Abstract
C24H23FeNO, monoclinic, P21/c (no. 14), a = 15.4240(10) Å, b = 10.2113(7) Å, c = 12.2776(7) Å, β = 105.808(2)°, V = 1860.6(2) Å3, Z = 4, Rgt(F) = 0.0564 wRref(F2) = 0.1804, T = 170 K.
The molecular structure is shown in the figure. Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif- file attached to this article.
1 Source of materials
To a solution of 1–ferrocenyl-3-(4-isopropylphenyl)-2-propen-1-one (3.58 g, 10 mmol) and tosylmethyl isocyanide (2.15 g, 11 mmol) in N,N-dimethylformamide (25 mL) was added potassium tert-butoxide (2.24 g, 20 mmol). The mixture was stirred at room temperature for 12 h, until the TLC indicated the reaction was completed. The mixture was diluted with brine, and then extracted with ethyl acetate (3 × 30 mL). The organic phase was washed with brine (30 mL), dried with anhydrous sodium sulphate, and then concentrated under pressure. The title compound was separated by silica-gel column chromatography with ethyl acetate-petroleum ether (25 %) gradient solvent system. The target product was obtained as a yellow solid. For crystal growth, the product was dissolved in a minimal amount of hot ethanol and slowly cooled to room temperature
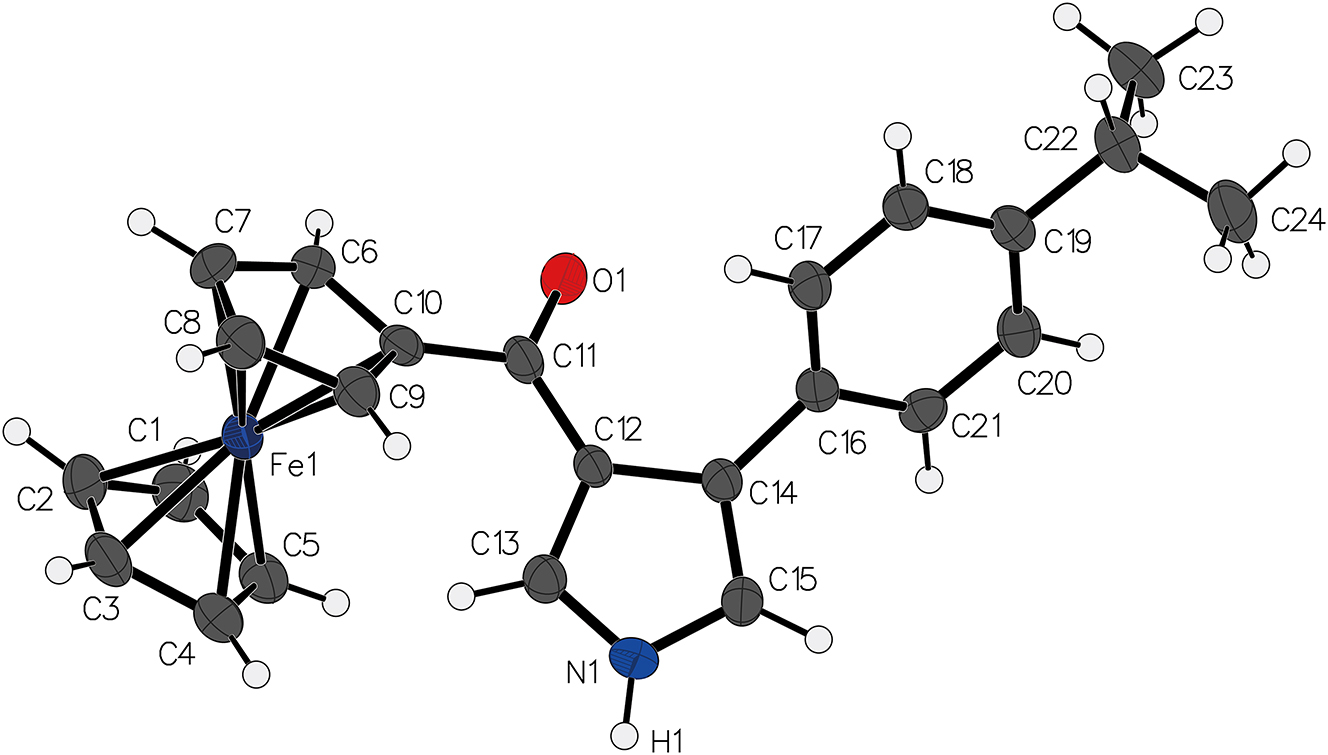
.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.09 × 0.05 × 0.04 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.82 mm−1 |
| Diffractometer, scan mode: | Bruker D8 VENTURE, φ and ω scans |
| θmax, completeness: | 26.4°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 13720, 3742, 0.093 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2,268 |
| N(param)refined: | 246 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
2 Experimental details
The crystal structure was determined using Direct Methods with the SHELXT program. 2 The initial solution was refined using full-matrix least squares on F2 with the SHELXL program, 3 implemented within the Olex2 software. 4 The hydrogen atoms were placed at their idealized positions based on standard geometrical constraints.
3 Comment
Ferrocene, consisting of a central iron atom sandwiched between two cyclopentadienyl rings, exhibits remarkable stability and symmetry, making it a model compound for understanding metal-organic interactions and the behavior of metallocenes. 5 Over the years, various functionalized ferrocene derivatives have been synthesized to explore their potential in a wide range of applications, including catalysis, materials science, and organic electronics. 6 , 7 , 8 , 9 , 10 Crystallographic studies of these compounds are particularly valuable as they provide detailed insights into the molecular arrangement, intermolecular interactions, and packing behavior within the solid state, which are critical for tailoring their properties for specific applications.
The structure of the title compound is characterized by a ferrocenyl moiety conjugated with a pyrrole core via a ketone bridge. The ferrocene unit exhibits an almost ideal sandwich structure, with the iron center symmetrically coordinated between the two cyclopentadienyl rings. 11 , 12 , 13 , 14 , 15 , 16 , 17 The Fe–C bond lengths range from 2.04 to 2.07 Å, consistent with previously reported ferrocenyl derivatives. The ketone (C=O) bond length is approximately 1.24 Å, indicative of a typical carbonyl functional group, while the pyrrole ring remains nearly planar, suggesting strong conjugation within the system.
The pyrrole core is functionalized at the 3-position with the ferrocenyl ketone group and at the 4-position with a para-substituted isopropylphenyl unit. The torsion angle between the pyrrole and phenyl rings is 44.3°. The isopropyl group is positioned in a staggered conformation, with the adjacent phenyl hydrogen atoms. The C–N bond in the pyrrole ring remains within the expected range 1.34–1.37 Å.
Intermolecular interactions in the crystal lattice are primarily governed by weak N–H⃛O interaction (N1–H1⃛O1), which contribute to the overall packing stability. The bond length of H1⃛O1 is 2.08 Å, and the angle of N1–H1⃛O1 is 138.0°. The torsion angle between the pyrrole and phenyl rings is 44.3°, and the torsion angle between the pyrrole and cyclopentadienyl rings is 52.4°. This contrasts with the corresponding dihedral angle in a similar structure, (4-(2-chlorophenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, 12 due to differences in the substituents and the intermolecular hydrogen bonding, which exert a significant influence on the molecular stereochemistry.
Acknowledgments
This work was financially supported by the projects of Social Development in Shaanxi Province Science and Technology Department (2023–YBSF-036), the 2023 research and development project of the Xianyang Science and Technology Bureau (L2023–ZDYF–SF-030), Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang city (2021QXNL–PT-0008), School-level Scientific and Technological Innovation Team for Design, Synthesis and Structural Modification of Drug Molecules (2024KCTD04).
References
1. Bruker. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2012.Suche in Google Scholar
2. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. Olex2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
5. Štěpnička, P. Forever Young: the First Seventy Years of Ferrocene. Dalton Trans. 2022, 51, 8085–8102; https://doi.org/10.1039/d2dt00903j.Suche in Google Scholar PubMed
6. Champaka, G.; Senthilkumar, K.; David, E.; Shanmugan, S.; Palanisami, N. Monomeric Zinc Ferrocene Carboxylate [Zn(FcCOO)(3,5-dmp)2Cl] Derived from 3, 5-dimethylpyrazole: Structural, Optical, Electrochemical and Antimicrobial Studies. J. Mol. Struct. 2021, 1228, 129749; https://doi.org/10.1016/j.molstruc.2020.129749.Suche in Google Scholar
7. Kang, T.; Kim, N.; Cheng, P. T.; Zhang, H.; Foo, K.; Engle, K. M. Nickel–Catalyzed 1,2–Carboamination of Alkenyl Alcohols. J. Am. Chem. Soc. 2021, 143, 13962–13970; https://doi.org/10.1021/jacs.1c07112.Suche in Google Scholar PubMed
8. Erb, W.; Wen, M.; Pierre Hurvois, J.; Mongin, F.; Halauko, Y. S.; Ivashkevich, O. A.; Matulis, V. E.; Roisnel, T. O–Isopropylferrocenesulfonate: Synthesis of Polysubstituted Derivatives and Electrochemical Study. Eur. J. Inorg. Chem. 2021, 2021, 3165–3176; https://doi.org/10.1002/ejic.202100448.Suche in Google Scholar
9. Ramírez-Gómez, A.; Gutiérrez-Hernández, A. I.; Alvarado-Castillo, M. A.; Toscano, R. A.; Ortega-Alfaro, M. C.; López-Cortés, J. G. Selenoamides as Powerful Scaffold to Build Imidazo[1,5-a]pyridines Using a Grinding Protocol. J. Organomet. Chem. 2020, 919, 121315; https://doi.org/10.1016/j.jorganchem.2020.121315.Suche in Google Scholar
10. Savani, C. J.; Roy, H.; Verma, S. K.; Vennapu, D. R.; Singh, V. K. Synthesis, Characterization and Evaluation of Novel Ferrocenylmethylamine Derivatives as Cytotoxic Agents. Appl. Organomet. Chem. 2021, 35, e6137; https://doi.org/10.1002/aoc.6137.Suche in Google Scholar
11. Wang, X. –L.; Li, H. –M. Crystal Structure of 4-(1H-Imidazol-1-yl) -6-pyrimidinylferrocene. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 141–143; https://doi.org/10.1515/ncrs-2015-0062.Suche in Google Scholar
12. Zhang, C.; Zhu, Z.; Tang, W.; Xu, X.; Liu, B. Crystal Structure of (4- (2-Chlorophenyl)-1H-Pyrrol-3-yl)(ferrocenyl) Methanone. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 375–377; https://doi.org/10.1515/ncrs-2024-0007.Suche in Google Scholar
13. Du, M.; Xu, X.; Wang, Q.; Tang, W.; Liu, B. Crystal Structure of (E)-3- (4-butoxyphenyl)acryloylferrocenee. Z. Kristallogr. N. Cryst. Struct. 2025, 240 (2), 261–262; https://doi.org/10.1515/ncrs-2024-0457.Suche in Google Scholar
14. Wang, Q.; Xu, X.; Du, M.; Tang, W.; Liu, B. Crystal Structure of Cinnamoyl Ferrocene. Z. Kristallogr. N. Cryst. Struct 2025, 240 (2), 257–259; https://doi.org/10.1515/ncrs-2024-0457.Suche in Google Scholar
15. Tian, H.; Chen, G.; Chen, S.; Tang, W.; Liu, B. Crystal Structure of Methyl 1-Phenyl-9H-Pyrido[3,4-b]indole-3-Carboxylate. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 1105–1107; https://doi.org/10.1515/ncrs-2024-0320.Suche in Google Scholar
16. Chamkin, A. A.; Krivykh, V. V.; Nikitin, O. M.; Kreindlin, A. Z.; Shteltser, N. A.; Dolgushin, F. M.; Artyushin, O. I.; Ikonnikov, N. S.; Borisov, Y. A.; Belousov, Y. A.; Ustynyuk, N. A. Direct Phosphination of Ferrocenium Ion with Tertiary Phosphines by the Mechanism of Oxidative Nucleophilic Substitution. Eur. J. Inorg. Chem. 2018, 2018, 4494–4504; https://doi.org/10.1002/ejic.201800961.Suche in Google Scholar
17. Shameem, M. A.; Esfandiarfard, K.; Öberg, E.; Ott, S.; Orthaber, A. Direct, Sequential, and Stereoselective Alkynylation of C,C–Dibromophosphaalkenes. Chem.–Eur. J. 2016, 22, 10614–10619; https://doi.org/10.1002/chem.201601955.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

