs may reach the cell. Fullerenes vary in their graphite cylinder arrangement and the central structure of a high number of conjugated double bonds. Fullerene studies have demonstrated their use in supply therapeutics as antiviral, anticancer, and antibiotic agents [55,56,57,58]. In addition, by providing free radicals, they can support damaged mitochondria [59]. This approach enables selective targeting of mitochondria to deliver drug molecules [60].
2.2.2 Nanodiamonds
Nanodiamonds (NDs) are components of carbon-based nanoparticles that are less than 100 nm. They come in two distinct facet forms, formed by different techniques, such as chemical vapor deposition (CVD), detonation, and high-temperature/pressure techniques [61,62]. The detonation process of nanodiamonds involves triggering a controlled explosion on carbon-containing precursors in a closed chamber, the oldest and most widely used nanodiamond preparation technique. Nanodiamonds prepared by this technique normally include sp2 carbon on the surface, and the electrostatic potential of the surface depends on the form and the composition of the NDs [63,64,65,66]. To deposit NDs on diverse substrates, such as thin films, the CVD form is preferable. With few defects, nanodiamond thin films are high quality [67]. Nanodiamonds have special features, such as electrostatic surface properties, low chemically inert core cytotoxicity, and low photobleaching. They immobilize various biomolecules, making them exceptional for medical applications such as magnetic resonance imaging (MRI), contact lens synthesis, and drug delivery. Furthermore, nanodiamonds can be coupled with gadolinium (Gd)(iii) as a contrast agent for MRI. The signal produced from this complex is superior to that of other contrast agents based on Gd(iii) [61,62,63].
2.3 Metallic nanoparticles
Metallic nanoparticles are 1–100 nm in size and are often composed of gold, cobalt, nickel, iron, and their respective oxides such as chromium dioxide, maghemite, cobalt ferrite, and magnetite. They can be easily synthesized and modified, which allows them to be decorated with different molecules, including medicinal agents and biological molecules such as DNA, proteins, and peptides [68,69,70,71,72,73]. As carriers, they are stable, biocompatible, and have unique characteristics such as magnetic properties. Magnetic nanoparticles may be guided to a particular location in the body using an external magnetic field. A significant parameter for their medical use is magnetic susceptibility, defined as the ratio of the induced magnetization to the area applied. Superparamagnetic iron oxide nanoparticles (SPIONs) have a high magnetic susceptibility and are commonly used as contrast agents for MRIs in hospitals [74]. Similarly, superparamagnetic properties promote the secure distribution of therapeutic agents to the body and proper accumulation in the target tissue, providing a reproducible and safe approach [57,75]. They can create heat in a process called magnetic hyperthermia, as metallic nanoparticles are affected by an alternating magnetic field, which allows them to be used in the ablation of tumors [76].
Gold nanoparticles (AuNPs) are used in the diagnosis and treatment of cancer. This is due to the unique optical and localized surface plasmon resonance (LSPR) and lower cytotoxicity resulting from the inert nature of gold. Because of the LSPR, when light with the proper wavelength is provided to gold nanoparticles as an external stimulus, they undergo photo-thermal transition. They target tumor tissues in a heat-specific manner to kill cancer cells. Gold nanoparticles are also used to deliver drugs, with light irradiation triggering drug release at the targeted site [73,77,78,79,80,81].
2.3.1 Quantum dots
Quantum dots (QDs) are tiny semiconducting particles or nanocrystals with a size range of 2–10 nm. These particles are composed of an inorganic core semiconductor, such as CdSe, and an organic-coated aqueous shell, such as ZnS [70,82]. Quantum dots fluoresce with distinctive colors that are partially the product of exceptionally high surface-to-volume ratios. The color produced is determined by the core structure of quantum dots, while the outer aqueous shell may be used to conjugate biomolecules such as peptides, proteins, and DNA [83,84,85]. A cap can also be borne by QDs, which increases their solubility in aqueous buffers. Quantum dots can be used to detect therapeutic agents inside cells/tissues because of their narrow emission, light fluorescence, and high photostability. Their surface’s flexible bioconjugation, adaptable photophysical features for multiplexed detection, and superior stability for longer examination times make them outstanding candidates compared to other fluorescent substances. However, the medicinal use of QDs is still controversial [86,87].
2.3.2 Silica-based nanoparticles
Silica-based nanoparticles provide significant advantages in nanomedicine owing to their applicability to modeling complex structures and their cost-effectiveness. Their particular surface properties, porosity, and functionalization characterize them as appealing therapeutic delivery tools [88]. The broad surface area of silica nanoparticles is protected by polar silanol units, which are favorable for water adsorption and enhance the stability of therapeutic agents. Furthermore, nanoparticles based on silica can interact with nucleic acids, allowing them to be used as targeted delivery vehicles [89]. In addition, therapeutic agent encapsulation inside silica-based nanoparticles offers a solid agent distribution medium. The pores of silica nanoparticles can be capped with different stimulus-responsive molecules to enhance drug release in the desired target tissue. Mesoporous silica nanoparticles capped with β-cyclodextrin were used to release the encapsulated drug into acidic tumor tissue. In biological systems, nanoparticles are combined with contrast agents such as silver, iron oxide, organic dyes, gold, and quantum dots [90]. In addition, these nanoparticles are used in pharmaceutical processing as additives to enhance the mechanical characteristics and biocompatibility of the substance.
2.4 Lipid-based nanoparticles
2.4.1 Liposomes
Liposomes are particles formed by hydrating dried phospholipids. They are made with lipid particles and surface adjustment to produce particles of various shapes, compositions, scales, and versatility. Liposomes have the significant benefit of being able to fuse with the cell membrane and spill their contents into the cytoplasm, making them appropriate carrier systems for selective delivery. The simplest liposomes are composed of a lipid bilayer surrounding a hollow center of 50–1,000 nm. Therapeutic molecules can be loaded into this hollow center [91,92,93]. Liposomes are categorized into three specific forms based on the number of bilayers: large unilamellar, multilamellar, and small unilamellar. Multilamellar particles are composed of multiple bilayers of lipids that are separated by aqueous spaces. Unilamellar vesicles, by comparison, are composed of a single bilayer covering the trapped aqueous area. They can carry both hydrophobic and hydrophilic particles owing to their structural properties. In the liposome’s aqueous interior, hydrophilic particles can be stored, and in lipid membrane hydrophobic particles, they can be dissolved [94].
Furthermore, more than one form of the drug may be loaded in liposomes in two compartments (lipid and aqueous) or in the multiple aqueous layers of multilamellar liposomes. They also enable the release of various drug molecules through the dissociation of layers from the outer shell to the inner center [95]. Compared to large, unmodified liposomes, neutral or positively charged smaller liposomes have a better circulation time [96]. Surface modifications, such as coating with a functionalized polymer or PEG chains, facilitate specific target distribution and improve circulation time in biological systems. Liposomes have been studied for a broad range of therapeutic uses, including tumor detection and treatment, antibacterial therapy, immunization, and brain-targeted drug delivery [93,97].
2.4.2 Exosomes
Exosomes are normally shaped and secreted by different cell types. They are extracellular vesicles originating from endosomes with a scale of 30–150 nm that are typically present in various body fluids such as blood, urine, saliva, and breast milk [98]. Exosomes are lipid bilayer vesicles that are cell membrane-like and contain diverse compounds, including DNA, glycolipids, RNA, and proteins [99,100]. Transmission of different compounds to physiological pathways such as neural connectivity, immune response, and antigen presentation can help in conditions such as cardiovascular disease, diabetes, cancer, and inflammation. Exosomes are essential for intracellular communication [98,101]. Allogenic exosomes benefit the immune system. These vesicles can be separated from body fluids and used to effectively shield cargo from accelerated clearance and enhance drug distribution to the targeted sites [102]. Exosomes are being studied for their potential as drug carriers for tumors and autoimmune diseases and as diagnostic biomarkers for tumors and even for tissue regeneration [103,104].
2.4.3 Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are aqueous colloidal dispersions that contain a solid lipid matrix at room and body temperatures. Surfactants improve their stability, while the particular lipid used has an impact on the drug delivery characteristics. SLNs range in size from 10 to 1,000 nm, depending on the manufacturing method [105]. SLNs are a type of lipid carrier that can encapsulate large amounts of lipophilic, hydrophilic, and nucleic acids, making them a versatile drug delivery vehicle [106,107]. Antibodies, magnetic nanoparticles, and pH-sensitive lipids/polymers are among the various moieties that can be loaded onto SLNs to modulate targeted delivery and stimuli-responsive drug release [108,109]. They have been demonstrated to be efficient carriers for tumor, pulmonary, and oral drug delivery [110,111].
2.4.4 Nanocrystalline particles
Nanocrystalline spheres (nanocrystals) have a crystallite dimension of only a few nanometers, and they are carrier-free drug particles. As a highly cost-effective solution, nanocrystal products are commonly used for poorly water-soluble drugs, which would normally have low bioavailability and absorption [112,113,114]. Size reduction is usually an effective method to increase the bioavailability of drugs, in which the rate-limiting stage is the dissolution velocity. The crystal structure results in a larger total surface area, which increases the dissolution velocity. This trait increases solubility, particularly when the agent’s therapeutic index is reduced because of difficulties with absorption. Owing to their rapid breakdown, nanocrystalline particles allow the rapid absorption of therapeutic agents, providing a benefit for agents that rely on rapid action. The sustained or selective release can be accomplished by changing the nanocrystal structure, enabling therapeutics to be used at smaller concentrations, and reducing adverse effects [115].
2.5 Viral vector vaccines
Despite the fact that the use of viral vectors for therapeutic purposes began in the late 1990s, the application of these vectors for disease treatment was mostly overtaken by the death of Jesse Gelsinger, who died after receiving an adenoviral vector for adenovirus infection [116], and leukemia in children with severe combined immunodeficiency (SCID) who were treated with retroviral vectors [117,118]. Although significant progress has been made in the development of viral vector vaccines in recent years, the results have been encouraging with regard to dendritic cells, and an increasing number of studies have begun to focus on the use of different viral vectors, including RNA (retroviral and lentiviral), adenoviral, and AAV vectors [119,120,121,122]. In viral vector vaccines, the immunogenicity-causing antigen is cloned in a pseudovirus that cannot proliferate or transfer in dendritic cells, creating higher immune stimulation than recombinant proteins [123].
2.5.1 Retrovirus and lentivirus-based vectors
Retroviruses contain a single-stranded RNA genome that encodes all replication-related proteins [124]. MMLV-based vectors are among the most efficient engineered vectors, with high transduction efficiency in dividing cells, and good integration and expression of transgenes [125]. Retroviral expression vectors can carry up to 8 kb genes and use a variety of envelope proteins to package them. These envelope proteins can be modified to recognize only mouse and rat cell receptors (ecotropic) or be amphoteric, allowing targeting of a wide range of mammalian cell receptors. Encapsulating envelope proteins from other virus strains, such as the broad-spectrum tropism VSV-G protein (VSV-G), can also be used to pseudotype retroviral vectors [126]. Retroviral vector therapy is effective in treating X-linked SCID (SCID-X1), chronic granulomatous disease (CGD), and adenosine deaminase-deficient SCID [127,128,129,130]. Nonintegrating lentiviral vectors (NILVs) [131] can express transgenes transiently in dividing cells or episomes into nondividing cells and can be introduced into diverse cell types. With relation to COVID-19 infections, the Shenzhen Geno-immune Medical Institute recently produced two lentiviral vector-based vaccines (Covid-19/aAPC and LV-SMENP-DC) [132]. These vectors have been engineered to express several viral genes as antigens, including conserved structural, structural, and protease protein domains.
2.5.2 Adenovirus-based vectors
Adenoviruses are nonenveloped, double-stranded DNA viruses with a diameter of 90 nm. They have been found to infect humans, birds, reptiles, fish, amphibians, and non-human primates [133,134,135]. Adenoviruses cause respiratory infections, conjunctivitis, and gastroenteritis in humans [133,136]. The linear double-stranded DNA of the virus measures between 25 and 48 kb and contains noncoding inverted terminal repeats (ITRs) at both ends and genes encoding approximately 35 proteins. The early genes are involved in host cell gene regulation and virus replication, while the late genes encode structural proteins required for capsid assembly [136]. Preclinical and clinical studies have shown that human adenovirus serotype 5 (AdHu5) is effective in inducing immune responses as well as in gene therapy applications [137,138,139,140]. Recently, a new COVID-19 vaccine was developed using an adenovirus-based vector [132]. The use of adenovirus-based vectors has a number of advantages, including enhanced transgene expression and immune responses via induction of innate immunity, scalability, and purification and safety for human application. In addition, they can be delivered mucosally or systemically [141,142,143].
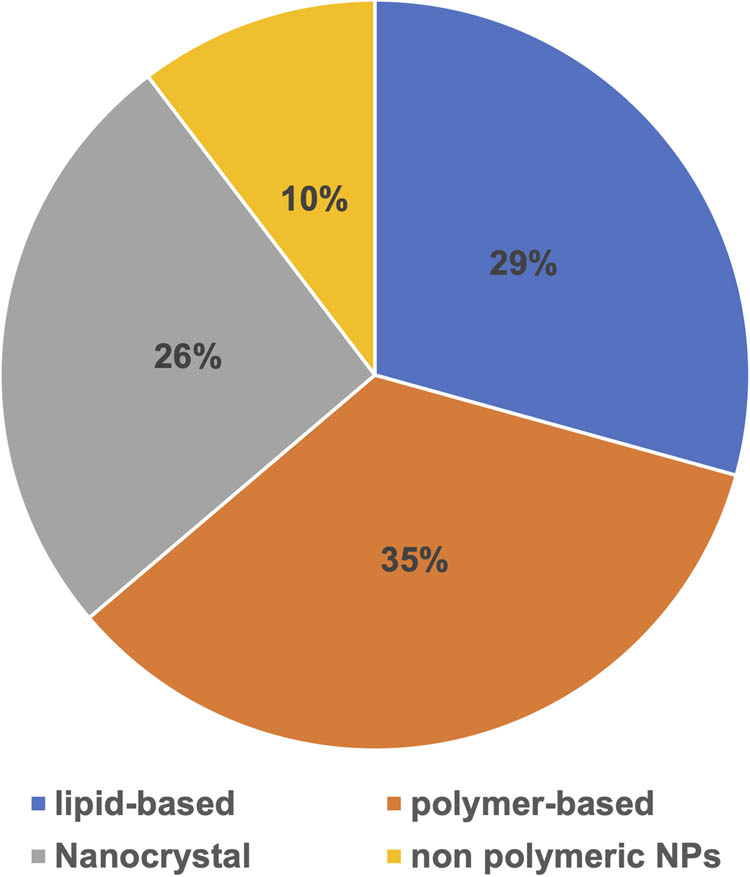
The percentage distribution of approved nanoparticle medications in the global market.
2.5.3 Adeno-associated virus vectors as a platform for vaccination
Adeno-associated viruses (AAVs) are tiny nonenveloped viruses in the Parvoviridae genus Dependovirus [144,145,146]. The genome structure [147,148,149,150], infection latency [151,152,153,154], replication/transcription, and virion assembly [155,156,157] were all described throughout the ensuing 20 years of study [158]. The AAV virion is a 4.7 kb single-stranded particle encased in a 25 nm capsid [144]. The AAV genome has an ITR at each end that serves as a replication and packaging origin. There is one replication gene (rep4) that encodes four proteins (Rep4, Rep52, and Rep68), one capsid gene (cap) that encodes three subunits via differential splicing and translation and one assembly activating protein (AAP) that promotes virion assembly [159,160]. Several preclinical and clinical trials for the treatment of genetic disorders, including hemophilia, spinal muscular atrophy, hereditary retinal disease, and lipoprotein lipase deficiency [161,162,163], have demonstrated the effectiveness and safety of rAAVs in delivering genes to target cells [164,165]. Recent research has focused on creating an AAV-type COVID-19 vaccine [132].
2.5.4 RNA-based vaccines and nanoparticle formulations
RNA-based vaccines are the latest advancement in the quest for safe and effective immunization. The poor stability of RNA has been one of the primary obstacles to its application in immunization. Because RNA is only transiently expressed and negatively charged, it requires extra substrates to enter cells. RNA-based techniques are now among the most efficient methods of immunization due to recent developments in mRNA stability and RNA transport into cells. However, because mRNA is negatively charged, its transfection efficiency is typically very low, necessitating the use of alternative substrates to facilitate mRNA delivery into cells, of which lipid nanoparticles (LNPs) are one of the most widely used [166,167]. The first COVID-19 vaccine to start clinical testing was an RNA-based vaccine, which was expected to make a significant contribution to the ongoing pandemic struggle [168]. In addition, it was discovered that administering 50 ng of taRNA to mice resulted in the development of antibodies against the influenza hemagglutinin antigen [169]. These vaccines have shown promise in clinical studies against the Ebola virus using saRNA viral vectors. RNA-based vaccines can be generated in a few weeks, with clinical trials starting in a few months depending on the gene sequence. A wide range of NP methods can also be used to promote RNA transport within cells, thereby extending therapeutic possibilities. Given the urgency of the need for an effective COVID-19 vaccine, RNA-based vaccines appear to be the most viable option available [170].
2.6 Biomimetic nanoparticles
Molecular biomimetics is a new area in which hybrid technologies are being produced by combining the capabilities of molecular biology with nanotechnology to create new applications. Polypeptides may now be genetically designed to precisely attach to chosen inorganic compounds, allowing them to be used in nanotechnology and biotechnology applications. These advances are based on biological principles [171,172]. It is becoming increasingly common to use biomimetic NPs, which combine the functionality of biological materials with the flexibility of synthetic materials to achieve effective navigation and interfacing in complex biological systems. Biomimetic NPs are a new class of particles that combine the functionality of biological materials with the flexibility of synthetic materials to achieve efficient navigation and interfacing in complex biological systems. Biomimetic systems, such as cell membrane-coated NPs, combine the functionality of cell membranes with the ability of synthetic core structures to transport imaging reporters and medicinal cargo [173]. It mainly referred to the development of any new substance or technology that resembles or is derived from nature. Biomimetic NPs are a new type of nanoparticle that aims to overcome the existing difficulties in nanomedicine by mimicking the biomimicry of natural cells. Research in this area provides a wide range of NPs, ranging from such mimicking blood cells, to immunologic cells, and even tumor cells. Traditional NP platforms may now leverage the characteristics of natural cells to accomplish particular functions while preserving the enhanced delivery capabilities of a synthetic NP due to the application of these biomimetic methods [174]. Biomimetic nanoparticles can be designed and built through the use of interaction forces to move lipid accumulation from bilayers onto the spherical solid cores. Nanoparticles, such as polystyrene nanospheres, silica particles, and hydrophobic drug aggregates, have been coated with lipid bilayers, yielding functional hybrid assemblies. Many uses have been developed including biomolecular recognition, drug delivery, and vaccine development [175].
Current nanoparticle platforms have the potential to be considerably improved and enhanced using biomimetic design methodologies. Many of the distinct capabilities performed by different cell types are largely due to the composition of cell membranes. Several alternative techniques for using the cell membrane as a source of inspiration for nanoparticle development. These include the use of protein and glycans, and also its derivatives, and the use of natural cell membranes as nanocarriers or as a coating material for synthesized nanoparticulate cores [176]. Recently, researchers throughout the world are becoming interested in biomimetic nanoparticles because they can combine the benefits of both synthetic nanomaterials and natural materials, allowing for molecular imaging and precise drug delivery via a biomimetic approach [177].
3 Approved nanoparticles in the global market
3.1 Lipid-based nanoparticles
3.1.1 Doxorubicin (Doxil)
In 1995, the FDA-approved doxorubicin (Doxil), also known as Caelyx. It is a nanodrug used to treat multiple cancers, ranging from metastatic ovarian cancer to Kaposi sarcoma (KS) associated with AIDS. A doxorubicin (adriamycin) mixture is enclosed in unilamellar liposomes coated with PEG (polyethylene glycol) and are known as “PEGylated liposomes.” The sizes of these structures vary between 80 and 90 nm. This DDS increases the half-life of circulation, leading to a boost in the bioavailability of medications. The first company to develop injectable Doxil was an Indian pharmaceutical company called Sun Pharma Global FZE, which obtained FDA approval in 2013. Doxil has two distinct mechanisms of action: intercalation into the DNA molecule, causing topoisomerase and DNA repair destruction, and intracellular production of reactive oxygen species and free radicals, causing lipid peroxidation and destruction of cell membranes. The second-generation PEGylated liposomal doxorubicin (Doxil or Lipobox) works by a passive targeting technique to enhance the EPR effect of tumors. The other main advantage of liposomal doxorubicin is its ability to reduce adverse drug effects, which can be toxic to several parts of the body, especially the skin and heart (Table 1) [178,179].
Approved marketed drug-loaded lipid-based nanoparticle
| No. | Drug loaded | Type of nanoparticle | Trade name (company name) | Applications | Approval date | Ref. |
|---|---|---|---|---|---|---|
| 1 | Daunorubicin | Liposomal daunorubicin | DaunoXome (Galen) | Karposi sarcoma | 1996 | [307,308] |
| 2 | Cytarabine | Liposomal cytarabine | DepoCyt© (Pacira Pharms Inc) | Lymphoma | 1999 | [307,308] |
| 3 | Vincristine | Liposomal vincristine | Marqibo (Acrotech) | Acute lymphocytic blood clot | 2012 | [309] |
| 4 | Irinotecan | Liposomal irinotecan | Onivyde (Ipsen Inc) | Pancreatic cancer | 2015 | [181] |
| 5 | Amphotericin B | Liposomal amphoteric B | AmBisome (Astellas) | Fungal infection | 1997 | [307,308] |
| 6 | Verteporfin | Liposomal verteporfin | Visudyne (Valeant Luxembourg) | Decreased vision, ophthalmic hiscomaplastia | 2000 | [307,308] |
| 7 | Doxorubicin | Liposomal doxorubicin | Doxil (Baxter Hlthcare Corp) | Karposi sarcoma, ovarian cancer, multiple myeloma | 1995 | [307,308] |
| 8 | Amphotericin B lipid complex | Liposomal amphotericin B lipid complex | Abelcet (Leadiant Biosci Inc.) | Fungal infection | 1995 | [307,308] |
| 9 | Poractant Alfa | Liposome-proteins SP-band SP-C | Curosurf (Chiesi USA Inc) | Lung activator for stress disorder | 1999 | [307,308] |
| 10 | Perflutren | Perflutren lipid microspheres | Definity (Lantheus Medcl) | Ultrasound contrast agent | 2001 | [194] |
| 11 | Octocog alfa | Liposome | Advate | Hemophilia A | 2004 | [310] |
| 12 | Mifamurtide | Liposomes | Mepact (Takeda) | Myosarcoma | 2009 | [311] |
| 13 | Propofol | Liposomes | Diprivan/Propofol-Lipuro/Propofol | Anesthesia | 1989 | [194] |
| 14 | Cytarabine: daunorubicin | Liposomes | VYXEOS (Celator Pharms) | Acute myeloid leukemia | 2017 | [194] |
| 15 | Pfizer-BioNTech | Liposomal nanoparticle | Pfizer-BioNTech | COVID-19 | 2020 (EUAs) | [170,278] |
| 16 | Moderna | Liposomal nanoparticle | Moderna | COVID-19 | 2020 (EUAs) | [170,278] |
| 17 | Patisiran sodium | Lipid nanoparticle | Onpattro | Transthyretin (TTR)-mediated amyloidosis | 2018 | [194] |
EUAs, emergency use authorization; NPs; nanoparticles.
3.1.2 Daunorubicin (DaunoXome)
Liposomal daunorubicin, commercially known as DaunoXome, was approved by the FDA in 1996. DaunoXome, another anthracycline drug, is used to treat cancer and HIV-associated Kaposi’s sarcoma (KS). In addition, different clinical trials have demonstrated the applicability and efficacy of daunorubicin for various types of leukemia. Because of its potency and lower side effects compared with alternative cytotoxic drugs, such as adriamycin, bleomycin, and vincristine, DaunoXome has been approved as a first-line cytotoxic therapy in advanced KS. The liposomes have an approximate diameter of 45 nm and consist of cholesterol and distearoyl phosphatidylcholine compound lipid bilayers with a molar ratio of 1:2. In DaunoXome, the lipid-to-drug weight ratio is 18.7:11 (lipid:daunorubicin). Although the exact mechanism of DaunoXome specificity is not known, it is thought to be the consequence of enhanced permeability of tumor neovasculature (EPR effect). In preclinical studies, DaunoXome has been shown to increase the concentration of daunorubicin in tumors while reducing its concentration in the brain, liver, spleen, and intestine, compared to the treatment with free daunorubicin. However, myelosuppression, which can cause fever, nausea, and vomiting, is a primary toxic effect of DaunoXome (see Table 1) [178,180].
3.1.3 Irinotecan (Onivyde)
Onivyldan was approved by the FDA in 2015 as an irinotelatinoid nanoform (MM-398) derivative. Liposomal irinotecan has also been shown to exhibit synergistic effects with other anticancer agents. Irinosuccinate, 5-fluoracylinic acid, folinic acid, and oxalate folinamide treatment showed superior outcomes in patients with advanced pancreatic cancer. Nanoliposomal formulations have improved circulation time, passive tumor targeting, and fewer side effects due to tumor overexpression. However, there are still some side effects of Onivyde, including diarrhea, vomiting, stomach pain, and alopecia (Table 1) [178,181].
3.1.4 Cytarabine (DepoCyt)
DepoCyt was authorized under accelerated approval regulation in 1999. It is a cytarabine liposomal formulation that is produced using Depofoam technology. In 2007, the FDA approved it for the treatment of a life-threatening condition called lymphomatic meningitis. In this case, liposomal medicine was only delivered intrathecally into the spine. The liposomal formulation comprises dioleoyl phosphatidylcholine, dipalmitoyl phosphatidylglycerol, triolein, and cholesterol. This special liposomal preparation has a half-life 40 times longer than that of normal cytarabine. DepoCyt is a sustained-release formulation intended for immediate administration into the cerebrospinal fluid. This antineoplastic agent can affect cells during S-phase cell division and inhibit DNA polymerase (Table 1) [178,182].
3.1.5 Vincristine (Marqibo)
In 2012, the FDA-approved liposomal vincristine sulfate, also known as Marqibo. Vincristine is an alkaloid anticancer agent that binds to tubulin and interferes with the cell division. Marqibo is vincristine encapsulated in sphingomyelin/cholesterol liposomes. When administered to adult patients with Philadelphia chromosome-negative chronic myelogenous leukemia, Marqibo (2.25 mg/m2) induced a positive reaction in 35% of the patients. In comparison, conventional vincristine clearance is slower (area under the plasma drug concentration vs time curve). Liposomal vincristine offers other benefits, such as increased circulation time and low systemic toxicity. Significant adverse effects include constipation, nausea, weakness, diarrhea, and insomnia (Table 1) [178,182].
3.1.6 Amphotericin B (AmBisome)
AmBisome, the liposomal form of amphotericin B (AmB) or L-AmB, is an antifungal agent administered to treat a broad spectrum of fungal pathogens. Because it does not work through enzyme inhibition, AmB does not lead to the emergence of resistant fungal species, like other antifungal agents do. Fungizone and standard injectable water-soluble AmB have been developed in an attempt to find new formulations that alleviate toxicity. Studies have been carried out on murine models concentrating on particle size, AmB content, physicochemical stability, and toxicity. The final optimized mixture consists of phosphatidylcholine-hydrogenated soybeans, cholesterol, and AmB-containing di-stearoyl phosphatidylglycerol at a molar ratio of 2:1:0.8:0.4. The benefits of liposomal AmBisome include improved pharmacokinetic properties and circulation stability, decreased accumulation in normal uninfected tissue, and decreased mammalian cell toxicity compared to fungal cells, making it far safer than Fungizone. AmBisome allows the drug to cross the liposome membrane and then bind to ergosterol in the fungal membrane, resulting in ion leakage and, ultimately, fungal cell death [178,183].
3.1.7 Daunorubicin: cytarabine (Vyxeos)
Daunorubicin and cytarabine encapsulated in liposomes, known as Vyxeos, received FDA approval in 2017 to treat adults with acute myeloid leukemia resulting from previous therapy or changes in myelodysplasia [178,184]. Cytarabine and daunorubicin were loaded into the liposomal structure in a 5:1 molar ratio. Daunorubicin influences DNA and RNA syntheses by forming complexes with DNA that control gene expression and generate free radicals. Cytarabine decreases DNA synthesis by controlling DNA polymerase. Vyxeos significantly increases plasma exposure and reduces distribution to ordinary tissues. However, it also has side effects such as hyperviscosity, cardiotoxicity, and tissue necrosis (Table 1) [178,184,185,186,187,188].
3.1.8 Amphotericin B lipid complex (Abelcet)
In 1995, another AmB lipid complex, Abelcet, was approved. It is an antifungal medication, similar to AmBisome. It is used to treat severe leishmaniasis and fungal infections such as aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis. In a 1:1 drug to lipid molar ratio, Abelcet contains AmB and two phospholipids. It also produces less severe side effects compared with free AmB (Table 1) [178,189].
3.1.9 Verteporfin (Visudyne)
Visudyne is a liposomal formulation of the benzoporphyrin analog monoacid ring A photosensitizer (PS). In 2000, the FDA approved it for the treatment of choroidal neovascularization caused by wet macular degeneration associated with age. This proliferation of unwanted blood vessels in the back of the eye is one of the leading causes of blindness in adults. Visudyne is injected intravenously, followed by shining of a red laser through the pupil into the eye after 10 min. The PS absorbs light and is boosted into an excited state, where its energy is transferred to ambient oxygen, and singlet oxygen is produced. This reactive oxygen species harms the recently created leaky blood vessels, thus halting and reversing progressive vision loss. In addition to its use for age-related macular degeneration, a mixture of Visudyne photodynamic therapy and immunosuppression was recommended to help treat sub-foveal choroidal neovascularization that may occur as a side effect of inflammatory conditions. The side effects of Visudyne therapy are usually moderate and can include slight changes in vision; light flashes; dryness, redness, or swelling in the eyes; and headache (Table 1) [178,190].
3.1.10 Poractant Alfa (Curosurf)
Curosurf is a porcine minced pulmonary surfactant extract called Poractant Alfa, which was approved in 1995. Multivesicular vesicles are formed in the range of 100 nm to 5 μm in scale. It is formulated as a phospholipid and protein suspension of 80 g/L containing phosphatidylcholine lipids, phosphatidylglycerol, and SP-B and SP-C membrane proteins [191]. Curosurf is recommended and administered at a dosage of 200 mg/kg for the rescue treatment of respiratory distress syndrome (RDS) in premature infants. The use of nanoparticles for Poractant Alfa has been shown to increase delivery with lower volume and reduced toxicity. Acquired pneumonia, acquired septicemia, bronchopulmonary dysplasia, intracranial hemorrhage, patent ductus arteriosus, pneumothorax, and pulmonary interstitial emphysema are side effects of Curosurf (Table 1) [192].
3.1.11 Perflutren (Definity)
Definity (perflutren lipid microsphere) injectable suspension is a contrast agent used to brighten and clarify the image of the heart during echocardiograms [193]. FDA approval was obtained for Definity as an ultrasound contrast agent in 2001 [194]. The perflutren lipid microspheres are composed of octa-fluoro propane encapsulated in an outer lipid shell consisting of DPPA, DPPC, and DPPE. The microsphere particle size ranges from 1.1 to 3.3 μm. However, it has several side effects, the most common being potential prolongation of QTc and headache (Table 1) [193].
3.1.12 Octocog alfa (Advate)
Advate is a drug used in patients of all ages with hemophilia A (an inherited bleeding disorder caused by a lack of factor VIII) to treat and prevent bleeding. It contains octocog alfa (human coagulation factor VIII) as the active material and was approved in 2004. Advate (antihemophilic factor (recombinant), plasma/albumin-free method) is a purified glycoprotein consisting of 2,332 amino acids. It is synthesized in a genetically engineered Chinese hamster ovary (CHO) cell line. CHO cells synthesize recombinant antihemophilic factor (rAHF) with the same biological effects as human AHF (hAHF). The recombinant protein has a mixture of heterogeneous heavy and light chains identical to those in hAHF. rAHF is secreted into cell culture medium by the CHO cells. It is then purified from the culture medium using a series of chromatography columns. The purification approach involves an immunoaffinity chromatography step, in which the rAHF is selectively removed from the medium, and a monoclonal antibody directed against Factor VIII is used. No additives of human or animal origin are used in the cell culture and purification processes. A dedicated viral inactivation solvent detergent treatment stage is included in the manufacturing process (Table 1) [195,196,197,198].
3.1.13 Mifamurtide (Mepact)
Mifamurtide is a drug used to treat osteosarcoma, a form of bone cancer that mainly affects children and young adults and is lethal in more than half of cases [199,200]. The medicine was approved in March 2009 in Europe. Mifamurtide is a phosphatidylethanolamine (MTP-PE) muramyl tripeptide, a synthetic muramyl dipeptide analog. The side chains of the molecule have a longer half-life than the specific product. The drug is encapsulated in liposomes to produce L-MTP-PE. The lipid bilayer of liposomes accumulates in the form of infusion or a micelle shape (Table 1) [201].
3.1.14 Propofol (Diprivan)
Diprivan (propofol) injectable emulsion is a sedative-hypnotic agent used with surgery or other surgical treatments to calm patients before and after general anesthesia. It is often administered to chronically ill patients who need a breathing tube attached to a ventilator. Propofol is partially soluble in water and is thus formed as an emulsion of white oil-in-water. Its pK a is 11. For propofol, the octanol/water partition coefficient is 6,761:1 at a pH of 6–8.5. The formula also includes soybean oil (100 mg/mL), glycerol (22.5 mg/mL), egg lecithin (12 mg/mL), and disodium edetate (0.005 percent) in addition to the active ingredient propofol, with sodium hydroxide to modify the pH. Diprivan injectable emulsion, USP, has a pH of 7–8.5, and is isotonic. Propofol was discovered in 1977 and was approved for use in 1989 in the United States (Table 1) [202,203,204,205].
3.2 Polymer-based nanoparticles
3.2.1 Certolizumab pegol (Cimzia)
Certolizumab pegol was approved by the FDA in 2008 and is a PEGylated blocker of tumor necrosis factor-alpha (TNF-α). Cimzia is a PEGylated Fab fragment (part of a humanized IgG antibody without the Fc region) that specifically recognizes and binds to TNF-α, thus neutralizing its activity. Cimzia is used to treat arthritis in rheumatoid, psoriatic, and ankylosing spondylitis. These diseases are all related to autoimmunity, an unhealthy immune response of the patient to healthy cells. Strictly speaking, Crohn’s disease is not an autoimmune disease, since it seems that the content of the gut lumen, and not self-antigens, triggers the response. TNF-α is a pluripotent pro-inflammatory cytokine and may be one of the main cytokines responsible for the autoimmune attack (Table 2) [178,206].
Approved marketed drug-loaded polymer-based nanoparticles
| No. | Drug loaded | Type of nanoparticle | Trade name (Company name) | Applications | Approval date | Ref. |
|---|---|---|---|---|---|---|
| 1 | Certolizumab pegol | PEGylated antibody fragment (Certolizumab) | Cimzia (UCB) | Chron’s disease, rheumatoid arthritis, psoriasis, ankylosing spondylitis | 2008 | [307,308] |
| 2009 | ||||||
| 2013 | ||||||
| 2 | Glatiramer acetate | Random copolymer of l-glutamate, l-alanine, l-lysine and l-tyrosine | Copaxone (Teva) | Multiple sclerosis | 1996 | [307,308] |
| 3 | Leuprolide acetate | Leuprolide acetate and polymer [PLGH (poly (dl-lactide-coglycolide)] | Eligard (Tolmar) | Prostate cancer | 2002 | [307,308] |
| 4 | Methoxy PEG glycol-epoetin β | Chemically synthesized ESA (erythropoiesis-stimulating agent) | Mircera (Hoffman-LaRoche) | Anemia with chronic renal failure | 2007 | [307,308] |
| 5 | Pegfilgrastim | PEGylated GCSF protein | Neulasta (Amgen) | Leukopenia by chemotherapy | 2002 | [307,308] |
| 6 | Peginterferon alfa-2A | PEGylated IFN alpha-2a protein | Pegasys (Hoffman-La Roche) | Hepatitis B and C | 2002 | [307,308] |
| 7 | Peginterferon alfa-2B | PEGylated IFN alpha-2b protein | PegIntron (Schering) | Hepatitis C | 2001 | [307,308] |
| 8 | Pegvisomant | PEGylated HGH receptor antagonist | Somavert (Pharmacia) | Acromegaly | 2003 | [307,308] |
| 9 | Pegaspargase | Polymer–protein conjugate PEGylated l-asparaginase | Oncaspar (Sigma Tau) | Acute lymphocytic blood clot | 1994 | [307,308] |
| 10 | Pegloticase | Polymer–protein conjugate (PEGylated porcine-likeuricase) | Krystexxa (Horizon) | Chronic gout | 2010 | [307,308] |
| 11 | Peginterferon beta-1A | Polymer–protein conjugate (PEGylated IFNbeta-1a) | Plegridy (Biogen) | Multiple sclerosis | 2014 | [307,308] |
| 12 | PEGylated factor VIII | Polymer–protein conjugate (PEGylated factor VIII) | Adynovate (Baxalta) | Hemophilia | 2015 | [307,308] |
| 13 | Factor IX | Glycopegylated coagulation factor IX | Rebinyn (Novo Nordisk Inc.) | Hemophilia | FDA 2017 | [178] |
| 14 | Triamconolone acetonide | PLGA hydrogel | Zilretta (Flexion Theraps Inc) | Osteoarthritis | 2017 | [178] |
| 15 | Trastuzumab | Maytansine derivative, DM1 | Kadcyla | Breast cancer | 2013 | [312] |
| 16 | Paclitaxel | Protein NP | Abraxane (Abraxis Bioscience) | Breast cancer | 2005 | [194] |
| 17 | Denileukin diftitox | Protein NP | Ontak (Eisai Inc) | T-cell lymphoma | 1999 | [307,308] |
| 18 | Docetaxel | Micelle | Taxotere (Sanofi Aventis) | Antineoplastic | 1996 | [313] |
| 19 | Verapamil HCl | PLGA nanoparticles | Verelan PM (Schwarz Pharma) | Hypertension, angina, and rhythm disorders | 1998 | [15] |
| 20 | Ibritumomab tiuxetan | Suspension | Zevalin (Spectrum Pharms) | Lymphoma | 2002 | [307,308] |
EUAs, emergency use authorization; NPs, nanoparticles.
3.2.2 PEGfilgrastim (Neulasta)
Neulasta, a PEGylated form of filgrastim, was approved by the FDA in 2002. Neutropenia (low white blood cell counts) is a common adverse effect found in patients with nonmyeloid cancer who receive chemotherapy. As a leukocyte growth factor, Neulasta is used for the treatment of febrile neutropenia and consequent infections arising due to a lack of neutrophils. Filgrastim (the parent molecule) is a recombinant methionyl human granulocyte colony-stimulating factor (r-metHuG-CSF) produced from Escherichia coli. Neulasta is synthesized by attachment of a monomethoxy-PEG aldehyde chain (20 kDa) to the N-terminal methionine residue of Filgrastim. The resulting imine is then reduced using sodium cyanoborohydride. PEGylation of filgrastim results in increased circulation time and higher solubility of the parent molecule. The half-life of the native molecule (filgrastim) is about 3.5–3.8 h, but Neulasta remains in circulation for up to 42 h (Table 2) [178,207].
3.2.3 Pegaspargase (Oncaspar)
Oncaspar, with the generic name pegaspargase, is a PEGylated-l-asparaginase approved by the FDA in 1994. Acute lymphoblastic leukemia and chronic myelogenous leukemia are treated with this agent. It can also be used as a replacement in patients with leukemia who display hypersensitivity to E. coli-derived l-asparaginase. Oncaspar is beneficial as it is only administered every 2 weeks, while the native compound, l-asparaginase, must be administered three times per week. The less frequent administration of Oncaspar is simply the result of a longer half-life due to PEGylation. Furthermore, Oncaspar demonstrates decreased hypersensitivity and considerable total cost savings for patients (Table 2) [178].
3.2.4 Peginterferon alfa-2a (Pegasys)
Pegasys, with the generic name peginterferon alfa-2a, was approved by the FDA in 2002. Pegasys is a recombinant human alfa-2a interferon conjugated to branched PEG for the treatment of hepatitis C and HBeAg-positive chronic hepatitis B. The increased half-life of Pegasys due to PEGylation makes it possible to administer a subcutaneous injection only every 12 weeks. Free interferon must be administered three times per week. Pegasys has been used with ribavirin, resulting in better hepatitis C therapy outcomes. In HBeAg-positive chronic hepatitis B patients, the combination of lamivudine and Pegasys also resulted in more remarkable survival (Table 2) [178,208].
3.2.5 Pegvisomant (Somavert)
Somavert (pegvisomant [B2036-PEG]) is a PEGylated analog of human growth hormone (GH) for acromegaly treatment that was approved by the FDA in 2003. The pituitary gland secretes excessive quantities of growth hormone in patients with acromegaly, resulting in abnormal enlargement of the forehead, jaw, hands, and feet. Somavert is a GH receptor antagonist that blocks GH binding and interferes with GH signal transduction pathways, thereby reducing IGF-I serum concentration (one of the essential mediators of GH activity) by at least 50%. As expected, PEGylation of the active ingredient of Somavert (B2036) results in reduced clearance (estimated to be 28 mL/h for subcutaneous injection of 10–20 mg) and an increased half-life (approximately 6 days) (Table 2) [178,209].
3.2.6 Methoxy PEG glycol-epoetin β (Mircera)
Mircera or epoetin β (EPO) conjugated to methoxy-PEG is a drug formulation used to treat anemia. Both the European Commission and the FDA-approved Mircera in 2007. EPO is a genetically recombinant form of erythropoietin capable of stimulating erythropoiesis by functioning on the erythropoietin receptors of bone marrow progenitor cells. To create Mircera, the PEG moiety (∼60 kD) is first linked with butanoic acid, and the NHS-modified structure is connected to the lysine moiety of the EPO structure via amide bonds. This formulation provides a controlled release system with a half-life of ∼135 h. Naked EPO has a half-life of 7–20 h. The main benefit is the less frequent administration of Mircera. The administration of Mircera usually entails intravenous or subcutaneous injection of 0.6 μg/kg every 2 weeks (Table 2) [178,210].
3.2.7 Peginterferon alfa-2b (PEG-INTRON)
PEG interferon alfa-2b is a long-acting interferon; its structure contains an alpha interferon (INF) molecule conjugated to a mono PEG chain through succinimidyl carbonate. Compared to the regular interferon molecule, its longer half-life and slower elimination lead to less frequent administration. PEG interferon alfa-2b was approved by the FDA in 2001 and is commonly used as a monotherapy or in combination with other medicines, such as ribavirin, to treat chronic hepatitis C (Table 2) [178,211].
3.2.8 Pegloticase (Krystexxa)
Krystexxa [212] is a treatment for patients with chronic refractory gout. In September 2010, FDA approval was obtained following investigations into its efficacy in reducing uric acid levels and reducing concentrations of uric acid crystals in joints and soft tissues. The EMA also approved this medication for the treatment of tophaceous gout disorder in January 2013. Tophi are nodular masses of uric acid crystals. Pegloticase is a recombinant porcine-like uricase that can metabolize uric acid to allantoin, similar to the nonpegylated Rasburicase. Pegloticase is composed of four uniform chains of approximately 300 amino acids; 9 of the 30 lysine residues in each chain are pegylated (PEG chains MW: 10 kDa). The improved solubility of pegloticase removes the risk of precipitate formation. Furthermore, the PEG molecules in pegloticase result in an increased drug half-life to about 10 days (in comparison with Rasburicase, which has a half-life of 8 h) and a reduction in its immunogenicity. Accordingly, pegloticase is a better choice than other treatments, especially for chronic therapy. The side effects of Krystexxa include infusion and allergic reactions, which should be noted before repeating the treatment. Other minor side effects include sore throat, vomiting, nausea, and chest pain (Table 2) [178,213].
3.2.9 Peginterferon beta-1a (Plegridy)
Plegridy contains the active material PEG-IFN-β-1a, a glycosylated recombinant PEG-conjugated IFN-β modified with a single, linear 20 kDa methoxy-PEG-O-2-methylpropionaldehyde molecule (mPEG). In 2014, the FDA-approved Plegridy to treat recurrent remitting multiple sclerosis in adult patients. Plegridy has been used to observe elevations in hepatic enzymes and liver damage. Patients must be checked for signs of hepatic impairment, and therapy must be stopped if icterus or other clinical symptoms of hepatic dysfunction occur (Table 2) [178,214].
3.2.10 PEGylated factor VIII (Adynovate)
Adynovate is a recombinant pegylated antihemophilic factor used to treat hemophilia A in patients with repeated bleeding events. Adynovate functions by temporarily raising blood clotting factor VIII levels in the blood. In the human body, Adynovate has an extended circulation time, which means it requires a decreased injection frequency. The structure consists of coagulation factor VIII conjugated to PEG. Clinical studies have demonstrated the safety and effectiveness of this drug against hemophilia. Common side effects include diarrhea, nausea, headache, vomiting, rash, and other common allergic reactions (Table 2) [178,215].
3.2.11 Glatiramer acetate (Copaxone)
Copaxone or glatiramer acetate (GA; known as COP-1) is a synthetic polymer consisting of l-alanine, l-glutamic acid, l-tyrosine, and l-lysine in a ratio of 0.141:0.338:0.427:0.095, respectively. The FDA has approved its use for the treatment of multiple sclerosis (MS). Although the mechanism of action of this random copolymer is not fully known, it may suppress inflammatory responses by blocking MHC II and modifying the T-cell population phenotype, according to some studies. Its therapeutic advantage (particularly in autoimmune disorders) can be inferred from its marketing approval in many countries, including the United States, Canada, and some European countries. However, clinical studies have shown that GA has various side effects in a number of organs, including the heart, eyes, skin, and gastrointestinal and immune systems (Table 2) [178,216,217].
3.2.12 Leuprolide acetate (Eligard)
Eligard is a formulation of leuprolide acetate (Atrigel) as a polymeric injectable nanosuspension agent that received FDA approval in 2002 as a palliative agent for prostate cancer treatment. Leuprolide acetate (known as Lupron) is a gonadotropin-releasing hormone (GnRH) synthetic peptide-type analog capable of interacting with the GnRH receptor and altering gonadotropin secretion. The decline in gonadotropin release decreases other hormones, such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The outcomes are hypogonadism and decreased levels of estradiol and testosterone. Artigel is a DDS based on a mixture of polymers (usually polylactic and polyglycolide). Eligard is an example of a nanopharmaceutical that acts as a sophisticated system for Lupron delivery. It is an injectable liquid formulation that solidifies in the body and slowly releases the drug over 1 month as it degrades. Irritation and erythema are the most common adverse effects of Eligrad after subcutaneous injection (Table 2) [178,218].
3.2.13 Triamcinolone acetonide (Zilretta)
Triamcinolone acetonide that has been embedded in a PLGA hydrogel is known as Zilretta and was approved by it’s the FDA in 2017 for the treatment of knee osteoarthritis. This formulation is administered by intra-articular injection to alleviate knee osteoarthritis pain, but its overall beneficial effects are still being studied. Despite the benefits seen in clinical trials, Zilretta demonstrated no significant advantage over the immediate release of triamcinolone in a clinical trial. In clinical trials, 32 mg of Zilretta was administered to 424 patients, 143 of whom were aged 65 years or older, and the adverse effects did not vary between old and young patients (Table 2) [178,219].
3.2.14 Factor IX (Rebinyn)
Rebinyn is an injectable drug used to replace clotting factor IX, which is absent in patients with hemophilia B. It was approved by the FDA in 2017. In all age groups, hemophilia B is an inherited bleeding disorder that usually prevents blood from clotting. In patients with hemophilia B, Rebinyn is used to treat and control bleeding. It was developed in CHO cells using recombinant DNA technology. No additives of human or animal origin are used in cell culture, purification, conjugation, or preparation of Rebinyn. The rFIX protein is isolated by a sequence of chromatographic steps to selectively extract rFIX from the cell culture media, including an affinity chromatography step using a monoclonal antibody (produced in CHO cells). Two dedicated viral clearance steps are used in the development process, namely, a detergent treatment stage for inactivation and a 20 nm filtration step for virus elimination. During the purification process, conjugation of the PEG group is carried out by an enzymatic reaction, followed by the final purification of Rebinyn (Table 2) [220,221].
3.2.15 Paclitaxel (Abraxane)
Abraxane is paclitaxel formulated as albumin-bound nanoparticles with a mean particle size of approximately 130 nm, also known as nanoparticle albumin-bound paclitaxel. Paclitaxel exists in a noncrystalline, amorphous form in these particles. ABRAXANE is provided as a 20 mL injection in 0.9% sodium chloride (USP). It is a white to yellow, clean, lyophilized powder that is reconstituted before use. It is used to treat breast cancer, lung cancer, and pancreatic cancer, among others. By avoiding the natural breakup of microtubules during cell division, paclitaxel destroys cancer cells. Paclitaxel is bonded to albumin as a transport vehicle in this formulation. It is manufactured under the trade name Abraxane, which is designated as an orphan drug for first-line treatment, combined with gemcitabine, for metastatic adenocarcinoma of the pancreas. This treatment was approved in the United States in 2005 and the European Union in 2008 for breast cancer cases that did not respond to other chemotherapy or had relapsed (Table 2) [178,222].
3.2.16 Denileukin diftitox (Ontak)
Denileukin diftitox, known as Ontak, was approved by the FDA in 1999 to treat T-cell lymphoma. It consists of an IL-2-conjugated recombinant diphtheria toxin protein and has been developed to bind to the IL-2 receptor. In leukemia and lymphoma therapy, Ontak is used because it selectively transmits diphtheria toxin to target cells expressing interleukin-2 receptors. Some studies have shown that Ontak may be used to treat fungal mycosis, the most common type of cutaneous T-cell lymphoma. The side effects include hypersensitivity reactions during infusion with low blood pressure, back pain, headache, shortness of breath, nausea and vomiting, blood test anomalies and liver issues, fatigue, rash, and poor appetite. In 2006, severe problems with loss of vision emerged, and the FDA added a black box warning to the drug labeling, including a description of ophthalmologic adverse events. In 2014, the marketing of Ontak was discontinued in the United States (Table 2) [178,223].
3.2.17 Docetaxel (Taxotere)
Docetaxel is an antineoplastic agent belonging to the taxoid family. It is prepared by semi-synthesis, starting with a precursor derived from renewable needle biomass of yew plants. Docetaxel (C43H53NO14) is a white to near-white powder with a molecular weight of 861.9 kDa. In water, it is strongly lipophilic and insoluble. Taxotere is recommended to treat patients with locally advanced or metastatic breast cancer when previous chemotherapy has failed (Table 2) [224,225].
3.2.18 Ibritumomab tiuxetan (Zevalin)
Zevalin (ibritumomab tiuxetan) is an immunoconjugate between the monoclonal antibody ibritumomab and the linker-chelator tiuxetan arising from the stable thiourea covalent link. This linker-chelator offers an Indium-111 or Yttrium-90.0 site for high affinity, conformationally limited chelation. Ibritumomab tiuxetan has an average molecular mass of 148 kDa. Ibritumomab, a murine IgG1 kappa monoclonal antibody directed against the CD20 antigen, is the antibody moiety of zevalin. Ibritumomab tiuxetan is a transparent, colorless, sterile, preservative-free, pyrogen-free solution containing fine particles. It is provided in single-use vials containing 3.2 mg of ibritumomab in 2 mL of 0.9% sodium chloride. Zevalin (ibritumomab tiuxetan) is a protein used in conjunction with other drugs to treat non-Hodgkin lymphoma that attacks white blood cells (Table 2) [226,227].
3.3 Crystalline nanoparticles
3.3.1 Aprepitant (Emend)
Emend is the nanocrystalline form of the antiemetic drug aprepitant, approved by the FDA in 2003. It is used to prevent nausea and vomiting during chemotherapy, especially in courses containing high-dose cis-platin. Cis-platin is known to be highly emetogenic, which means it induces vomiting and nausea in patients. Aprepitant is a human substance-P-ligand antagonist with a high affinity for neurokinin-1 (NK1) receptors in the postrema region, known as the brain’s vomiting area. The benefit of aprepitant over other NK1 antagonists is that it has minimal or no preference for receptors of serotonin (5-HT3), corticosteroids, or dopamine. Thus, there is no interference from the activity of other pharmaceutical agents targeting those receptors. Furthermore, aprepitant absorption occurs in the upper gastrointestinal (GI) tract. It is insoluble in water. By applying a technology called Elan Drug Delivery Nanocrystals, these problems can be solved. Oral Emend possesses better water solubility, greater absorption in the upper GI, and better bioavailability. In 2008, the FDA approved the IV injectable form of Emend. The active ingredient of this formula is fosaprepitant dimeglumine, a prodrug of aprepitant. Fosaprepitant is water soluble and can be converted to aprepitant within 30 min of iv administration (Table 3) [178,228].
Approved marketed drug-loaded crystalline nanoparticles
| No. | Drug loaded | Type of nanoparticle | Trade name (company name) | Applications | Approval date | Ref. |
|---|---|---|---|---|---|---|
| 1 | Aprepitant | Nanocrystal | Emend (Merck) | Vomiting agent | 2003 | [307,308] |
| 2 | Fenofibrate | Nanocrystal | Tricor (Abbvie) | Hyperlipidemia | 2004 | [307,308] |
| 3 | Sirolimus | Nanocrystal | Rapamune (PF Prism CV) | Immunosuppressant | 2000 | [307,308] |
| 4 | Megestrol acetate | Nanocrystal | MegaceES (Endo Pharms Inc) | Anorexia | 2005 | [307,308] |
| 5 | Dexmethylphenidate HCl | Nanocrystal | Focalin XR /(Novartis) | Mental stimulant | 2005 | [307,308] |
| 6 | Metyhlphenidate HCl | Nanocrystal | Ritalin LA (Novartis) | Mental stimulant | 1955 | [307,308] |
| 7 | Tizanidine HCl | Nanocrystal | Zanaflex (Covis) | Muscle relaxant | 2002 | [307,308] |
| 8 | Paliperidone Palmitate | Nanocrystal | Invega Sustenna (Janssen) | Schizoaffective disorder | 2009 | [314] |
| 2014 | ||||||
| 9 | Dantrolene sodium | Nanocrystal | Ryanodex (Eagle Pharmaceutical) | Malignant benign hypothermia | 2014 | [315] |
| 10 | Olanzapine | Nanocrystal | Zyprexa (Lilly Pharma) | Schizophrenia | 1996 | [307,308] |
| 11 | Nabilone | Nanocrystal | Cesamet (Bausch) | Anti-emetic | 1985 | [15] |
| 12 | Naproxen sodium | Nanocrystal | Naprelan (Almatica) | Anti-inflammation | 1996 | [15] |
| 13 | Theophylline | Nanocrystal | Elixophyllin Nostrum Labs Inc | Bronchial dilation | 1979 | [15] |
| 14 | Brinzolamide | Nanocrystal | Azopt (Novartis) | Glaucoma | 1998 | [15] |
| 15 | Griseofulvin | Nanocrystal | Gris-Peg (Novartis) | Fungal infection | 1982 | [15] |
EUAs, emergency use authorization; NPs, nanoparticles.
3.3.2 Sirolimus (Rapamune)
Rapamune, with the generic name sirolimus (rapamycin), was the first nanocrystalline-type drug that was approved by the FDA in 2000. It is used to prevent the rejection of kidney transplants. Sirolimus is a macrocyclic triene antibiotic derived from Streptomyces hygroscopicus, and it works as an immunosuppressant. The release profile of poorly soluble drugs increases dramatically using the Elan Drug Delivery Nanocrystals technology involving bead/pearl milling, enabling better bioavailability. Although extended release for Rapamune is not entirely critical, nanoformulation leads to more convenient storage and enables oral administration. Rapamune blocks T-lymphocyte proliferation induced by stimuli operating via either Ca2+-dependent or Ca2+-independent pathways, resulting in a weakened cellular immune system to accept the transplanted organ. In 2015, the FDA approved the use of Rapamune to treat lymphangioleiomyomatosis, a rare progressive lung disease (Table 3) [178,229].
3.3.3 Methylphenidate (Ritalin)
Ritalin, also known as methylphenidate, was approved by the FDA in 1955 to treat hyperactivity disorders in children. The medication is primarily used to treat attention deficit hyperactivity disorder (ADHD). Since the 1990s, the drug has been increasingly administered for more prevalent ADHD diagnoses. In 2007, in England, general practitioners administered this drug to around 420,000 individuals, and by 2012, the amount had increased to 657,000, reflecting a 56% increase. According to research, in disorders such as ADHD, norepinephrine and dopamine release are imbalanced, and the brain’s dopamine and norepinephrine receptor control is dysregulated. Ritalin inhibits dopamine reuptake, thus increasing the concentration of dopamine in the synaptic cleft [178,230,231,232,233].
Consequently, there is an increase in cognitive and executive functions in the brain. In addition, it can increase the overall alertness of the central nervous system, resulting in short-term benefits as a cost-effective therapy. Similar to most drugs, ritalin also displays side effects; these include irritability, anxiety, disturbance in motor function, and poor appetite (Table 3) [178,234].
3.3.4 Fenofibrate (TriCor)
TriCor was approved in 2004 to reduce triglyceride and cholesterol levels to prevent atherosclerosis development by reducing plaques on the inner wall of arteries, leading to strokes and heart attacks. Fenofibrate is metabolized in the intestine by the oral administration of fenofibric acid. Fenofibric acid can activate peroxisome proliferator-activated receptor alpha (PPAR-alpha). Apolipoprotein C-III (an inhibitor of lipoprotein lipase activity) decreases the lipolysis of triglyceride-rich molecules and increases when lipoprotein lipase is enabled. This process finally reduces circulating total cholesterol and triglycerides, as well as triglyceride-rich low-density lipoprotein, very low-density lipoprotein, and apolipoprotein B. It also increases apo A-I and apo A-II of high-density lipoprotein and apolipoproteins. Using Elan drug delivery nanocrystal technology, water-insoluble fenofibrate can be made more water soluble in TriCor. Without this technology, fenofibrate must be taken with food, and it passes unchanged through the varying pH conditions of the GI tract because it lacks ionizable groups. Fenofibrate should be taken with food due to the presence of surfactants and lipids in food that can emulsify it. The higher water solubility of TriCor allows this drug to be taken with or without food (Table 3) [178,235].
3.3.5 Megestrol acetate (Megace ES)
Megace ES oral suspension includes megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically referred to as 17-hydroxy-6-methyl pregna-4,6-diene-3,20-dione acetate. Its solubility in water at 37°C is 2 μg/mL, and its solubility in plasma is 24 μg/mL. It has a molecular weight of 384.52. Megace ES oral suspension is indicated in individuals with a history of acquired immunodeficiency syndrome (AIDS) to prevent anorexia, cachexia, or unexpected substantial weight loss. Several investigators have documented the appetite-stimulating effects of megestrol acetate and its potential use in cachexia. Currently, the exact mechanism by which megestrol acetate treats anorexia and cachexia symptoms remains unclear (Table 3) [236,237].
3.3.6 Dexmethylphenidate HCl (Focalin XR)
Focalin XR is a dexmethylphenidate extended-release formulation with a bimodal release profile. The patented SODAS (spheroidal oral drug absorption system) technology is used in Focalin XR. Each focal XR bead-filled capsule contains half the dose as immediate-release beads and half as entero-coated delayed-release beads, giving an immediate release of dexmethylphenidate and a second delayed release of dexmethylphenidate. Dexmethylphenidate hydrochloride is a white to off-white powder, which is acidic in solution. It is freely soluble in methanol and water, soluble in alcohol, and slightly soluble in acetone and chloroform. In patients aged 6 years and older, focal XR is recommended for the treatment of ADHD (Table 3) [233,238].
3.3.7 Tizanidine HCl (Zanaflex)
Zanaflex (tizanidine hydrochloride) is a central alpha-2-adrenergic agonist. Tizanidine HCl is a fine crystalline powder that is white to off-white and odorless, although it sometimes has a faint characteristic odor. In water and methanol, tizanidine is slightly soluble, and its solubility in water decreases as the pH increases. The molecular formula of izanidine is C9H8ClN5S–HCl, with a molecular weight of 290.2. Zanaflex has been suggested for the treatment of spasticity. Due to its limited duration of therapeutic benefit, Zanaflex therapy should be reserved for particular everyday tasks and periods when relief of spasticity is necessary. Tizanidine is a key agonist of the alpha-2-adrenergic receptor, which probably decreases spasticity by increasing motor neuron presynaptic inhibition. Tizanidine’s effects on polysynaptic pathways are strong. The cumulative influence of these actions is assumed to decrease spinal motor neuron facilitation (Table 3) [239,240,241].
3.3.8 Paliperidone palmitate (Invega Sustenna)
Invega Sustenna is an atypical antipsychotic containing paliperidone palmitate. Paliperidone is a psychotropic agent belonging to the benzisoxazole family. In Invega Sustenna, a racemic mixture of (+)-and (−)-paliperidone palmitate is used. Paliperidone palmitate is only partially soluble in ethanol and methanol, practically insoluble in polyethylene glycol 400 and propylene glycol, and slightly soluble in ethyl acetate. Invega Sustenna is a white or off-white sterile aqueous extended-release suspension prescribed for the treatment of schizophrenia (Table 3) [242,243,244,245,246].
3.3.9 Dantrolene sodium (Ryanodex)
Ryanodex (dantrolene sodium) [247] is a skeletal muscle relaxant used to treat malignant hyperthermia and prevent malignant hyperthermia in high-risk patients, in combination with effective supportive interventions [248,249,250,251,252]. Advanced nanosuspension technology is paired with a lyophilized formulation that makes it possible to reconstitute and deliver Ryanodex in substantially less time than other dantrolene sodium formulations. Ryanodex (dantrolene sodium) is a sterile lyophilized powder. The unique nanosuspension technology enables fast reconstitution within 10 s with less sterile water than high-volume low-concentration dantrolene sodium formulations because it does not need to be dissolved before administration. Dantrolene has been shown to induce relaxation in isolated nerve muscle preparations by manipulating the contractile reaction of the muscle at a position outside the myoneural junction. One of the potential pathways involves the breakdown of skeletal muscle tissue by inhibiting the release of Ca2+ from the sarcoplasmic reticulum. Evidence points to an underlying abnormality in skeletal muscle tissue in anesthetic-induced malignant hyperthermia syndrome. In affected humans, “triggering agents,” such as general anesthetics and depolarizing neuromuscular blocking agents, have been postulated to induce a transition within the cell that increases myoplasmic calcium. Malignant hyperthermia can be initiated by elevated myoplasmic calcium syndrome, which can also progress to an elevation in the amount of intracellular calcium. The addition of dantrolene can restore a normal level of ionized calcium in the myoplasm of the “triggered” malignant hyperthermal muscle cells. Inhibition by dantrolene of the release of calcium from the sarcoplasmic reticulum restores the myoplasmic balance of calcium, thereby increasing the percentage of bound calcium. Thus, it is possible to counteract or attenuate hormonal, metabolic, and biochemical changes associated with malignant hyperthermia (Table 3) [252,253].
3.3.10 Olanzapine (Zyprexa)
Olanzapine, sold under the trade name Zyprexa, is an atypical antipsychotic that is primarily used to treat schizophrenia and bipolar disorder. It can be used for both new-onset schizophrenia and long-term maintenance. Weight gain, movement disturbances, dizziness, exhaustion, constipation, and dry mouth are typical side effects. Olanzapine was patented in 1971 and approved in the United States in 1996 for clinical use (Table 3) [254,255,256].
3.3.11 Nabilone (Cesamet)
Cesamet (nabilone) [257,258] is a synthetic orally delivered cannabinoid. Nabilone is a white to off-white polymorphic crystalline powder. The solubility of nabilone is less than 0.5 mg/L in aqueous media, with pH values varying from 1.2 to 7.0. Cesamet capsules are administered in patients who have not successfully responded to traditional antiemetic medications for the treatment of nausea and vomiting resulting from cancer chemotherapy. This limitation is appropriate because a large proportion of patients administered Cesamet undergo alarming psychotomimetic responses that have not been seen with other antiemetic agents. Sleepiness, vertigo, dry throat, euphoria (feeling “high”), ataxia, headache, and attention issues are the most frequently encountered side effects (Table 3).
3.3.12 Naproxen sodium (Naprelan)
Naprelan (naproxen sodium) controlled-release tablets are nonsteroidal anti-inflammatory drugs. Naproxen sodium is an odorless crystalline powder that is white to creamy in texture. This compound is soluble in methanol and water. The following inactive components are also used in Naprelan: copolymer ammoniomethacrylate type A, copolymer ammoniomethacrylate type B, citric acid, crospovidone, magnesium stearate, copolymer methacrylate type A, microcrystalline cellulose, povidone, and talc. The covering of the tablet includes hydroxypropyl methylcellulose, polyethylene glycol, and titanium dioxide. NSAIDs have been used to treat medical problems such as multiple types of arthritis, menstrual cramps, and other types of short-term discomfort, including pain, redness, swelling, and heat (inflammation) (Table 3) [259,260].
3.3.13 Theophylline (Elixophyllin)
Elixophyllin [261,262,263,264] (theophylline anhydrous liquid) is a methylxanthine indicated for treating symptoms and reversible airflow obstruction associated with chronic asthma and other chronic lung diseases, such as emphysema and chronic bronchitis. Theophylline is structurally classified as methylxanthine. It is a white, odorless, crystalline powder with a bitter taste (Table 3).
3.4 Nonpolymeric nanoparticles
3.4.1 Ferumoxytol (Feraheme)
Ferumoxytol, known as Feraheme, approved by the FDA in 2009, is an intravenous drug formulation with a neutral pH for the treatment of anemia. It is recommended for adult patients with evidence of iron overload. Clinical trials have shown that 510 mg intravenous ferumoxytol injection is well tolerated. According to the AMAG label for Feraheme, hypersensitivity reactions and hypotension were encountered in 0.2 and 1.9% of patients, respectively. Some of the severe side effects of Feraheme seen in clinical trials include nausea, diarrhea, hypotension, constipation, dizziness, and peripheral edema (Table 4) [178].
Approved marketed drugs loaded non polymeric nanoparticles
| No. | Drug loaded | Type of nanoparticle | Trade name/(company name) | Applications | Approval date | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ferumoxytol | Inorganic/metallic NPs | Feraheme™/(Amag) | Chronic renal failure with iron deficiency | 2009 | [307,308] |
| 2 | Iron sucrose | Inorganic/metallic NPs | Venofer (Am Regent) | Chronic renal failure with iron deficiency | 2000 | [307,308] |
| 3 | Sodium ferric gluconate | Inorganic/metallic NPs | Ferrlecit (Sanofi Avertis) | Chronic renal failure with iron deficiency | 1999 | [307,308] |
| 4 | Iron dextran (low MW) | Inorganic/metallic NPs | INFeD (Allergan) | Chronic renal failure with iron deficiency | 1974 | [307,308] |
| 5 | Ferric carboxymaltose | Inorganic/metallic NPs | Injectafer/(Am Regent) | Iron deficiency anemia in chronic kidney disease | 2013 | [316] |
| 6 | Lanreotide acetate | Nanotube | Somatuline depot/(Ipsen Pharma) | Acromegaly | 2007 | [313] |
EUAs, emergency use authorization; NPs, nanoparticles.
3.4.2 Iron sucrose (Venofer)
Venofer (iron sucrose) was the first colloidal particle-based nano-sized medication. It was developed as a substitute for oral iron supplements to treat iron deficiency [265]. Venofer (iron sucrose injection, USP), an iron replacement component, is a brown, sterile, aqueous complex of polynuclear iron(iii)-hydroxide in sucrose. It has a molecular weight between 34,000 and 60,000 Da. Venofer injection is an iron substitute medication used in patients with kidney disease to treat iron deficiency anemia. Venofer is typically provided with another drug to stimulate red blood cell formation (Table 4) [266,267,268].
3.4.3 Sodium ferric gluconate (Ferrlecit)
Ferrlecit is an iron replacement medication used to treat iron deficiency anemia in adults and children aged 6 years and older with severe kidney disease who are undergoing hemodialysis and receiving supplementary epoetin treatment. Ferrlecit is a sodium ferric gluconate complex in sucrose. It is a macromolecular complex with an apparent molecular weight of 289–440 kDa. At alkaline pH, the macromolecular complex is negatively charged and is found in solution with sodium cations. The substance has a deep red color, caused by the ferric oxide bonds. The chemical name of Ferrlecit is d-gluconic acid, iron (3+) sodium salt (Table 4) [269,270,271].
3.4.4 Iron dextran (INFeD)
INFeD (Iron Dextran Injection USP) is a dark brown, slightly viscous sterile liquid complex of ferric hydroxide and dextran for intravenous or intramuscular use. Intravenous or intramuscular injections of INFeD are recommended for the treatment of patients with confirmed iron deficiency who are unsatisfactory or unable to undergo oral administration (Table 4) [272,273,274,275].
3.4.5 Ferric carboxymaltose (Injectafer)
Iron is required for red blood cell formation. When the body does not receive enough iron, it cannot produce enough red blood cells. This disease is known as iron deficiency anemia. Injectafer (ferric carboxymaltose injection) is used to treat anemia caused by iron deficiency. Several medical problems including excessive menstrual bleeding, breastfeeding, miscarriage, inflammatory bowel disease, other malabsorption diseases, bariatric surgery, or chronic kidney disease may be responsible for iron deficiency anemia. Injectafer is a dark brown, clean, aqueous, isotonic colloidal solution. Each 1 mL contains 50 mg of iron in water for injection as ferric carboxymaltose. Injectafer is available in 15 mL single-use vials. Sodium hydroxide or hydrochloric acid may be added to change the pH to 5.0–7.0 (Table 4) [276,277].
3.5 COVID-19 vaccines
3.5.1 Pfizer-BioNTech
By reporting that their coronavirus vaccine was over 90% successful, New York-based Pfizer and the German firm BioNTech made history on November 9, 2021. On December 11, just over a month later, the FDA gave it the first emergency use permit ever issued to a coronavirus vaccine by the United States. BioNTech’s researchers initiated the work on the vaccine in January 2020, based on a genetic molecule called messenger RNA (mRNA). The vaccine includes genetic instructions to develop a coronavirus protein, known as a spike. The vaccine allows cells to produce spike proteins, which are then released into the circulation and provoke an immune response. In March, to scale up the study, BioNTech partnered with Pfizer, initiating a clinical trial in May [170,278]. They gave the vaccine the generic name tozinameran and the brand name Comirnaty. Tozinameran is loaded in liposomal nanoparticles (LNPs) or PEGylated liposomes (PEGLips). PEGLips are artificial phospholipid vesicles that are effective in stabilizing pharmaceutical products and enhancing their pharmacological properties [279]. In the case of COVID-19 vaccine, this liposome formulation enables mRNA to be stabilized due to its lability [280,281]. Lipid nanoparticle (LNP)-formulated mRNA vaccine technology enables antigen-presenting cells to be given correct genetic information along with an adjuvant [282]. Preclinical models have shown the prophylactic efficacy of this technology against several viral targets [283,284,285]. In clinical trials, LNP and liposome-formulated RNA vaccines to prevent infectious diseases or treat cancer have been safe and well tolerated [286]. The mRNA is transiently expressed and is not incorporated into the genome. It is well established, free from animal-derived materials, and synthesized from DNA templates by an efficient, cell-free in vitro transcription process [283,286,287,288]. The rapid and highly versatile processes of mRNA production and LNP formulation allow several vaccine doses to be developed quickly [284,285,289], making it ideal for the rapid development of vaccines and the delivery of pandemic vaccines (Tables 5–7) [286].
COVID-19 vaccines loaded nanoparticles [317]
| Vaccine | Technology | Dosing | Efficacy | Storage |
|---|---|---|---|---|
| Pfizer-BioNTech | mRNA | 2 doses, 3 weeks apart | 95% symptomatic illness, 90% severe COVID-19 | −70°C. 5 days at 2–8°C |
| Moderna | mRNA | 2 doses, 4 weeks apart | 94.1% symptomatic illness, 100% severe COVID-19 | −20°C for 6 months |
| 30 days at 2–8°C | ||||
| Oxford-AstraZeneca | Viral vector | 3 doses, 4 weeks apart | 62% symptomatic illness, 100% severe COVID-19 | Refrigerated at 2–8°C |
| Johnson & Johnson | Viral vector | Single dose | 66% symptomatic illness, 85% severe COVID-19 | 2 years at −20°C, 3 months at 2–8°C |
| Novavax | Protein | 2 doses, 3 weeks apart | 89.3% symptomatic illness, 49% symptomatic illness in South Africa phase 2b | Refrigerated at 2–8°C |
| Sinopharm | Inactivated virus | 2 doses, 3 weeks apart | 79% symptomatic illness, 100% severe COVID-19 | Refrigerated at 2–8°C |
Characteristics of the Pfizer/BioNTech, Oxford University/AstraZeneca and Moderna vaccines [290–294]
| Characteristics | Pfizer/BioNTech | Oxford University/AstraZeneca | Moderna |
|---|---|---|---|
| Therapeutic indication | For effective immunization to suppress SARS-CoV-2 virus-induced COVID-19 in persons 16 years of age and older. | For effective immunization for the prevention of COVID-19 in persons 18 years of age and older. | For effective immunization to prevent SARS-CoV-2 virus-induced COVID-19 in persons 18 years of age and older. |
| Type of vaccine | Messenger RNA (mRNA). | Adenovirus vector. | Messenger RNA (mRNA) |
| Number of doses | A multidose vial. | One dose. | multidose. |
| Pharmaceutical form | Concentrate for solution for injection. | Solution for injection. | Dispersion for injection. |
| Color | White to off-white frozen solution. | Colorless to slightly brown, clear to slightly opaque, and particle free. | White to off-white frozen dispersion (pH: 7.0–8.0). |
| Dosage schedule | Two doses (0.3 mL each) with an interval of between 3 and 12 weeks. | Two doses (0.5 mL each) with an interval of between 4 and 12 weeks. | Two doses (0.5 mL each). It is recommended to administer the second dose 28 days after the first dose. |
| Route of administration | Intramuscular | Intramuscular | Intramuscular |
| Contraindication | Hypersensitivity | Hypersensitivity | Hypersensitivity |
| Special warnings and precautions | Hypersensitivity and anaphylaxis | Hypersensitivity | Anaphylaxis: events of anaphylaxis have been reported |
| Traceability: to enhance the traceability of biological medicinal products, the name, and the batch number of the administered product should be clearly recorded. | Acute severe febrile illness | Traceability: to enhance the traceability of biological medicinal products, the name, and the batch number of the administered product should be clearly recorded. | |
| Acute severe febrile illness | Traceability: to enhance the traceability of biological medicinal products, the name, and the batch number of the administered product should be clearly recorded. | Anxiety-related reactions | |
| Anticoagulant therapy and bleeding disorders: the vaccine should not be given to people receiving anticoagulant treatment or those with a bleeding condition that contraindicates intramuscular injection unless the possible benefit clearly outweighs the hazard of administration. | Anticoagulant therapy and bleeding disorders: the vaccine should not be given to people receiving anticoagulant treatment or those with a bleeding condition that contraindicates intramuscular injection unless the possible benefit clearly outweighs the hazard of administration | Immunocompromised individuals | |
| Immunocompromised individuals. | Immunocompromised individuals. | Coagulation disorders: should be given with caution in individuals with bleeding disorders. | |
| Acute severe febrile illness | |||
| Interactions with other medicinal products | Interaction studies have not been performed. | Interaction studies have not been performed. | Interaction studies have not been performed. |
| Shelf life and special precautions for storage | Store for 6 months in a freezer at −80°C to −60°C shelf life. Stored at −90°C to −60°C in a thermal container. | Unopened multidose vial: in the refrigerator for 6 months (2°C to 8°C). Don't freeze. To protect against light, hold vials in the outer carton. | Store shelf life of 7 months at −25°C to −15°C. Do not store or move on or below −40°C dry ice. Should be protected from light. |
| Stored to protect from light in the original package. | After first use: use as soon as practically possible and within 6 h. The vaccine may be preserved during the in-use time between 2°C and 25°C. | The vaccine cannot be refrozen after thawing and should be kept refrigerated at 2°C to 8°C, protected from light for up to 30 days, unless used (needle punctured). | |
| When taken from the fridge, the undiluted vaccine may be kept at 2°C to 8°C for up to 5 days and at temperatures up to 25°C for up to 2 h until usage. | The chemical and physical stability of an unopened vial was illustrated for 12 h at 8 °C to 25°C following release from refrigerated environments. Don't refreeze. | ||
| Minimize exposure to room light during storage and prevent exposure to direct sunlight and ultraviolet light. | Punctured vial: after first puncture, chemical and physical in-use stability was demonstrated for 6 h at 2°C to 25°C. From a microbiological standpoint, the products can be automatically used. | ||
| Thawed vials can be handled in room light conditions. | When not used instantly, in-use storage times and situations are the responsibility of the user. | ||
| Stock the vaccine at 2°C to 25°C after dilution and use as soon as technically possible and within 6 h. | |||
| The vials must be labeled with the dilution time when diluted then discarded during 6 h of dilution. | |||
| Once thawed, the vaccine cannot be refrozen. | |||
| Nature and contents of container | Five doses of concentrate solution for injection in a 2 mL clear vial. Pack size of 195 vials. | In 10-dose vials, 5 mL of solution, or in 8-dose vials, 4 mL of solution. The box size is 10 vials. Not all pack sizes are permitted to be sold. | A limit of 10 doses of 0.5 mL each are found in each vial. The box size is 10 vials. |
| Suitability in pediatrics | In children younger than 16 years, safety and effectiveness have not yet been identified. | In children younger than 18 years, safety and effectiveness have not yet been identified. | In children younger than 18 years, safety and effectiveness have not yet been identified. |
| Suitability in pregnancy | There is insufficient evidence of the vaccine in pregnant women. | The experience for pregnant women is limited. | The experience for pregnant women is limited. |
| Administration must be considered only once the possible benefits outweigh any possible risks. | Pregnancy administration can only be taken into consideration where the possible advantages outweigh the possible risks. | Animal studies do not suggest that breastfeeding, embryo/fetal growth, parturition or postnatal development have direct or indirect adverse consequences. | |
| The Joint Committee on Vaccination and Immunization (JCVI) has recognized that the possible benefits of vaccination are particularly important for some pregnant women. This includes those at very high risk of getting the infection or those with health problems that place them at high risk of severe COVID-19 complications. | Pregnancy administration can only be taken into consideration where the possible advantages outweigh the possible risks. | ||
| Suitable in breastfeeding | Unknown | Unknown | Unknown |
| Effects on fertility | Animal experiments do not show any direct or indirect harmful fertility outcomes. | Animal experiments do not show any direct or indirect harmful fertility outcomes. | Animal experiments do not show any direct or indirect harmful fertility outcomes. |
| Alcohol content | In the production process, ethanol is used and then extracted down to trace amounts. Consequently, it is not used in the final product formulation. | A very small quantity (0.002 mg of alcohol (ethanol) per 0.5 mL dose) of alcohol. That's not enough to cause any noticeable effects. | Awaiting confirmation |
| Interchangeability | To complete the vaccination sequence, individuals who have received one dose of COVID-19 mRNA Vaccine BNT162b2 should receive a second dose of COVID-19 mRNA Vaccine BNT162b2. | Individuals receiving the first dose of the AstraZeneca COVID-19 vaccine should get a second dose of the AstraZeneca COVID-19 vaccine to complete the vaccination sequence. | To complete the vaccination sequence, individuals who have received one dose of COVID-19 Vaccine Moderna should receive a second dose of COVID-19 Vaccine Moderna. |
| Vaccine | Antibiotic free | Preservative free | Sodium free | Gluten free | Nut free | Soy free | Egg free | Vegetarian and vegan suitability | Halal certification |
|---|---|---|---|---|---|---|---|---|---|
| Pfizer/BioNTech | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Oxford University/AstraZeneca | Awaiting confirmation | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Awaiting confirmation |
| Moderna | Awaiting confirmation | Yes | Yes | Awaiting confirmation | Awaiting confirmation | Awaiting confirmation | Awaiting confirmation | Awaiting confirmation | Awaiting confirmation |
3.5.2 Moderna vaccine
The FDA issued emergency use permission on December 18, a week after the vaccine produced by Pfizer and BioNTech, for a vaccine developed by Moderna, a Boston-based firm. The Moderna vaccine was the second COVID-19 vaccine approved by the FDA. Moderna produces the vaccine from mRNA, similar to the Pfizer and BioNTech vaccines. The organization has been testing mRNA vaccines for various diseases in recent years, but they have yet to put one on the market. They started developing a vaccine for coronavirus in January 2020 [279,286]. Moderna packages this vaccine in LNPs or PEGLips. Packaging these vaccines in liposomes aids in preserving the mRNA and conferring its lability (Tables 5–7) [280].
3.5.3 Oxford-AstraZeneca
To create and validate the coronavirus vaccine known as ChAdOx1 nCoV-19 or AZD1222, the University of Oxford collaborated with the British-Swedish Corporation AstraZeneca. Clinical trials showed that when two doses were administered 12 weeks apart the vaccine was 82.4% effective. Despite confusion regarding the outcome of some trials in December 2020, Britain approved the vaccine for emergency use. In January 2021, India approved a variant of the Covishield vaccine. Like the Pfizer-BioNTech vaccine, the Oxford-AstraZeneca vaccine is based on the genetic instructions for the construction of the virus spike protein. However, the Oxford vaccine uses double-stranded DNA, unlike the Pfizer-BioNTech and Moderna vaccines, which store the instructions in single-stranded RNA. The researchers attached an adenovirus to the gene encoding the coronavirus spike protein. Adenoviruses are natural viruses that generally cause flu-like symptoms or colds. A modified version of a chimpanzee adenovirus, known as ChAdOx1, was used by the Oxford-AstraZeneca team. It can enter cells but cannot replicate. The Oxford-AstraZeneca vaccine for Covid-19 is more rigorous than the Pfizer and Moderna mRNA vaccines. Although DNA is not as fragile as mRNA, the protective protein coating of the adenovirus helps coat and protect the genetic material. As a result, the Oxford vaccine does not have to be stored frozen. The vaccine is expected to last for at least 6 months when refrigerated at 2–8°C (Tables 5–7) [290–294].
3.5.4 Sinopharm
An inactivated coronavirus vaccine, known as BBIBP-CorV, was developed by the Beijing Institute of Biological Products by the end of 2020. A state-owned corporation, Sinopharm performed clinical trials and found that BBIBP-CorV had a 79% efficacy score. The vaccine was approved by China and rapidly distributed to other countries. When researchers began producing greater quantities of coronavirus, they preserved them in droplets with a chemical called beta-propiolactone. The compound disables coronavirus particles by binding to their DNA. The inactivated coronaviruses can no longer replicate. However, their proteins, including spikes, remain intact. BBIBP-CorV functions by training the immune system to produce antibodies against SARS-CoV-2. The antibodies bind to viral proteins, such as the spike proteins that stud their surface (Table 5) [295–299].
3.5.5 Novavax
Recombinant nanoparticle technology was used to generate antigens engineered from the genetic sequence of COVID-19 spike proteins. In combination with the proprietary Matrix-M™ adjuvant, NVX-CoV2373 has been demonstrated in preclinical studies to bind efficiently with human receptors targeted by the virus, a critical aspect for effective vaccine protection (Table 5) [300].
3.5.6 Johnson & Johnson
A coronavirus vaccine known as JNJ-78436735 or Ad26.COV2 has been studied by Johnson & Johnson. Scientific trials have shown that the effectiveness of a single dose of the vaccine was as high as 72%. In partnership with the Beth Israel Deaconess Medical Center, Janssen Pharmaceutical (a Belgian-based division of Johnson & Johnson) manufactures the vaccine. The researchers placed the gene for the coronavirus spike protein into an adenovirus. Other vaccines for coronaviruses are also based on adenoviruses, such as the Oxford-AstraZeneca vaccine. A modified adenovirus, which can enter cells but cannot replicate or induce illness, was used by the Johnson & Johnson team. The vaccine from Johnson & Johnson comes out of decades of adenovirus-based vaccine research. The first was an Ebola vaccine approved for general use in July and was also developed by Johnson & Johnson. The organization is now performing trials on vaccines based on adenoviruses for other illnesses, including HIV and Zika (Table 5) [301].
4 Discussion
Currently, 58 nanoparticle therapies and imaging agents have been approved for clinical use, whether in the United States by the FDA or in the European Union by the European Medicines Agency (EMA). The different categories of FDA-approved nanomedicines are shown in Figure 2. Many clinically approved nanoparticle formulations are used for the treatment of different cancers at various stages. All drugs use liposomal nanoparticle systems except Abraxane. The first approved cancer nanomedicine (FDA 1995) was Doxil, polyethylene glycol (PEG)-functionalized liposomal doxorubicin. Shortly thereafter, the FDA approved other liposomal formulations, including liposomal daunorubicin (DaunoXome), liposomal vincristine (Marqibo), and liposomal irinotecan (Onivyde), while the EMA approved non-PEGylated liposomal doxorubicin (Myocet) and liposomal mifamurtide (MEPACT). Abraxane is an albumin-bound paclitaxel nanoparticle. With the exception of Doxil and Onivyde, most of these formulations are not PEGylated, which may be surprising considering the well-known advantages that even small amounts of PEG have been shown to confer on nanoparticle delivery systems. In addition, with no active or chemical-based targeting moieties, all of these formulations passively target the diseases. Other benefits, such as minimized toxicity resulting from their selective accumulation at tumor sites and minimal off-target side effects through the enhanced permeation and retention (EPR) effect, are likely to be responsible for the success and improved efficacy of these approved particles over their free drug equivalents [229,302–306] (Figure 2).
Another clinical field where nanoparticles have made a large impact is iron-replacement therapeutics for the treatment of anemia. In these applications, nanoparticles (iron-oxide colloids) can be used to increase iron concentrations in the body. The need to address toxicity concerns associated with the injection of free iron has led to these nanoparticle approaches. Many of these toxicity problems have been overcome using sugar-coated colloidal iron. It should be mentioned that nanoparticles indicated for iron replacement undergo significantly different approval procedures, by both the FDA and EMA, as they are nonbiological complex medications. It is a common belief that additional factors, stemming from their colloidal and nanoparticle nature, need to be considered during their approval (e.g., manufacturing conditions) [303]. The main advantages of nanoparticles are as follows: (1) enhanced bioavailability by improving water solubility, (2) increased resistance time in the body (increasing half-life for clearance/increasing specificity for its cognate receptors), and (3) drug targeting to a specific region in the body (its site of action).
A variety of other clinical situations also require the use of nanoparticles or liposomes. Diprivan, a general anesthetic, was the first liposome delivery system approved by the FDA in 1989. Two vaccines packaged in liposomal systems have received authorization for emergency use: the Pfizer/BioNTech and Moderna COVID-19 vaccines. Both are formed in liposomal nanoparticles (LNPs) or PEGLips. PEGlips are artificial phospholipid vesicles that are effective in stabilizing pharmaceutical products and enhancing their pharmacological properties. In the case of these COVID-19 vaccines, this liposome formulation enables mRNA to be stabilized due to its lability [290–294]. Liposomes or lipid-based nanoformulations have also been clinically approved for fungal and parasitic infections. For example, liposomes have been formulated for the highly toxic antifungal drug amphotericin B (AmBisome), which is used to treat systemic fungal infections. As the pharmacokinetics and tissue distribution are improved through liposomal encapsulation, toxicity is significantly decreased. In addition, liposomal formulation solves a major problem related to the free drug formulation of amphotericin B, i.e., its insolubility in pH 7 saline. There are other lipid-complexed formulations of amphotericin B, such as Abelcet, approved by the FDA. Visudyne is a liposomal formulation of verteporfin that is triggered by light. Liposomal encapsulation enhances absorption by proliferating cells, which improves specific targeting and absorption in neovascular areas. The endothelium is damaged upon light stimulation, and local blood vessels are blocked to prevent and treat neovascularization [303].
Bobo et al. [229] published an article in 2016 that counted the number of approved nanoparticles used in the clinic; in 2016, 51 nanomedicines were being used. In this review, we have updated the list, as some of the drugs have been discontinued. Discontinued nanomedicines include Feridex, GastroMARK, Dexferrum, Avinza, Estrasorb, DepoDur, Renagel, Macugen, and Adagen. Currently used drugs include Definity, Diprivan, Vyxeos, Pfizer-BioNTech, Moderna, Zilretta, Kadcyla, Taxotere, Verelan, Zevalin, Zyprexa, Cesamet, Naprelan, Elixophyllin, Azopt, Gris-Peg, INjectafer, and Somatuline depot. Polymeric nanoparticles are the most commonly approved nanomedicine, but there is a noticeable increase in the approval of lipid nanoparticles, and they are expected to be the most commonly used in the future.
5 Conclusion
Several nanoparticle drugs that are used in the clinic to treat or diagnose disease have been authorized by the FDA or EMA. Researchers are pushing these same technologies even further by seeking approval for additional indications. Beyond these efforts, there are a significant number of clinical trials researching new nanoparticle systems that are, in several ways, more developed than those that have already been authorized. Moreover, the nanoparticle systems undergo continued progress in clinical trials for future use, such as active targeting methods to enhance biodistribution or even the use of stimuli-responsive mechanisms to enhance drug release in specific parts of the body. NPs have greater stability that help to extend shelf life, large carrier capacity that can be used to integrate several drug molecules in the particle medium, capability of incorporating both hydrophilic and hydrophobic compounds, and feasibility of multiple routes of administration. Nanoparticles can be developed to allow sustained drug release. Nanoparticles have properties that enhance solubility, allowing for greater drug bioavailability and reduced dose frequency, as well as resolving the problem of poor adherence. Direct delivery of the drug to the site of action can minimize systemic toxicity while also increasing drug concentration at the target site. This development in the clinic over the past 20 years (since the approval of Doxil) has been made possible by comprehensive efforts in preclinical, industrial, and clinical trials. In addition, the overall outlook of nanoparticle drug delivery systems is encouraging, as liposomal nanoparticles are also being used in both the Pfizer/BioNTech and Moderna vaccines to treat COVID-19.
-
Funding information: The researchers would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Narang AS, Chang RK, Hussain MA. Pharmaceutical development and regulatory considerations for nanoparticles and nanoparticulate drug delivery systems. J Pharm Sci. 2013;102(11):3867–82.10.1002/jps.23691Suche in Google Scholar PubMed
[2] Thabet AF, Boraei HA, Galal OA, El-Samahy MFM, Mousa KM, Zhang YZ, et al. Silica nanoparticles as pesticide against insects of different feeding types and their non-target attraction of predators. Sci Rep. 2021;11(1):14484.10.1038/s41598-021-93518-9Suche in Google Scholar PubMed PubMed Central
[3] Harms C, Datwyler AL, Wiekhorst F, Trahms L, Lindquist R, Schellenberger E, et al. Certain types of iron oxide nanoparticles are not suited to passively target inflammatory cells that infiltrate the brain in response to stroke. J Cereb Blood Flow Metab. 2013;33(5):e1–9.10.1038/jcbfm.2013.22Suche in Google Scholar PubMed PubMed Central
[4] Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813–27.10.1038/nrd4333Suche in Google Scholar PubMed PubMed Central
[5] Liu CH, Grodzinski P. Nanotechnology for cancer imaging: advances, challenges, and clinical opportunities. Radiol Imaging Cancer. 2021;3(3):e200052.10.1148/rycan.2021200052Suche in Google Scholar PubMed PubMed Central
[6] Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12(1):137.10.1186/s13045-019-0833-3Suche in Google Scholar PubMed PubMed Central
[7] Maniam G, Mai CW, Zulkefeli M, Dufes C, Tan DM, Fu JY. Challenges and opportunities of nanotechnology as delivery platform for tocotrienols in cancer therapy. Front Pharmacol. 2018;9:1358.10.3389/fphar.2018.01358Suche in Google Scholar PubMed PubMed Central
[8] Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71.10.1038/nrc1566Suche in Google Scholar PubMed
[9] Sanches BMA, Ferreira EI. Is prodrug design an approach to increase water solubility? Int J Pharm. 2019;568:118498.10.1016/j.ijpharm.2019.118498Suche in Google Scholar PubMed
[10] Jermain SV, Brough C, Williams 3rd RO. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery – An update. Int J Pharm. 2018;535(1–2):379–92.10.1016/j.ijpharm.2017.10.051Suche in Google Scholar PubMed
[11] Sosnik A, Augustine R. Challenges in oral drug delivery of antiretrovirals and the innovative strategies to overcome them. Adv Drug Deliv Rev. 2016;103:105–20.10.1016/j.addr.2015.12.022Suche in Google Scholar PubMed
[12] Stella VJ, Nti-Addae KW. Prodrug strategies to overcome poor water solubility. Adv Drug Deliv Rev. 2007;59(7):677–94.10.1016/j.addr.2007.05.013Suche in Google Scholar PubMed
[13] von Helmholtz R. Untersuchungen über Dämpfe und Nebel, besonders über solche von Lösungen. Ann der Phys. 1886;263(4):508–43.10.1002/andp.18862630403Suche in Google Scholar
[14] Hattori Y, Haruna Y, Otsuka M. Dissolution process analysis using model-free Noyes–Whitney integral equation. Colloids Surf B Biointerfaces. 2013;102:227–31.10.1016/j.colsurfb.2012.08.017Suche in Google Scholar PubMed
[15] Bhakay A, Rahman M, Dave RN, Bilgili EJP. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: formulation–Processing aspects and challenges. Pharmaceutics. 2018;10(3):86.10.3390/pharmaceutics10030086Suche in Google Scholar PubMed PubMed Central
[16] Merkel TJ, Herlihy KP, Nunes J, Orgel RM, Rolland JP, DeSimone JM. Scalable, shape-specific, top-down fabrication methods for the synthesis of engineered colloidal particles. Langmuir. 2010;26(16):13086–96.10.1021/la903890hSuche in Google Scholar PubMed PubMed Central
[17] Chan HK, Kwok PC. Production methods for nanodrug particles using the bottom-up approach. Adv Drug Deliv Rev. 2011;63(6):406–16.10.1016/j.addr.2011.03.011Suche in Google Scholar PubMed
[18] Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25(9):2193.10.3390/molecules25092193Suche in Google Scholar PubMed PubMed Central
[19] Abolmaali SS, Tamaddon AM, Salmanpour M, Mohammadi S, Dinarvand R. Block ionomer micellar nanoparticles from double hydrophilic copolymers, classifications and promises for delivery of cancer chemotherapeutics. Eur J Pharm Sci. 2017;104:393–405.10.1016/j.ejps.2017.04.009Suche in Google Scholar PubMed
[20] Purbia R, Paria S. Yolk/shell nanoparticles: classifications, synthesis, properties, and applications. Nanoscale. 2015;7(47):19789–873.10.1039/C5NR04729CSuche in Google Scholar PubMed
[21] Bharali DJ, Khalil M, Gurbuz M, Simone TM, Mousa SA. Nanoparticles and cancer therapy: a concise review with emphasis on dendrimers. Int J Nanomed. 2009;4:1–7.10.2147/IJN.S4241Suche in Google Scholar
[22] Hsu HJ, Bugno J, Lee SR, Hong S. Dendrimer-based nanocarriers: a versatile platform for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(1):e1409.10.1002/wnan.1409Suche in Google Scholar PubMed
[23] Palmerston Mendes L, Pan J, Torchilin VP. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22(9):1401.10.3390/molecules22091401Suche in Google Scholar PubMed PubMed Central
[24] Crucho CIC, Barros MT. Polymeric nanoparticles: a study on the preparation variables and characterization methods. Mater Sci Eng C Mater Biol Appl. 2017;80:771–84.10.1016/j.msec.2017.06.004Suche in Google Scholar PubMed
[25] Letchford K, Liggins R, Wasan KM, Burt H. In vitro human plasma distribution of nanoparticulate paclitaxel is dependent on the physicochemical properties of poly(ethylene glycol)-block-poly(caprolactone) nanoparticles. Eur J Pharm Biopharm. 2009;71(2):196–206.10.1016/j.ejpb.2008.08.003Suche in Google Scholar PubMed
[26] Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, et al. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Rel. 2014;174:15–26.10.1016/j.jconrel.2013.11.001Suche in Google Scholar PubMed
[27] Liu Q, Wang X, Liu X, Kumar S, Gochman G, Ji Y, et al. Use of polymeric nanoparticle platform targeting the liver to induce treg-mediated antigen-specific immune tolerance in a pulmonary allergen sensitization model. ACS Nano. 2019;13(4):4778–94.10.1021/acsnano.9b01444Suche in Google Scholar PubMed PubMed Central
[28] Abou-El-Naga AM, Mutawa G, El-Sherbiny IM, Mousa SA. Activation of polymeric nanoparticle intracellular targeting overcomes chemodrug resistance in human primary patient breast cancer cells. Int J Nanomed. 2018;13:8153–64.10.2147/IJN.S182184Suche in Google Scholar PubMed PubMed Central
[29] Bressler EM, Kim J, Shmueli RB, Mirando AC, Bazzazi H, Lee E, et al. Biomimetic peptide display from a polymeric nanoparticle surface for targeting and antitumor activity to human triple-negative breast cancer cells. J Biomed Mater Res A. 2018;106(6):1753–64.10.1002/jbm.a.36360Suche in Google Scholar PubMed PubMed Central
[30] Xu L, Xu S, Wang H, Zhang J, Chen Z, Pan L, et al. Enhancing the efficacy and safety of doxorubicin against hepatocellular carcinoma through a modular assembly approach: the combination of polymeric prodrug design, nanoparticle encapsulation, and cancer cell-specific drug targeting. ACS Appl Mater Interfaces. 2018;10(4):3229–40.10.1021/acsami.7b14496Suche in Google Scholar PubMed
[31] Gad A, Kydd J, Piel B, Rai P. Targeting cancer using polymeric nanoparticle mediated combination chemotherapy. Int J Nanomed Nanosurg. 2016;2(3):1–12.10.16966/2470-3206.116Suche in Google Scholar
[32] Ahmad Z, Shah A, Siddiq M, Kraatz H-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4(33):17028–38.10.1039/C3RA47370HSuche in Google Scholar
[33] Panouille M, Benyahia L, Durand D, Nicolai T. Dynamic mechanical properties of suspensions of micellar casein particles. J Colloid Interface Sci. 2005;287(2):468–75.10.1016/j.jcis.2005.02.007Suche in Google Scholar PubMed
[34] Stolnik S, Heald CR, Neal J, Garnett MC, Davis SS, Illum L, et al. Polylactide-poly(ethylene glycol) micellar-like particles as potential drug carriers: production, colloidal properties and biological performance. J Drug Target. 2001;9(5):361–78.10.3109/10611860108998772Suche in Google Scholar PubMed
[35] Markovsky E, Baabur-Cohen H, Satchi-Fainaro R. Anticancer polymeric nanomedicine bearing synergistic drug combination is superior to a mixture of individually-conjugated drugs. J Control Rel. 2014;187:145–57.10.1016/j.jconrel.2014.05.025Suche in Google Scholar PubMed
[36] Yang R, Mondal G, Wen D, Mahato RI. Combination therapy of paclitaxel and cyclopamine polymer-drug conjugates to treat advanced prostate cancer. Nanomedicine. 2017;13(2):391–401.10.1016/j.nano.2016.07.017Suche in Google Scholar PubMed
[37] Pang X, Jiang Y, Xiao Q, Leung AW, Hua H, Xu C. pH-responsive polymer-drug conjugates: design and progress. J Control Rel. 2016;222:116–29.10.1016/j.jconrel.2015.12.024Suche in Google Scholar PubMed
[38] Lv S, Tang Z, Zhang D, Song W, Li M, Lin J, et al. Well-defined polymer-drug conjugate engineered with redox and pH-sensitive release mechanism for efficient delivery of paclitaxel. J Control Rel. 2014;194:220–7.10.1016/j.jconrel.2014.09.009Suche in Google Scholar PubMed
[39] Chen Z, Zhang P, Cheetham AG, Moon JH, Moxley Jr JW, Lin YA, et al. Controlled release of free doxorubicin from peptide-drug conjugates by drug loading. J Control Rel. 2014;191:123–30.10.1016/j.jconrel.2014.05.051Suche in Google Scholar PubMed
[40] Tu Y, Zhu L. Enhancing cancer targeting and anticancer activity by a stimulus-sensitive multifunctional polymer-drug conjugate. J Control Rel. 2015;212:94–102.10.1016/j.jconrel.2015.06.024Suche in Google Scholar PubMed
[41] Hill BD, Zak A, Khera E, Wen F. Engineering virus-like particles for antigen and drug delivery. Curr Protein Pept Sci. 2018;19(1):112–27.10.2174/1389203718666161122113041Suche in Google Scholar PubMed
[42] Lopez-Sagaseta J, Malito E, Rappuoli R, Bottomley MJ. Self-assembling protein nanoparticles in the design of vaccines. Comput Struct Biotechnol J. 2016;14:58–68.10.1016/j.csbj.2015.11.001Suche in Google Scholar PubMed PubMed Central
[43] Neek M, Kim TI, Wang SW. Protein-based nanoparticles in cancer vaccine development. Nanomedicine. 2019;15(1):164–74.10.1016/j.nano.2018.09.004Suche in Google Scholar PubMed PubMed Central
[44] Tarhini M, Greige-Gerges H, Elaissari A. Protein-based nanoparticles: from preparation to encapsulation of active molecules. Int J Pharm. 2017;522(1–2):172–97.10.1016/j.ijpharm.2017.01.067Suche in Google Scholar PubMed
[45] Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014;2014:180549.10.1155/2014/180549Suche in Google Scholar PubMed PubMed Central
[46] Niu Y, Yu M, Meka A, Liu Y, Zhang J, Yang Y, et al. Understanding the contribution of surface roughness and hydrophobic modification of silica nanoparticles to enhanced therapeutic protein delivery. J Mater Chem B. 2016;4(2):212–9.10.1039/C5TB01911GSuche in Google Scholar PubMed
[47] Herrera Estrada LP, Champion JA. Protein nanoparticles for therapeutic protein delivery. Biomater Sci. 2015;3(6):787–99.10.1039/C5BM00052ASuche in Google Scholar PubMed
[48] Alemán JV, Chadwick AV, He J, Hess M, Horie K, Jones RG, et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl Chem. 2007;79(10):1801–29.10.1351/pac200779101801Suche in Google Scholar
[49] Tahara Y, Akiyoshi K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv Drug Deliv Rev. 2015;95:65–76.10.1016/j.addr.2015.10.004Suche in Google Scholar PubMed
[50] Sharma A, Garg T, Aman A, Panchal K, Sharma R, Kumar S, et al. Nanogel – An advanced drug delivery tool: current and future. Artif Cell Nanomed Biotechnol. 2016;44(1):165–77.10.3109/21691401.2014.930745Suche in Google Scholar PubMed
[51] Neamtu I, Rusu AG, Diaconu A, Nita LE, Chiriac AP. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017;24(1):539–57.10.1080/10717544.2016.1276232Suche in Google Scholar PubMed PubMed Central
[52] Akiyoshi K, Deguchi S, Moriguchi N, Yamaguchi S, Sunamoto J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules. 2002;26(12):3062–8.10.1021/ma00064a011Suche in Google Scholar
[53] Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–8.10.1038/354056a0Suche in Google Scholar
[54] Reilly RM. Carbon nanotubes: potential benefits and risks of nanotechnology in nuclear medicine. J Nucl Med. 2007;48(7):1039–42.10.2967/jnumed.107.041723Suche in Google Scholar
[55] Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, et al. Functionalized fullerenes mediate photodynamic killing of cancer cells: type I versus type II photochemical mechanism. Free Radic Biol Med. 2007;43(5):711–9.10.1016/j.freeradbiomed.2007.05.005Suche in Google Scholar
[56] Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12(10):1127–35.10.1016/j.chembiol.2005.08.014Suche in Google Scholar
[57] Bosi S, Da Ros T, Castellano S, Banfi E, Prato M. Antimycobacterial activity of ionic fullerene derivatives. Bioorg Med Chem Lett. 2000;10(10):1043–5.10.1016/S0960-894X(00)00159-1Suche in Google Scholar
[58] Ji H, Yang Z, Jiang W, Geng C, Gong M, Xiao H, et al. Antiviral activity of nano carbon fullerene lipidosome against influenza virus in vitro. J Huazhong Univ Sci Technol Med Sci. 2008;28(3):243–6.10.1007/s11596-008-0303-6Suche in Google Scholar PubMed
[59] Cai X, Jia H, Liu Z, Hou B, Luo C, Feng Z, et al. Polyhydroxylated fullerene derivative C(60)(OH)(24) prevents mitochondrial dysfunction and oxidative damage in an MPP(+)-induced cellular model of Parkinson’s disease. J Neurosci Res. 2008;86(16):3622–34.10.1002/jnr.21805Suche in Google Scholar PubMed
[60] Markovic Z, Trajkovic V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials. 2008;29(26):3561–73.10.1016/j.biomaterials.2008.05.005Suche in Google Scholar PubMed
[61] Torres Sangiao E, Holban AM, Gestal MC. Applications of nanodiamonds in the detection and therapy of infectious diseases. Mater (Basel). 2019;12(10):1639.10.3390/ma12101639Suche in Google Scholar PubMed PubMed Central
[62] Man HB, Ho D. Nanodiamonds as platforms for biology and medicine. J Lab Autom. 2013;18(1):12–8.10.1177/2211068212456198Suche in Google Scholar
[63] Ho D, Wang CH, Chow EK. Nanodiamonds: the intersection of nanotechnology, drug development, and personalized medicine. Sci Adv. 2015;1(7):e1500439.10.1126/sciadv.1500439Suche in Google Scholar
[64] Chauhan S, Jain N, Nagaich U. Nanodiamonds with powerful ability for drug delivery and biomedical applications: recent updates on in vivo study and patents. J Pharm Anal. 2020;10(1):1–12.10.1016/j.jpha.2019.09.003Suche in Google Scholar
[65] van der Laan K, Hasani M, Zheng T, Schirhagl R. Nanodiamonds for in vivo applications. Small. 2018;14(19):e1703838.10.1002/smll.201703838Suche in Google Scholar
[66] Tinwala H, Wairkar S. Production, surface modification and biomedical applications of nanodiamonds: a sparkling tool for theranostics. Mater Sci Eng C Mater Biol Appl. 2019;97:913–31.10.1016/j.msec.2018.12.073Suche in Google Scholar
[67] Chakrabarti K, Chakrabarti R, Chattopadhyay KK, Chaudhuri S, Pal AK. Nano-diamond films produced from CVD of camphor. Diam Relat Mater. 1998;7(6):845–52.10.1016/S0925-9635(97)00312-9Suche in Google Scholar
[68] Abdellatif AAH, Alturki HNH, Tawfeek HM. Different cellulosic polymers for synthesizing silver nanoparticles with antioxidant and antibacterial activities. Sci Rep. 2021;11(1):84.10.1038/s41598-020-79834-6Suche in Google Scholar PubMed PubMed Central
[69] Abdellatif AAH, Rasheed Z, Alhowail AH, Alqasoumi A, Alsharidah M, Khan RA, et al. Silver citrate nanoparticles inhibit PMA-induced TNFalpha expression via deactivation of NF-kappaB activity in human cancer cell-lines, MCF-7. Int J Nanomed. 2020;15:8479–93.10.2147/IJN.S274098Suche in Google Scholar PubMed PubMed Central
[70] Abdellatif AAH, Hennig R, Pollinger K, Tawfeek HM, Bouazzaoui A, Goepferich A. Fluorescent nanoparticles coated with a somatostatin analogue target blood monocyte for efficient leukaemia treatment. Pharm Res. 2020;37(11):217.10.1007/s11095-020-02938-1Suche in Google Scholar PubMed
[71] Abdellatif AAH. A plausible way for excretion of metal nanoparticles via active targeting. Drug Dev Ind Pharm. 2020;46(5):744–50.10.1080/03639045.2020.1752710Suche in Google Scholar PubMed
[72] Abdellatif AAH, Aldalaen SM, Faisal W, Tawfeek HM. Somatostatin receptors as a new active targeting sites for nanoparticles. Saudi Pharm J. 2018;26(7):1051–9.10.1016/j.jsps.2018.05.014Suche in Google Scholar PubMed PubMed Central
[73] Abdellatif AAH, Tawfeek HM. Development and evaluation of fluorescent gold nanoparticles. Drug Dev Ind Pharm. 2018;44(10):1679–84.10.1080/03639045.2018.1483400Suche in Google Scholar PubMed
[74] Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006;107(3):459–66.10.1002/cncr.22035Suche in Google Scholar PubMed
[75] Fan C, Gao W, Chen Z, Fan H, Li M, Deng F, et al. Tumor selectivity of stealth multi-functionalized superparamagnetic iron oxide nanoparticles. Int J Pharm. 2011;404(1–2):180–90.10.1016/j.ijpharm.2010.10.038Suche in Google Scholar PubMed
[76] Andocs G, Renner H, Balogh L, Fonyad L, Jakab C, Szasz A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther Onkol. 2009;185(2):120–6.10.1007/s00066-009-1903-1Suche in Google Scholar PubMed
[77] Abdellatif AA, Zayed G, El-Bakry A, Zaky A, Saleem IY, Tawfeek HM. Novel gold nanoparticles coated with somatostatin as a potential delivery system for targeting somatostatin receptors. Drug Dev Ind Pharm. 2016;42(11):1782–91.10.3109/03639045.2016.1173052Suche in Google Scholar PubMed
[78] Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003.10.1038/nmat3776Suche in Google Scholar PubMed
[79] Tian L, Lu L, Qiao Y, Ravi S, Salatan F, Melancon MP. Stimuli-responsive gold nanoparticles for cancer diagnosis and therapy. J Funct Biomater. 2016;7(3):19.10.3390/jfb7030019Suche in Google Scholar
[80] Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979.10.3390/ijms19071979Suche in Google Scholar PubMed PubMed Central
[81] Masse F, Ouellette M, Lamoureux G, Boisselier E. Gold nanoparticles in ophthalmology. Med Res Rev. 2019;39(1):302–27.10.1002/med.21509Suche in Google Scholar PubMed
[82] Abdellatif AAH, Abou-Taleb HA, Abd El Ghany AA, Lutz I, Bouazzaoui A. Targeting of somatostatin receptors expressed in blood cells using quantum dots coated with vapreotide. Saudi Pharm J. 2018;26(8):1162–9.10.1016/j.jsps.2018.07.004Suche in Google Scholar PubMed PubMed Central
[83] Iga AM, Robertson JH, Winslet MC, Seifalian AM. Clinical potential of quantum dots. J Biomed Biotechnol. 2007;2007(10):76087.10.1155/2007/76087Suche in Google Scholar PubMed PubMed Central
[84] Medintz IL, Mattoussi H, Clapp AR. Potential clinical applications of quantum dots. Int J Nanomed. 2008;3(2):151–67.10.2147/IJN.S614Suche in Google Scholar
[85] Santra S. The potential clinical impact of quantum dots. Nanomed (Lond). 2012;7(5):623–6.10.2217/nnm.12.45Suche in Google Scholar PubMed
[86] Matea CT, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia C, et al. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomed. 2017;12:5421–31.10.2147/IJN.S138624Suche in Google Scholar PubMed PubMed Central
[87] Pleskova S, Mikheeva E, Gornostaeva E. Using of quantum dots in biology and medicine. Adv Exp Med Biol. 2018;1048:323–34.10.1007/978-3-319-72041-8_19Suche in Google Scholar PubMed
[88] Chen F, Hableel G, Zhao ER, Jokerst JV. Multifunctional nanomedicine with silica: role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J Colloid Interface Sci. 2018;521:261–79.10.1016/j.jcis.2018.02.053Suche in Google Scholar PubMed PubMed Central
[89] Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci U S A. 2005;102(32):11539–44.10.1073/pnas.0504926102Suche in Google Scholar PubMed PubMed Central
[90] Bagheri E, Ansari L, Abnous K, Taghdisi SM, Charbgoo F, Ramezani M, et al. Silica based hybrid materials for drug delivery and bioimaging. J Control Rel. 2018;277:57–76.10.1016/j.jconrel.2018.03.014Suche in Google Scholar PubMed
[91] Leucuta SE. Nanotechnology for delivery of drugs and biomedical applications. Curr Clin Pharmacol. 2010;5(4):257–80.10.2174/157488410793352003Suche in Google Scholar PubMed
[92] Buse J, El-Aneed A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: current research and advances. Nanomed (Lond). 2010;5(8):1237–60.10.2217/nnm.10.107Suche in Google Scholar PubMed
[93] Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif Cell Nanomed Biotechnol. 2016;44(1):381–91.10.3109/21691401.2014.953633Suche in Google Scholar PubMed
[94] Oberholzer T, Luisi PL. The use of liposomes for constructing cell models. J Biol Phys. 2002;28(4):733–44.10.1023/A:1021267512805Suche in Google Scholar
[95] Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8–18.10.1016/j.chemphyslip.2013.10.011Suche in Google Scholar PubMed
[96] Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286.10.3389/fphar.2015.00286Suche in Google Scholar PubMed PubMed Central
[97] Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54(4):987–92.Suche in Google Scholar
[98] He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–55.10.7150/thno.21945Suche in Google Scholar PubMed PubMed Central
[99] Shimasaki T, Yamamoto S, Arisawa T. Exosome research and co-culture study. Biol Pharm Bull. 2018;41(9):1311–21.10.1248/bpb.b18-00223Suche in Google Scholar PubMed
[100] Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull. 2018;41(6):835–42.10.1248/bpb.b18-00133Suche in Google Scholar PubMed
[101] Urbanelli L, Buratta S, Sagini K, Ferrara G, Lanni M, Emiliani C. Exosome-based strategies for diagnosis and therapy. Recent Pat CNS Drug Discov. 2015;10(1):10–27.10.2174/1574889810666150702124059Suche in Google Scholar PubMed
[102] Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Rel. 2015;219:396–405.10.1016/j.jconrel.2015.07.030Suche in Google Scholar PubMed PubMed Central
[103] Familtseva A, Jeremic N, Tyagi SC. Exosomes: cell-created drug delivery systems. Mol Cell Biochem. 2019;459(1–2):1–6.10.1007/s11010-019-03545-4Suche in Google Scholar PubMed
[104] Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291–301.10.1016/j.jcyt.2017.11.002Suche in Google Scholar PubMed
[105] de Jesus MB, Zuhorn IS. Solid lipid nanoparticles as nucleic acid delivery system: properties and molecular mechanisms. J Control Rel. 2015;201:1–13.10.1016/j.jconrel.2015.01.010Suche in Google Scholar PubMed
[106] Doktorovova S, Souto EB, Silva AM. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers – A systematic review of in vitro data. Eur J Pharm Biopharm. 2014;87(1):1–18.10.1016/j.ejpb.2014.02.005Suche in Google Scholar PubMed
[107] Ezzati Nazhad Dolatabadi J, Valizadeh H, Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharm Bull. 2015;5(2):151–9.10.15171/apb.2015.022Suche in Google Scholar PubMed PubMed Central
[108] Rostami E, Kashanian S, Azandaryani AH, Faramarzi H, Dolatabadi JE, Omidfar K. Drug targeting using solid lipid nanoparticles. Chem Phys Lipids. 2014;181:56–61.10.1016/j.chemphyslip.2014.03.006Suche in Google Scholar PubMed
[109] Shen H, Shi S, Zhang Z, Gong T, Sun X. Coating solid lipid nanoparticles with hyaluronic acid enhances antitumor activity against melanoma stem-like cells. Theranostics. 2015;5(7):755–71.10.7150/thno.10804Suche in Google Scholar PubMed PubMed Central
[110] Weber S, Zimmer A, Pardeike J. Solid lipid nanoparticles (SLN) and Nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22.10.1016/j.ejpb.2013.08.013Suche in Google Scholar PubMed
[111] Luo Y, Teng Z, Li Y, Wang Q. Solid lipid nanoparticles for oral drug delivery: chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr Polym. 2015;122:221–9.10.1016/j.carbpol.2014.12.084Suche in Google Scholar PubMed
[112] Du R, Yi W, Li W, Yang H, Bai H, Li J, et al. Quasi-metal microwave route to MoN and Mo2C ultrafine nanocrystalline hollow spheres as surface-enhanced raman scattering substrates. ACS Nano. 2020;14(10):13718–26.10.1021/acsnano.0c05935Suche in Google Scholar PubMed
[113] Li X, Song T, Chen X, Wang M, Yang X, Xiao Y, et al. Osteoinductivity of Porous biphasic calcium phosphate ceramic spheres with nanocrystalline and their efficacy in guiding bone regeneration. ACS Appl Mater Interfaces. 2019;11(4):3722–36.10.1021/acsami.8b18525Suche in Google Scholar PubMed
[114] Lu W, Chen M, Wu L. Easy method for preparing nanocrystalline CdS hollow spheres using miniemulsion droplets as templates. J Colloid Interface Sci. 2008;324(1–2):220–4.10.1016/j.jcis.2008.04.059Suche in Google Scholar PubMed
[115] Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10(1):13–23.10.1016/j.ajps.2014.08.005Suche in Google Scholar
[116] Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80(1–2):148–58.10.1016/j.ymgme.2003.08.016Suche in Google Scholar PubMed
[117] McCormack MP, Rabbitts TH. Activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2004;350(9):913–22.10.1056/NEJMra032207Suche in Google Scholar PubMed
[118] Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132–42.10.1172/JCI35700Suche in Google Scholar PubMed PubMed Central
[119] Wang F, Wang Z, Tian H, Qi M, Zhai Z, Li S, et al. Biodistribution and safety assessment of bladder cancer specific recombinant oncolytic adenovirus in subcutaneous xenografts tumor model in nude mice. Curr Gene Ther. 2012;12(2):67–76.10.2174/156652312800099599Suche in Google Scholar PubMed PubMed Central
[120] Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol. 2014;1(1):427–51.10.1146/annurev-virology-031413-085355Suche in Google Scholar PubMed
[121] Epstein AL, Marconi P, Argnani R, Manservigi R. HSV-1-derived recombinant and amplicon vectors for gene transfer and gene therapy. Curr Gene Ther. 2005;5(5):445–58.10.2174/156652305774329285Suche in Google Scholar PubMed
[122] Ady JW, Johnsen C, Mojica K, Heffner J, Love D, Pugalenthi A, et al. Oncolytic gene therapy with recombinant vaccinia strain GLV-2b372 efficiently kills hepatocellular carcinoma. Surgery. 2015;158(2):331–8.10.1016/j.surg.2015.03.044Suche in Google Scholar PubMed PubMed Central
[123] Cohn L, Delamarre L. Dendritic cell-targeted vaccines. Front Immunol. 2014;5:255.10.3389/fimmu.2014.00255Suche in Google Scholar PubMed PubMed Central
[124] Schambach A, Morgan M. Retroviral vectors for cancer gene therapy. Recent Results Cancer Res. 2016;209:17–35.10.1007/978-3-319-42934-2_2Suche in Google Scholar
[125] Cone RD, Mulligan RC. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984;81(20):6349–53.10.1073/pnas.81.20.6349Suche in Google Scholar
[126] Yee JK, Friedmann T, Burns JC. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 1994;43(Pt A):99–112.10.1016/S0091-679X(08)60600-7Suche in Google Scholar
[127] Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay J-P, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346(16):1185–93.10.1056/NEJMoa012616Suche in Google Scholar PubMed
[128] Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296(5577):2410–3.10.1126/science.1070104Suche in Google Scholar PubMed
[129] Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–58.10.1056/NEJMoa0805817Suche in Google Scholar PubMed
[130] Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–9.10.1038/nm1393Suche in Google Scholar PubMed
[131] Luis A. The old and the new: prospects for non-integrating lentiviral vector technology. Viruses. 2020;12(10):1103.10.3390/v12101103Suche in Google Scholar PubMed PubMed Central
[132] Wang J, Peng Y, Xu H, Cui Z, Williams 3rd RO. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech. 2020;21(6):225.10.1208/s12249-020-01744-7Suche in Google Scholar PubMed PubMed Central
[133] Sayedahmed EE, Elkashif A, Alhashimi M, Sambhara S, Mittal SK. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines (Basel). 2020;8(4):574.10.3390/vaccines8040574Suche in Google Scholar PubMed PubMed Central
[134] Cheng C, Wang L, Ko SY, Kong WP, Schmidt SD, Gall JGD, et al. Combination recombinant simian or chimpanzee adenoviral vectors for vaccine development. Vaccine. 2015;33(51):7344–51.10.1016/j.vaccine.2015.10.023Suche in Google Scholar PubMed
[135] Zhang C, Chi Y, Zhou D. Development of novel vaccines against infectious diseases based on chimpanzee adenoviral vector. Methods Mol Biol (Clifton, NJ). 2017;1581:3–13.10.1007/978-1-4939-6869-5_1Suche in Google Scholar PubMed
[136] Liu L. Fields virology, 6th edn, Clin Infect Dis. 2014;59(4):613.10.1093/cid/ciu346Suche in Google Scholar
[137] Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013;13(6):421–33.10.2174/1566523213666131125095046Suche in Google Scholar PubMed PubMed Central
[138] Alonso-Padilla J, Papp T, Kaján GL, Benkő M, Havenga M, Lemckert A, et al. Development of novel adenoviral vectors to overcome challenges observed with HAdV-5-based constructs. Mol Ther. 2016;24(1):6–16.10.1038/mt.2015.194Suche in Google Scholar PubMed PubMed Central
[139] Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81(9):4654–63.10.1128/JVI.02696-06Suche in Google Scholar PubMed PubMed Central
[140] Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29(2):304–13.10.1016/j.vaccine.2010.10.037Suche in Google Scholar PubMed
[141] Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181(3):2134–44.10.4049/jimmunol.181.3.2134Suche in Google Scholar PubMed
[142] Fejer G, Freudenberg M, Greber UF, Gyory I. Adenovirus-triggered innate signalling pathways. Eur J Microbiol Immunol (Bp). 2011;1(4):279–88.10.1556/EuJMI.1.2011.4.3Suche in Google Scholar PubMed PubMed Central
[143] Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both toll-like receptor-dependent and -independent pathways. J Virol. 2007;81(7):3170–80.10.1128/JVI.02192-06Suche in Google Scholar PubMed PubMed Central
[144] Balakrishnan B, Jayandharan GR. Basic biology of adeno-associated virus (AAV) vectors used in gene therapy. Curr Gene Ther. 2014;14(2):86–100.10.2174/1566523214666140302193709Suche in Google Scholar
[145] Atchison RW, Casto BC, Hammon WM. Adenovirus-Associated Defective Virus Particles. Science. 1965;149(3685):754–6.10.1126/science.149.3685.754Suche in Google Scholar
[146] Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966;55(6):1467–74.10.1073/pnas.55.6.1467Suche in Google Scholar
[147] Crawford LV, Follett EA, Burdon MG, McGeoch DJ. The DNA of a minute virus of mice. J Gen Virol. 1969;4(1):37–46.10.1099/0022-1317-4-1-37Suche in Google Scholar
[148] Rose JA, Berns KI, Hoggan MD, Koczot FJ. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969;64(3):863–9.10.1073/pnas.64.3.863Suche in Google Scholar
[149] Carter BJ, Khoury G, Denhardt DT. Physical map and strand polarity of specific fragments of adenovirus-associated virus DNA produced by endonuclease R-EcoRI. J Virol. 1975;16(3):559–68.10.1128/jvi.16.3.559-568.1975Suche in Google Scholar
[150] Lusby E, Fife KH, Berns KI. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J Virol. 1980;34(2):402–9.10.1128/jvi.34.2.402-409.1980Suche in Google Scholar
[151] Berns KI, Pinkerton TC, Thomas GF, Hoggan MD. Detection of adeno-associated virus (AAV)-specific nucleotide sequences in DNA isolated from latently infected Detroit 6 cells. Virology. 1975;68(2):556–60.10.1016/0042-6822(75)90298-6Suche in Google Scholar
[152] Cheung AK, Hoggan MD, Hauswirth WW, Berns KI. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J Virol. 1980;33(2):739–48.10.1128/jvi.33.2.739-748.1980Suche in Google Scholar
[153] Kotin RM, Berns KI. Organization of adeno-associated virus DNA in latently infected Detroit 6 cells. Virology. 1989;170(2):460–7.10.1016/0042-6822(89)90437-6Suche in Google Scholar
[154] Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990;87(6):2211–5.10.1073/pnas.87.6.2211Suche in Google Scholar
[155] Carter BJ, Khoury G, Rose JA. Adenovirus-associated virus multiplication. IX. Extent of transcription of the viral genome in vivo. J Virol. 1972;10(6):1118–25.10.1128/jvi.10.6.1118-1125.1972Suche in Google Scholar
[156] Hauswirth WW, Berns KI. Origin and termination of adeno-associated virus DNA replication. Virology. 1977;78(2):488–99.10.1016/0042-6822(77)90125-8Suche in Google Scholar
[157] Marcus CJ, Laughlin CA, Carter BJ. Adeno-associated virus RNA transcription in vivo. Eur J Biochem. 1981;121(1):147–54.10.1111/j.1432-1033.1981.tb06443.xSuche in Google Scholar PubMed
[158] Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45(2):555–64.10.1128/jvi.45.2.555-564.1983Suche in Google Scholar PubMed PubMed Central
[159] Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci U S A. 2010;107(22):10220–5.10.1073/pnas.1001673107Suche in Google Scholar PubMed PubMed Central
[160] Sonntag F, Köther K, Schmidt K, Weghofer M, Raupp C, Nieto K, et al. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J Virol. 2011;85(23):12686–97.10.1128/JVI.05359-11Suche in Google Scholar PubMed PubMed Central
[161] Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–65.10.1056/NEJMoa1108046Suche in Google Scholar PubMed PubMed Central
[162] Gaudet D, Méthot J, Déry S, Brisson D, Essiembre C, Tremblay G, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20(4):361–9.10.1038/gt.2012.43Suche in Google Scholar PubMed PubMed Central
[163] Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(5):341–55.10.1038/nrg2988Suche in Google Scholar PubMed
[164] Büning H. Gene therapy enters the pharma market: the short story of a long journey. EMBO Mol Med. 2013;5(1):1–3.10.1002/emmm.201202291Suche in Google Scholar PubMed PubMed Central
[165] Hoy SM. Onasemnogene abeparvovec: first global approval. Drugs. 2019;79(11):1255–62.10.1007/s40265-019-01162-5Suche in Google Scholar PubMed
[166] Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27(4):710–28.10.1016/j.ymthe.2019.02.012Suche in Google Scholar PubMed PubMed Central
[167] Kauffman KJ, Dorkin JR, Yang JH, Heartlein MW, DeRosa F, Mir FF, et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15(11):7300–6.10.1021/acs.nanolett.5b02497Suche in Google Scholar PubMed
[168] Fuller DH, Berglund P. Amplifying RNA vaccine development. N Engl J Med. 2020;382(25):2469–71.10.1056/NEJMcibr2009737Suche in Google Scholar PubMed
[169] Beissert T, Perkovic M, Vogel A, Erbar S, Walzer KC, Hempel T, et al. A Trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol Ther. 2020;28(1):119–28.10.1016/j.ymthe.2019.09.009Suche in Google Scholar PubMed PubMed Central
[170] Bouazzaoui A, Abdellatif AAH, Al-Allaf FA, Bogari NM, Al-Dehlawi S, Qari SH. Strategies for vaccination: conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics. 2021;13(2):140.10.3390/pharmaceutics13020140Suche in Google Scholar PubMed PubMed Central
[171] Tamerler C, Sarikaya M. Molecular biomimetics: nanotechnology and bionanotechnology using genetically engineered peptides. Philos Trans A Math Phys Eng Sci. 2009;367(1894):1705–26.10.1098/rsta.2009.0018Suche in Google Scholar PubMed
[172] Sarikaya M, Tamerler C, Jen AK, Schulten K, Baneyx F. Molecular biomimetics: nanotechnology through biology. Nat Mater. 2003;2(9):577–85.10.1038/nmat964Suche in Google Scholar PubMed
[173] Jin J, Bhujwalla ZM. Biomimetic nanoparticles camouflaged in cancer cell membranes and their applications in cancer theranostics. Front Oncol. 2019;9:1560.10.3389/fonc.2019.01560Suche in Google Scholar PubMed PubMed Central
[174] Zinger A, Sushnitha M, Naoi T, Baudo G, De Rosa E, Chang J, et al. Enhancing inflammation targeting using tunable leukocyte-based biomimetic nanoparticles. ACS Nano. 2021;15(4):6326–39.10.1021/acsnano.0c05792Suche in Google Scholar
[175] Carmona-Ribeiro AM. Preparation and characterization of biomimetic nanoparticles for drug delivery. Methods Mol Biol. 2012;906:283–94.10.1007/978-1-61779-953-2_22Suche in Google Scholar
[176] Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83.10.1016/j.biomaterials.2017.02.041Suche in Google Scholar
[177] Jin K, Luo Z, Zhang B, Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8(1):23–33.10.1016/j.apsb.2017.12.002Suche in Google Scholar
[178] Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomed (Lond). 2019;14(1):93–126.10.2217/nnm-2018-0120Suche in Google Scholar
[179] Pillai G, Ceballos-Coronel ML. Science and technology of the emerging nanomedicines in cancer therapy: a primer for physicians and pharmacists. SAGE Open Med. 2013;1:2050312113513759.10.1177/2050312113513759Suche in Google Scholar
[180] Murphy T, Yee KWL. Cytarabine and daunorubicin for the treatment of acute myeloid leukemia. Expert Opin Pharmacother. 2017;18(16):1765–80.10.1080/14656566.2017.1391216Suche in Google Scholar
[181] Zhang H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016;9:3001–7.10.2147/OTT.S105587Suche in Google Scholar
[182] Mantripragada S. A lipid based depot (DepoFoam technology) for sustained release drug delivery. Prog Lipid Res. 2002;41(5):392–406.10.1016/S0163-7827(02)00004-8Suche in Google Scholar
[183] Stone NR, Bicanic T, Salim R, Hope W, Liposomal, Amphotericin B. AmBisome((R))): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs. 2016;76(4):485–500.10.1007/s40265-016-0538-7Suche in Google Scholar PubMed PubMed Central
[184] Krauss AC, Gao X, Li L, Manning ML, Patel P, Fu W, et al. FDA approval summary: (Daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019;25(9):2685–90.10.1158/1078-0432.CCR-18-2990Suche in Google Scholar PubMed
[185] Jensen IS, Wu E, Sacks NC, Cyr PL, Chung KC. Budget impact analysis of using daunorubicin-cytarabine liposome in patients with newly diagnosed therapy-related AML or AML and myelodysplasia-related changes. Am Health Drug Benefits. 2018;11(7):380–6.Suche in Google Scholar
[186] Blair HA. Daunorubicin/cytarabine liposome: a review in acute myeloid leukaemia. Drugs. 2018;78(18):1903–10.10.1007/s40265-018-1022-3Suche in Google Scholar PubMed PubMed Central
[187] Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–92.10.1200/JCO.2017.77.6112Suche in Google Scholar PubMed PubMed Central
[188] Kim M, Williams S. Daunorubicin and cytarabine liposome in newly diagnosed therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes. Ann Pharmacother. 2018;52(8):792–800.10.1177/1060028018764923Suche in Google Scholar PubMed
[189] Veerareddy PR, Vobalaboina V. Lipid-based formulations of amphotericin B. Drugs Today (Barc). 2004;40(2):133–45.10.1358/dot.2004.40.2.799425Suche in Google Scholar PubMed
[190] Wachtlin J, Heimann H, Behme T, Foerster MH. Long-term results after photodynamic therapy with verteporfin for choroidal neovascularizations secondary to inflammatory chorioretinal diseases. Graefes Arch Clin Exp Ophthalmol. 2003;241(11):899–906.10.1007/s00417-003-0734-5Suche in Google Scholar PubMed
[191] Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. Pharm Therap. 2017;42(12):742–55.Suche in Google Scholar
[192] Mousseau F, Puisney C, Mornet S, Borgne RL, Vacher A, Airiau M, et al. Supported pulmonary surfactant bilayers on silica nanoparticles: formulation, stability and impact on lung epithelial cells. Nanoscale. 2017;9(39):14967–78.10.1039/C7NR04574CSuche in Google Scholar PubMed
[193] Romagnuolo J, Hoffman B, Vela S, Hawes R, Vignesh S. Accuracy of contrast-enhanced harmonic EUS with a second-generation perflutren lipid microsphere contrast agent (with video). Gastrointest Endosc. 2011;73(1):52–63.10.1016/j.gie.2010.09.014Suche in Google Scholar PubMed
[194] Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):e10143.10.1002/btm2.10143Suche in Google Scholar PubMed PubMed Central
[195] Sottilotta G, Luise F, Massara E, Oriana V, Piromalli A. Efficacy of octocog alfa (Advate) in a child with type 3 von willebrand disease and alloantibodies. J Clin Med. 2017;6(9):85.10.3390/jcm6090085Suche in Google Scholar PubMed PubMed Central
[196] Klamroth R, Simpson M, von Depka-Prondzinski M, Gill JC, Morfini M, Powell JS, et al. Comparative pharmacokinetics of rVIII-SingleChain and octocog alfa (Advate((R))) in patients with severe haemophilia A. Haemophilia. 2016;22(5):730–8.10.1111/hae.12985Suche in Google Scholar PubMed
[197] Keating GM, Dhillon S. Octocog alfa (Advate(R)): a guide to its use in hemophilia A. BioDrugs. 2012;26(4):269–73.10.1007/BF03261885Suche in Google Scholar PubMed
[198] Dhillon S. Octocog alfa, antihaemophilic factor (recombinant), plasma/albumin free method (Advate(R)): a review of its use in the management of patients with haemophilia A. Drugs. 2012;72(7):987–1007.10.2165/11207480-000000000-00000Suche in Google Scholar PubMed
[199] Mudry P, Kyr M, Rohleder O, Mahdal M, Staniczkova Zambo I, Jezova M, et al. Improved osteosarcoma survival with addition of mifamurtide to conventional chemotherapy – Observational prospective single institution analysis. J Bone Oncol. 2021;28:100362.10.1016/j.jbo.2021.100362Suche in Google Scholar PubMed PubMed Central
[200] Punzo F, Bellini G, Tortora C, Pinto DD, Argenziano M, Pota E, et al. Mifamurtide and TAM-like macrophages: effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget. 2020;11(7):687–98.10.18632/oncotarget.27479Suche in Google Scholar PubMed PubMed Central
[201] Fidler IJ, Sone S, Fogler WE, Smith D, Braun DG, Tarcsay L, et al. Efficacy of liposomes containing a lipophilic muramyl dipeptide derivative for activating the tumoricidal properties of alveolar macrophages in vivo. J Immunotherapy. 1982;1(1):43–56.Suche in Google Scholar
[202] Wada H, Oshima T, Fukuda I, Karasawa F, Sato T. Total intravenous anesthesia with Diprivan (1% propofol emulsion) using a manual drip-infusion technique. Masui. 2000;49(6):611–4.Suche in Google Scholar
[203] Abdel-Zaher AO, Askar FG. The myoneural effects of propofol emulsion (Diprivan) on the nerve-muscle preparations of rats. Pharmacol Res. 1997;36(4):323–32.10.1006/phrs.1997.0237Suche in Google Scholar PubMed
[204] de Grood PM, Ruys AH, van Egmond J, Booij LH, Crul JF. Propofol (‘Diprivan’) emulsion for total intravenous anaesthesia. Postgrad Med J. 1985;61(Suppl 3):65–9.Suche in Google Scholar
[205] Cundy JM, Arunasalam K. Use of an emulsion formulation of propofol (‘Diprivan’) in intravenous anaesthesia for termination of pregnancy. A comparison with methohexitone. Postgrad Med J. 1985;61(Suppl 3):129–31.Suche in Google Scholar
[206] Connock M, Tubeuf S, Malottki K, Uthman A, Round J, Bayliss S, et al. Certolizumab pegol (CIMZIA(R)) for the treatment of rheumatoid arthritis. Health Technol Assess. 2010;14(Suppl. 2):1–10.10.3310/hta14suppl2-01Suche in Google Scholar
[207] Piedmonte DM, Treuheit MJ. Formulation of Neulasta (pegfilgrastim). Adv Drug Deliv Rev. 2008;60(1):50–8.10.1016/j.addr.2007.04.017Suche in Google Scholar PubMed
[208] Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–82.10.1142/9789814287005_0025Suche in Google Scholar
[209] Leonart LP, Tonin FS, Ferreira VL, Fernandez-Llimos F, Pontarolo R. Effectiveness and safety of pegvisomant: a systematic review and meta-analysis of observational longitudinal studies. Endocrine. 2019;63(1):18–26.10.1007/s12020-018-1729-7Suche in Google Scholar PubMed
[210] McGahan L. Continuous erythropoietin receptor activator (Mircera) for renal anemia. Issues Emerg Health Technol. 2008;113:1–6.Suche in Google Scholar
[211] Manns MP, Pockros PJ, Norkrans G, Smith CI, Morgan TR, Haussinger D, et al. Long-term clearance of hepatitis C virus following interferon alpha-2b or peginterferon alpha-2b, alone or in combination with ribavirin. J Viral Hepat. 2013;20(8):524–9.10.1111/jvh.12074Suche in Google Scholar PubMed
[212] Shannon JA, Cole SW. Pegloticase: a novel agent for treatment-refractory gout. Ann Pharmacother. 2012;46(3):368–76.10.1345/aph.1Q593Suche in Google Scholar PubMed
[213] Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym Chem. 2011;2(7):1442–8.10.1039/c1py00034aSuche in Google Scholar
[214] Chaplin S, Gnanapavan S. Plegridy for the treatment of RRMS in adults. Prescriber. 2015;26(9):29–31.10.1002/psb.1349Suche in Google Scholar
[215] Konkle BA, Stasyshyn O, Chowdary P, Bevan DH, Mant T, Shima M, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078–85.10.1182/blood-2015-03-630897Suche in Google Scholar PubMed PubMed Central
[216] Johnson KP. Glatiramer acetate for treatment of relapsing-remitting multiple sclerosis. Expert Rev Neurother. 2012;12(4):371–84.10.1586/ern.12.25Suche in Google Scholar PubMed
[217] Harrison DM, Gladstone DE, Hammond E, Cheng J, Jones RJ, Brodsky RA, et al. Treatment of relapsing-remitting multiple sclerosis with high-dose cyclophosphamide induction followed by glatiramer acetate maintenance. Mult Scler. 2012;18(2):202–9.10.1177/1352458511419701Suche in Google Scholar PubMed PubMed Central
[218] Wex J, Sidhu M, Odeyemi I, Abou-Setta AM, Retsa P, Tombal B. Leuprolide acetate 1-,3- and 6-monthly depot formulations in androgen deprivation therapy for prostate cancer in nine European countries: evidence review and economic evaluation. Clinicoecon Outcomes Res. 2013;5:257–69.10.2147/CEOR.S44855Suche in Google Scholar PubMed PubMed Central
[219] Kraus VB, Conaghan PG, Aazami HA, Mehra P, Kivitz AJ, Lufkin J, et al. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA). Osteoarthr Cartil. 2018;26(1):34–42.10.1016/j.joca.2017.10.003Suche in Google Scholar PubMed
[220] Ezban M, Hermit MB, Persson E. FIXing postinfusion monitoring: assay experiences with N9-GP (nonacog beta pegol; Refixia((R)); Rebinyn((R)). Haemophilia. 2019;25(1):154–61.10.1111/hae.13671Suche in Google Scholar PubMed
[221] Nielsen FS, Schmidt AS, Kristensen AK, Nielsen AD, Kristensen BK, Palm L. Characterisation of recombinant factor IX before and after GlycoPEGylation. Int J Pharm. 2020;588:119654.10.1016/j.ijpharm.2020.119654Suche in Google Scholar PubMed
[222] Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res. 2005;11(11):4136–43.10.1158/1078-0432.CCR-04-2291Suche in Google Scholar PubMed
[223] Foss F. Clinical experience with denileukin diftitox (ONTAK). Semin Oncol. 2006;33(1 Suppl 3):S11–6.10.1053/j.seminoncol.2005.12.017Suche in Google Scholar PubMed
[224] Hurtubise A, Momparler RL. Evaluation of antineoplastic action of 5-aza-2’-deoxycytidine (Dacogen) and docetaxel (Taxotere) on human breast, lung and prostate carcinoma cell lines. Anticancer Drugs. 2004;15(2):161–7.10.1097/00001813-200402000-00010Suche in Google Scholar PubMed
[225] Gueritte-Voegelein F, Guenard D, Dubois J, Wahl A, Potier P. Chemical and biological studies on Taxol (Paclitaxel) and Taxotere (Docetaxel), new antineoplastic agents. J Pharm Belg. 1994;49(3):193–205.Suche in Google Scholar
[226] Wahl RL, Frey EC, Jacene HA, Kahl BS, Piantadosi S, Bianco JA, et al. Prospective SPECT-CT organ dosimetry-driven radiation-absorbed dose escalation using the In-111 ((111)In)/Yttrium 90 ((90)Y) Ibritumomab Tiuxetan (Zevalin((R))) Theranostic pair in patients with lymphoma at myeloablative dose levels. Cancers (Basel). 2021;13(11):2828.10.3390/cancers13112828Suche in Google Scholar PubMed PubMed Central
[227] Mukherjee S, Ayanambakkam A, Ibrahimi S, Schmidt S, Charkrabarty JH, Khawandanah M. Ibritumomab tiuxetan (Zevalin) and elevated serum human anti-murine antibody (HAMA). Hematol Oncol Stem Cell Ther. 2018;11(3):187–8.10.1016/j.hemonc.2017.12.004Suche in Google Scholar PubMed
[228] Junghanns JU, Muller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomed. 2008;3(3):295–309.10.2147/IJN.S595Suche in Google Scholar
[229] Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–87.10.1201/9780429027819-7Suche in Google Scholar
[230] Wenthur CJ. Classics in chemical neuroscience: methylphenidate. ACS Chem Neurosci. 2016;7(8):1030–40.10.1021/acschemneuro.6b00199Suche in Google Scholar PubMed
[231] Jackson A. Impact of release mechanism on the pharmacokinetic performance of PAUC metrics for three methylphenidate products with complex absorption. Pharm Res. 2014;31(1):173–81.10.1007/s11095-013-1150-0Suche in Google Scholar PubMed
[232] Tucker JD, Suter W, Petibone DM, Thomas RA, Bailey NL, Zhou Y, et al. Cytogenetic assessment of methylphenidate treatment in pediatric patients treated for attention deficit hyperactivity disorder. Mutat Res. 2009;677(1–2):53–8.10.1016/j.mrgentox.2009.05.005Suche in Google Scholar PubMed
[233] Dexmethylphenidate – Novartis/Celgene. Focalin, D-MPH, D-methylphenidate hydrochloride, D-methylphenidate, dexmethylphenidate, dexmethylphenidate hydrochloride. Drugs R D. 2002;3(4):279–82.10.2165/00126839-200203040-00010Suche in Google Scholar PubMed
[234] Mott TF, Leach L. Is methylphenidate useful for treating adolescents with ADHD?. J Fam Pract. 2004;53(8):650–63.Suche in Google Scholar
[235] Alagona Jr P. Fenofibric acid: a new fibrate approved for use in combination with statin for the treatment of mixed dyslipidemia. Vasc Health Risk Manag. 2010;6:351–62.10.2147/VHRM.S6714Suche in Google Scholar
[236] Megestrol acetate NCD oral suspension – Par Pharmaceutical: megestrol acetate nanocrystal dispersion oral suspension, PAR 100.2, PAR-100.2. Drugs R D. 2007;8(6):403–6.10.2165/00126839-200708060-00009Suche in Google Scholar PubMed
[237] Deschamps B, Musaji N, Gillespie JA. Food effect on the bioavailability of two distinct formulations of megestrol acetate oral suspension. Int J Nanomed. 2009;4:185–92.10.2147/IJN.S6308Suche in Google Scholar
[238] Teo SK, Scheffler MR, Wu A, Stirling DI, Thomas SD, Stypinski D, et al. A single-dose, two-way crossover, bioequivalence study of dexmethylphenidate HCl with and without food in healthy subjects. J Clin Pharmacol. 2004;44(2):173–8.10.1177/0091270003261899Suche in Google Scholar PubMed
[239] Hord AH, Chalfoun AG, Denson DD, Azevedo MI. Systemic tizanidine hydrochloride (Zanaflex) relieves thermal hyperalgesia in rats with an experimental mononeuropathy. Anesth Analg. 2001;93(5):1310–5.10.1097/00000539-200111000-00057Suche in Google Scholar PubMed
[240] Hord AH, Denson DD, Azevedo MI. Systemic tizanidine hydrochloride (Zanaflex) partially decreases experimental postoperative pain in rats. Anesth Analg. 2001;93(5):1307–9.10.1097/00000539-200111000-00056Suche in Google Scholar PubMed
[241] FDA. Drugs@FDA: FDA-Approved Drugs.Suche in Google Scholar
[242] FDA. Paliperidone Palmitate (Invega sustenna®).Suche in Google Scholar
[243] Rise MB, Stolan LO, Erdner A, Hedberg B, Stahl K, Riise J, et al. Patients’ perspectives on three-monthly administration of antipsychotic treatment with paliperidone palmitate – a qualitative interview study. Nord J Psychiatry. 2021;75(4):257–65.10.1080/08039488.2020.1841289Suche in Google Scholar PubMed
[244] Bioque M, Parellada E, Garcia-Rizo C, Amoretti S, Fortea A, Oriolo G, et al. Clozapine and paliperidone palmitate antipsychotic combination in treatment-resistant schizophrenia and other psychotic disorders: a retrospective 6-month mirror-image study. Eur Psychiatry. 2020;63(1):e71.10.1192/j.eurpsy.2020.72Suche in Google Scholar PubMed PubMed Central
[245] Martinez-Andres JA, Garcia-Carmona JA. Switching from clozapine to paliperidone palmitate-3-monthly improved obesity, hyperglycemia and dyslipidemia lowering antipsychotic dose equivalents in a treatment-resistant schizophrenia cohort. Int Clin Psychopharmacol. 2020;35(3):163–9.10.1097/YIC.0000000000000300Suche in Google Scholar PubMed
[246] Brown B, Turkoz I, Mancevski B, Mathews M. Evaluation of paliperidone palmitate long-acting injectable antipsychotic therapy as an early treatment option in patients with schizophrenia. Early Interv Psychiatry. 2020;14(4):428–38.10.1111/eip.12868Suche in Google Scholar
[247] McAvoy JC, Brodsky JB, Brock-Utne J. Pennywise and a pound foolish: the advantage of dantrolene nanosuspension (ryanodex) in the treatment of malignant hyperthermia. Anesth Analg. 2019;129(6):e201–2.10.1213/ANE.0000000000004448Suche in Google Scholar
[248] Okwuasaba F, Ejike C, Parry O. Comparison of the skeletal muscle relaxant properties of Portulaca oleracea extracts with dantrolene sodium and methoxyverapamil. J Ethnopharmacol. 1987;20(2):85–106.10.1016/0378-8741(87)90082-1Suche in Google Scholar
[249] Gollan F, McDermott J. Effect of the skeletal muscle relaxant dantrolene sodium on the isolated, perfused heart. Proc Soc Exp Biol Med. 1979;160(1):42–5.10.3181/00379727-160-40384Suche in Google Scholar PubMed
[250] Ellis KO, Butterfield JL, Wessels FL, Carpenter JF. A comparison of skeletal, cardiac, and smooth muscle actions of dantrolene sodium – a skeletal muscle relaxant. Arch Int Pharmacodyn Ther. 1976;224(1):118–32.Suche in Google Scholar
[251] Ellis KO, Carpenter JF. Studies on the mechanism of action of dantrolene sodium. A skeletal muscle relaxant. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):83–94.10.1007/BF00505069Suche in Google Scholar PubMed
[252] Kim HJ, Koh WU, Choi JM, Ro YJ, Yang HS. Malignant hyperthermia and dantrolene sodium. Korean J Anesthesiol. 2019;72(1):78–9.10.4097/kja.d.18.00139Suche in Google Scholar PubMed PubMed Central
[253] FDA. Ryanodex (dantrolene sodium); 2014.Suche in Google Scholar
[254] Taylor D, Paton C, Paton C. The Maudsley prescribing guidelines, 10th ed. UK: CRC Press, Taylor & Francis group UK; 2009.10.3109/9780203092835Suche in Google Scholar
[255] Wang B, Studdert DM, Sarpatwari A, Franklin JM, Landon J, Kesselheim AS. The effect of federal and state off-label marketing investigations on drug prescribing: the case of olanzapine. PLoS One. 2017;12(4):e0175313.10.1371/journal.pone.0175313Suche in Google Scholar PubMed PubMed Central
[256] Elshafeey AH, Elsherbiny MA, Fathallah MM. A single-dose, randomized, two-way crossover study comparing two olanzapine tablet products in healthy adult male volunteers under fasting conditions. Clin Ther. 2009;31(3):600–8.10.1016/j.clinthera.2009.03.008Suche in Google Scholar PubMed
[257] Maida V, Ennis M, Irani S, Corbo M, Dolzhykov M. Adjunctive nabilone in cancer pain and symptom management: a prospective observational study using propensity scoring. J Support Oncol. 2008;6(3):119–24.Suche in Google Scholar
[258] Nabilone (Cesamet) for chemotherapy-induced nausea and vomiting. Med Lett Drugs Ther. 2006;48(1249/1250):103–4.Suche in Google Scholar
[259] Devane JG, Butler J, Mulligan S. IPDAS: a novel technology brings new benefits when applied to naproxen sodium. Am J Orthop (Belle Mead NJ). 1996;25(9 Suppl):7–13.Suche in Google Scholar
[260] Paulus HE. FDA Arthritis Advisory Committee Meeting: juvenile rheumatoid arthritis drug evaluation guidelines; over-the-counter naproxen? Arthritis Rheum. 1994;37(1):137–8.10.1002/art.1780370119Suche in Google Scholar PubMed
[261] Eisen AH, Bacal HL. Clinical evaluation of a theophylline solution (Elixophyllin) in children with bronchial asthma. Can Med Assoc J. 1962;86:444–6.Suche in Google Scholar
[262] Perlman E. Hydroalcoholic theophylline preparation (Elixophyllin) in the management of bronchial asthma. Ann Allergy. 1960;18:1350–8.Suche in Google Scholar
[263] Spielman AD. Comparative effectiveness of an alcohol-water solution of theophylline (elixophyllin), alcohol-water solution, and theophylline-water solution for the oral treatment of acute bronchial asthma. J Allergy. 1959;30(1):35–41.10.1016/0021-8707(59)90056-5Suche in Google Scholar
[264] Schluger J, McGinn JT, Burbank B. The treatment of the acute asthmatic attack with an oral alcohol-water solution of theophylline (elixophyllin). Am J Med Sci. 1957;234(1):28–30.10.1097/00000441-195707000-00002Suche in Google Scholar PubMed
[265] Nikravesh N, Borchard G, Hofmann H, Philipp E, Fluhmann B, Wick P. Factors influencing safety and efficacy of intravenous iron-carbohydrate nanomedicines: from production to clinical practice. Nanomedicine. 2020;26:102178.10.1016/j.nano.2020.102178Suche in Google Scholar PubMed
[266] Bhandari S, Kalra PA, Kothari J, Ambuhl PM, Christensen JH, Essaian AM, et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer(R)) compared with iron sucrose (Venofer(R)) as maintenance therapy in haemodialysis patients. Nephrol Dial Transpl. 2015;30(9):1577–89.10.1093/ndt/gfv096Suche in Google Scholar PubMed PubMed Central
[267] Manley HJ, Grabe DW. Determination of iron sucrose (Venofer) or iron dextran (DexFerrum) removal by hemodialysis: an in-vitro study. BMC Nephrol. 2004;5:1.10.1186/1471-2369-5-1Suche in Google Scholar PubMed PubMed Central
[268] Kosch M, Bahner U, Bettger H, Matzkies F, Teschner M, Schaefer RM. A randomized, controlled parallel-group trial on efficacy and safety of iron sucrose (Venofer) vs iron gluconate (Ferrlecit) in haemodialysis patients treated with rHuEpo. Nephrol Dial Transpl. 2001;16(6):1239–44.10.1093/ndt/16.6.1239Suche in Google Scholar PubMed
[269] Brandis JEP, Kihn KC, Taraban MB, Schnorr J, Confer AM, Batelu S, et al. Evaluation of the physicochemical properties of the iron nanoparticle drug products: brand and generic sodium ferric gluconate. Mol Pharm. 2021;18(4):1544–57.10.1021/acs.molpharmaceut.0c00922Suche in Google Scholar PubMed
[270] Sun D, Rouse R, Patel V, Wu Y, Zheng J, Karmakar A, et al. Comparative Evaluation of U.S. brand and generic intravenous sodium ferric gluconate complex in sucrose injection: physicochemical characterization. Nanomaterials (Basel). 2018;8(1):25.10.3390/nano8010025Suche in Google Scholar PubMed PubMed Central
[271] Wu M, Sun D, Tyner K, Jiang W, Rouse R. Comparative evaluation of U.S. brand and generic intravenous sodium ferric gluconate complex in sucrose injection: in vitro cellular uptake. Nanomaterials (Basel). 2017;7(12):451.10.3390/nano7120451Suche in Google Scholar PubMed PubMed Central
[272] Allan J, Plate P, Van, Winden S. The effect of iron dextran injection on daily weight gain and haemoglobin values in whole milk fed calves. Anim (Basel). 2020;10(5):853.10.3390/ani10050853Suche in Google Scholar PubMed PubMed Central
[273] Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol. 2011;86(10):860–2.10.1002/ajh.22153Suche in Google Scholar
[274] Fishbane S, Ungureanu VD, Maesaka JK, Kaupke CJ, Lim V, Wish J. The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis. 1996;28(4):529–34.10.1016/S0272-6386(96)90463-1Suche in Google Scholar
[275] Fletes R, Lazarus JM, Gage J, Chertow GM. Suspected iron dextran-related adverse drug events in hemodialysis patients. Am J Kidney Dis. 2001;37(4):743–9.10.1016/S0272-6386(01)80123-2Suche in Google Scholar
[276] Scott LJ. Ferric carboxymaltose: a review in iron deficiency. Drugs. 2018;78(4):479–93.10.1007/s40265-018-0885-7Suche in Google Scholar PubMed
[277] Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs. 2015;75(1):101–27.10.1007/s40265-014-0332-3Suche in Google Scholar PubMed
[278] Abdellatif AAH, Tawfeek HM, Abdelfattah A, El-Saber Batiha G, Hetta HF. Recent updates in COVID-19 with emphasis on inhalation therapeutics: nanostructured and targeting systems. J Drug Deliv Sci Technol. 2021;63:102435.10.1016/j.jddst.2021.102435Suche in Google Scholar PubMed PubMed Central
[279] Zamboni WC. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist. 2008;13(3):248–60.10.1634/theoncologist.2007-0180Suche in Google Scholar PubMed
[280] Kim H, Park Y, Lee JB. Self-assembled messenger RNA nanoparticles (mRNA-NPs) for efficient gene expression. Sci Rep. 2015;5:12737.10.1038/srep12737Suche in Google Scholar PubMed PubMed Central
[281] Caballero ML, Quirce S. Excipients as potential agents of anaphylaxis in vaccines: analyzing the formulations of currently authorized COVID-19 vaccines. J Investig Allergol Clin Immunol. 2021;31(1):92–3.10.18176/jiaci.0667Suche in Google Scholar PubMed
[282] Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215(6):1571–88.10.1084/jem.20171450Suche in Google Scholar PubMed PubMed Central
[283] Sahin U, Kariko K, Tureci O. mRNA-based therapeutics – Developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–80.10.1038/nrd4278Suche in Google Scholar PubMed
[284] Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–51.10.1038/nature21428Suche in Google Scholar PubMed PubMed Central
[285] Pardi N, LaBranche CC, Ferrari G, Cain DW, Tombácz I, Parks RJ, et al. Characterization of HIV-1 nucleoside-modified mRNA vaccines in rabbits and rhesus macaques. 2019;15:36–47.10.1016/j.omtn.2019.03.003Suche in Google Scholar PubMed PubMed Central
[286] Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–9.10.1038/s41586-020-2814-7Suche in Google Scholar PubMed
[287] Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963.10.3389/fimmu.2018.01963Suche in Google Scholar PubMed PubMed Central
[288] Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Rel. 2015;217:345–51.10.1016/j.jconrel.2015.08.007Suche in Google Scholar PubMed PubMed Central
[289] Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. 2018;9(1):1–12.10.1038/s41467-018-05482-0Suche in Google Scholar PubMed PubMed Central
[290] MHRA. Pfizer/BioNTech COVID-19 vaccine; 2020.Suche in Google Scholar
[291] MHRA. AstraZeneca COVID-19 vaccine; 2020.10.69645/WRSL8173Suche in Google Scholar
[292] MHRA. Moderna COVID-19 vaccine; 2020.Suche in Google Scholar
[293] PHE. COVID-19 vaccination guidance for healthcare practitioners; 2020.Suche in Google Scholar
[294] MHRA. Managing allergic reactions with Pfizer/BioNTech vaccine; 2020.Suche in Google Scholar
[295] Drulovic J, Ivanovic J, Martinovic V, Tamas O, Veselinovic N, Cujic D, et al. Humoral response to sars-CoV-2 and Covid-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult Scler Relat Disord. 2021;54:103150.10.1016/j.msard.2021.103150Suche in Google Scholar PubMed PubMed Central
[296] Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT, et al. Safety of Covid-19 vaccines. J Med Virol. 2021.10.1002/jmv.27214Suche in Google Scholar PubMed PubMed Central
[297] Abu-Hammad O, Alduraidi H, Abu-Hammad S, Alnazzawi A, Babkair H, Abu-Hammad A, et al. Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines (Basel). 2021;9(6):577.10.3390/vaccines9060577Suche in Google Scholar PubMed PubMed Central
[298] Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, et al. Side effects and perceptions following COVID-19 vaccination in Jordan: a randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines (Basel). 2021;9(6):556.10.3390/vaccines9060556Suche in Google Scholar PubMed PubMed Central
[299] Doroftei B, Ciobica A, Ilie OD, Maftei R, Ilea C. Mini-Review Discussing the Reliability and Efficiency of COVID-19 Vaccines. Diagnostics (Basel). 2021;11(4):579.10.3390/diagnostics11040579Suche in Google Scholar PubMed PubMed Central
[300] Novavax – Creating Tomorrow’s Vaccines Today. Clinical Stage Pipeline. 2020.Suche in Google Scholar
[301] FDA authorizes Johnson & Johnson COVID-19 vaccine. Med Lett Drugs Ther. 2021;63(1620):41–2.Suche in Google Scholar
[302] Abo-Zeid Y, Ismail NSM, McLean GR, Hamdy NM. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur J Pharm Sci. 2020;153:105465.10.1016/j.ejps.2020.105465Suche in Google Scholar PubMed PubMed Central
[303] Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1(1):10–29.10.1002/btm2.10003Suche in Google Scholar PubMed PubMed Central
[304] Castaneda RT, Khurana A, Khan R, Daldrup-Link HE. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J Vis Exp. 2011;57:e3482.10.3791/3482Suche in Google Scholar PubMed PubMed Central
[305] Eifler AC, Thaxton CS. Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. Methods Mol Biol. 2011;726:325–38.10.1007/978-1-61779-052-2_21Suche in Google Scholar PubMed
[306] Saha AK, Zhen MS, Erogbogbo F, Ramasubramanian AK. Design considerations and assays for hemocompatibility of FDA-approved nanoparticles. Semin Thromb Hemost. 2020;46(5):637–52.10.1055/s-0039-1688491Suche in Google Scholar PubMed
[307] Choi YH, Han HK. Correction to: nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Investig. 2019;49(1):201.10.1007/s40005-018-00412-0Suche in Google Scholar PubMed PubMed Central
[308] Choi YH, Han HK. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Investig. 2018;48(1):43–60.10.1007/s40005-017-0370-4Suche in Google Scholar PubMed PubMed Central
[309] Silverman JA, Deitcher SR. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71(3):555–64.10.1007/s00280-012-2042-4Suche in Google Scholar PubMed PubMed Central
[310] Morigi V, Tocchio A, Bellavite Pellegrini C, Sakamoto JH, Arnone M, Tasciotti E. Nanotechnology in medicine: from inception to market domination. J Drug Deliv. 2012;2012:389485.10.1155/2012/389485Suche in Google Scholar PubMed PubMed Central
[311] Venkatakrishnan K, Liu Y, Noe D, Mertz J, Bargfrede M, Marbury T, et al. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate renal impairment. Br J Clin Pharmacol. 2014;77(6):986–97.10.1111/bcp.12260Suche in Google Scholar PubMed PubMed Central
[312] Kim MT, Chen Y, Marhoul J, Jacobson F. Statistical modeling of the drug load distribution on trastuzumab emtansine (Kadcyla), a lysine-linked antibody drug conjugate. Bioconjug Chem. 2014;25(7):1223–32.10.1021/bc5000109Suche in Google Scholar PubMed
[313] Bajwa S, Munawar A, Khan WJPB. Nanotechnology in medicine: innovation to market. Pharm Bioprocess. 2017;5(2):11–5.Suche in Google Scholar
[314] Kaduk JA, Dmitrienko AO, Gindhart AM, Blanton TNJPD. Crystal structure of paliperidone palmitate (INVEGA SUSTENNA®). C39H57FN4O4. 2017;32(4):222.10.1017/S0885715617000896Suche in Google Scholar
[315] Patra JK, Das G, Fraceto LF, Campos EVR, del Pilar Rodriguez-Torres M, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. 2018;16(1):1–33.10.1186/s12951-018-0392-8Suche in Google Scholar PubMed PubMed Central
[316] Lyseng-Williamson KA, Keating GM. Ferric carboxymaltose: A review of its use in iron-deficiency anaemia. Drugs. 2009;69(6):739–56.10.2165/00003495-200969060-00007Suche in Google Scholar PubMed
[317] unbiasedscipod. unbiasedscipod; [Available from: https://www.unbiasedscipod.com/].Suche in Google Scholar
© 2021 Ahmed A. H. Abdellatif and Abdullah Fahad Alsowinea, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions
Artikel in diesem Heft
- Research Articles
- Improved impedance matching by multi-componential metal-hybridized rGO toward high performance of microwave absorption
- Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration
- Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries
- Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth
- Synergistic strengthening mechanism of copper matrix composite reinforced with nano-Al2O3 particles and micro-SiC whiskers
- Deformation mechanisms and plasticity of ultrafine-grained Al under complex stress state revealed by digital image correlation technique
- On the deformation-induced grain rotations in gradient nano-grained copper based on molecular dynamics simulations
- Removal of sulfate from aqueous solution using Mg–Al nano-layered double hydroxides synthesized under different dual solvent systems
- Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant
- Electrophoretic deposition of graphene on basalt fiber for composite applications
- Polyphenylene sulfide-coated wrench composites by nanopinning effect
- Thermal conductivity and thermoelectric properties in 3D macroscopic pure carbon nanotube materials
- An effective thermal conductivity and thermomechanical homogenization scheme for a multiscale Nb3Sn filaments
- Friction stir spot welding of AA5052 with additional carbon fiber-reinforced polymer composite interlayer
- Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating
- Quantum effects of gas flow in nanochannels
- An approach to effectively improve the interfacial bonding of nano-perfused composites by in situ growth of CNTs
- Effects of nano-modified polymer cement-based materials on the bending behavior of repaired concrete beams
- Effects of the combined usage of nanomaterials and steel fibres on the workability, compressive strength, and microstructure of ultra-high performance concrete
- One-pot solvothermal synthesis and characterization of highly stable nickel nanoparticles
- Comparative study on mechanisms for improving mechanical properties and microstructure of cement paste modified by different types of nanomaterials
- Effect of in situ graphene-doped nano-CeO2 on microstructure and electrical contact properties of Cu30Cr10W contacts
- The experimental study of CFRP interlayer of dissimilar joint AA7075-T651/Ti-6Al-4V alloys by friction stir spot welding on mechanical and microstructural properties
- Vibration analysis of a sandwich cylindrical shell in hygrothermal environment
- Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites
- Strong quadratic acousto-optic coupling in 1D multilayer phoxonic crystal cavity
- Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images
- Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes
- Xanthate-modified nanoTiO2 as a novel vulcanization accelerator enhancing mechanical and antibacterial properties of natural rubber
- Effect of steel fiber on impact resistance and durability of concrete containing nano-SiO2
- Ultrasound-enhanced biosynthesis of uniform ZnO nanorice using Swietenia macrophylla seed extract and its in vitro anticancer activity
- Temperature dependence of hardness prediction for high-temperature structural ceramics and their composites
- Study on the frequency of acoustic emission signal during crystal growth of salicylic acid
- Controllable modification of helical carbon nanotubes for high-performance microwave absorption
- Role of dry ozonization of basalt fibers on interfacial properties and fracture toughness of epoxy matrix composites
- Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface
- A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer
- Improving flexural strength of UHPC with sustainably synthesized graphene oxide
- The role of graphene/graphene oxide in cement hydration
- Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies
- Evaluation of the nanostructure of calcium silicate hydrate based on atomic force microscopy-infrared spectroscopy experiments
- Combined effects of nano-silica and silica fume on the mechanical behavior of recycled aggregate concrete
- Safety study of malapposition of the bio-corrodible nitrided iron stent in vivo
- Triethanolamine interface modification of crystallized ZnO nanospheres enabling fast photocatalytic hazard-free treatment of Cr(vi) ions
- Novel electrodes for precise and accurate droplet dispensing and splitting in digital microfluidics
- Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility
- Experimental and multiscale numerical investigations on low-velocity impact responses of syntactic foam composites reinforced with modified MWCNTs
- Comprehensive performance analysis and optimal design of smart light pole for cooperative vehicle infrastructure system
- Room temperature growth of ZnO with highly active exposed facets for photocatalytic application
- Influences of poling temperature and elongation ratio on PVDF-HFP piezoelectric films
- Large strain hardening of magnesium containing in situ nanoparticles
- Super stable water-based magnetic fluid as a dual-mode contrast agent
- Photocatalytic activity of biogenic zinc oxide nanoparticles: In vitro antimicrobial, biocompatibility, and molecular docking studies
- Hygrothermal environment effect on the critical buckling load of FGP microbeams with initial curvature integrated by CNT-reinforced skins considering the influence of thickness stretching
- Thermal aging behavior characteristics of asphalt binder modified by nano-stabilizer based on DSR and AFM
- Building effective core/shell polymer nanoparticles for epoxy composite toughening based on Hansen solubility parameters
- Structural characterization and nanoscale strain field analysis of α/β interface layer of a near α titanium alloy
- Optimization of thermal and hydrophobic properties of GO-doped epoxy nanocomposite coatings
- The properties of nano-CaCO3/nano-ZnO/SBR composite-modified asphalt
- Three-dimensional metallic carbon allotropes with superhardness
- Physical stability and rheological behavior of Pickering emulsions stabilized by protein–polysaccharide hybrid nanoconjugates
- Optimization of volume fraction and microstructure evolution during thermal deformation of nano-SiCp/Al–7Si composites
- Phase analysis and corrosion behavior of brazing Cu/Al dissimilar metal joint with BAl88Si filler metal
- High-efficiency nano polishing of steel materials
- On the rheological properties of multi-walled carbon nano-polyvinylpyrrolidone/silicon-based shear thickening fluid
- Fabrication of Ag/ZnO hollow nanospheres and cubic TiO2/ZnO heterojunction photocatalysts for RhB degradation
- Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application
- Investigation of the early-age performance and microstructure of nano-C–S–H blended cement-based materials
- Reduced graphene oxide coating on basalt fabric using electrophoretic deposition and its role in the mechanical and tribological performance of epoxy/basalt fiber composites
- Effect of nano-silica as cementitious materials-reducing admixtures on the workability, mechanical properties and durability of concrete
- Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion
- Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique
- A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications
- Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly
- Al 5251-based hybrid nanocomposite by FSP reinforced with graphene nanoplates and boron nitride nanoparticles: Microstructure, wear, and mechanical characterization
- Interlaminar fracture toughness properties of hybrid glass fiber-reinforced composite interlayered with carbon nanotube using electrospray deposition
- Microstructure and life prediction model of steel slag concrete under freezing-thawing environment
- Synthesis of biogenic silver nanoparticles from the seed coat waste of pistachio (Pistacia vera) and their effect on the growth of eggplant
- Study on adaptability of rheological index of nano-PUA-modified asphalt based on geometric parameters of parallel plate
- Preparation and adsorption properties of nano-graphene oxide/tourmaline composites
- A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers
- Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy
- Piezoresistive/piezoelectric intrinsic sensing properties of carbon nanotube cement-based smart composite and its electromechanical sensing mechanisms: A review
- Smart stimuli-responsive biofunctionalized niosomal nanocarriers for programmed release of bioactive compounds into cancer cells in vitro and in vivo
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study
- Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests
- Review Articles
- Advance on the dispersion treatment of graphene oxide and the graphene oxide modified cement-based materials
- Development of ionic liquid-based electroactive polymer composites using nanotechnology
- Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives
- Recent advances on the fabrication methods of nanocomposite yarn-based strain sensor
- Review on nanocomposites based on aerospace applications
- Overview of nanocellulose as additives in paper processing and paper products
- The frontiers of functionalized graphene-based nanocomposites as chemical sensors
- Material advancement in tissue-engineered nerve conduit
- Carbon nanostructure-based superhydrophobic surfaces and coatings
- Functionalized graphene-based nanocomposites for smart optoelectronic applications
- Interfacial technology for enhancement in steel fiber reinforced cementitious composite from nano to macroscale
- Metal nanoparticles and biomaterials: The multipronged approach for potential diabetic wound therapy
- Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application
- Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives
- Research progress on key problems of nanomaterials-modified geopolymer concrete
- Smart stimuli-responsive nanocarriers for the cancer therapy – nanomedicine
- An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment
- Effects of chemical modification and nanotechnology on wood properties
- Mechanisms, influencing factors, and applications of electrohydrodynamic jet printing
- Application of antiviral materials in textiles: A review
- Phase transformation and strengthening mechanisms of nanostructured high-entropy alloys
- Research progress on individual effect of graphene oxide in cement-based materials and its synergistic effect with other nanomaterials
- Catalytic defense against fungal pathogens using nanozymes
- A mini-review of three-dimensional network topological structure nanocomposites: Preparation and mechanical properties
- Mechanical properties and structural health monitoring performance of carbon nanotube-modified FRP composites: A review
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity
- Effects of alloying, heat treatment and nanoreinforcement on mechanical properties and damping performances of Cu–Al-based alloys: A review
- Recent progress in the synthesis and applications of vertically aligned carbon nanotube materials
- Thermal conductivity and dynamic viscosity of mono and hybrid organic- and synthetic-based nanofluids: A critical review
- Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth
- Layup sequence and interfacial bonding of additively manufactured polymeric composite: A brief review
- Quantum dots synthetization and future prospect applications
- Approved and marketed nanoparticles for disease targeting and applications in COVID-19
- Strategies for improving rechargeable lithium-ion batteries: From active materials to CO2 emissions

