Abstract
C11H4F6O5S, triclinic, P1̄ (no. 2), a = 8.7997(4) Å, b = 12.6338(4) Å, c = 12.9238(5) Å, α = 109.674(3)°, β = 95.183(3)°, γ = 98.301(3)°; V = 1323.77(9) Å3, Z = 4, Rgt(F) = 0.0449, wRref(F2) = 0.1294, T = 298 K.
1 Source of material
The title compound, 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate, was synthesized according to the literature method with a slight modification. 1 , 2 7-Hydroxy-4-(trifluoromethyl)coumarin (0.81 g, 3.5 mmol) was dissolved in pyridine (30 mL) and the solution was cooled to 0 °C. Trifuoromethanesulfonic anhydride (0.85 mL, 3.85 mmol) was added dropwisely to the above cooled solution and stirred for 30 min. Then, the mixture was warmed to room temperature and stirred for another 6 h. The reaction progress was monitored by thin—layer chromatography (TLC). When the starting 7-hydroxy-4-(trifluoromethyl)coumarin disappeared completely on the TLC, the reaction was diluted by adding acetic ether (30 mL). The resulting organic phase was washed with the saturated NaCl (3 × 30 mL), 5 % aqueous HCl (5 × 30 mL), and saturated NaCl (2 × 30 mL) successively. The collected organic phase was dried over anhydrous Na2SO4. The solvent was removed, yielding the crude product. The crude product was purified by flash column chromatography on silica gel (200–300 mesh), using dichloromethane and hexane (1:1) as the eluent to give the title compound as a yellowish solid. The purified 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate (25 mg) was dissolved in a mixed solvent 8 mL (dichloromethane/ethanol, 1:1) in a 10 mL vial with parafilm sealed. Several holes (3–5) were made with a needle on the parafilm cover, allowing a slow evaporation of the mixed solvent. After several days evaporation of the solvent slowly at room temperature, regular shaped crystals formed at the bottom of the vial. Suitable crystals were selected for X-ray diffraction and the data was collected for structure solving.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.17 × 0.15 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 3.16 mm−1 |
| Diffractometer, scan mode: | Rigaku SuperNova, ω scans |
| θmax, completeness: | 71.4°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 9366, 5022, 0.024 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 4,390 |
| N(param)refined: | 472 |
| Programs: | Rigaku, 3 Shelx, 4 , 5 , 6 Olex2 7 , Diamond 8 |
2 Experimental details
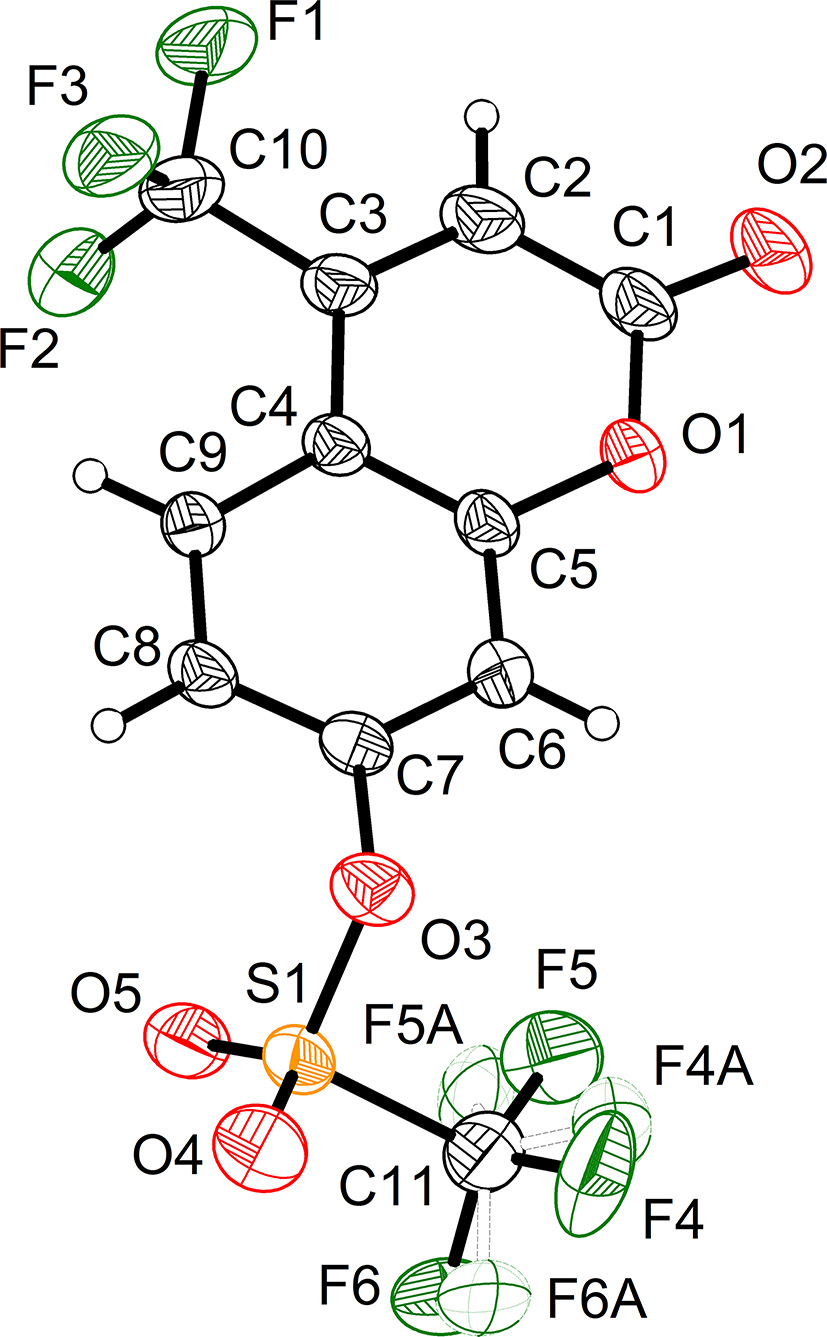
The structure of the 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate was solved using ShelXT and refined by ShelXL through the Olex2 interface. 4 , 5 Hydrogen atoms attached to C atoms were placed geometrically and refined using a riding model approximation, with d (C–H) = 0.93 Å(–CH). Uiso(H) = 1.2 Ueq(C) for CH. 4 There are two trifluoromethyl groups in the dye structure. The trifluoromethyl attaching to 4-position of coumarin is ordered. However, another one in 7-position of coumarin trifluoromethanesulfonate part is disordered, ascribing to its free rotation around the axis of C11–S2/C21–S1. Therefore, they are splitted into two parts around the axis of C11–S2/C21–S1, respectively. For C11/F4/F5/F6, the two parts accounted for 87 % and 13 %, respectively. The length of three C–F (F4/F5/F6) bond were restricted to be identical by SADI command. At the same time, the distances between F4/F5/F6 also was identically restrained by SADI instruction. RIGU command was used to restrain the whole group with the uncertainty 0.001. Furthermore, ISOR command was applied to F5 for its reasonalbe thermal ellipsoids. In case of another disordered trifluoromethyl (C11/F4/F5/F6), SAME instruction was used to the splitted two models with 48 % and 52 %, respectively. Each part was constrained with SIMU instruction. Specifically, F11 was also restricted by ISOR with 0.005 uncertainty for a reasonalbe thermal ellipsoid. The molecular graphics were drawn using the software DIAMOND with 50 % probability ellipsoids in Figure 1. 8 Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
3 Comment
The title compound, 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate, is a coumarin-containing fluorescent dye, which can emit bright blue fluorescence in the crystal state when irradiated with 365 nm UV light. Therefore, many fluorescent dyes with the build-in coumarin moiety were configured aiming for practical application in chemosenor, bioligical imaging, and dye-sensitized solar cells, etc. 9 , 10 , 11 , 12 , 13 In the title dye structure, there exists trifluoromethyl and trifluoromethanesulfonate serving as electron acceptor and donor, respectively. The build-in intramolecular electron “push-pull” effect, together with the π system of coumain, configure the specifically optical character of the title compound. Based on this molecule designing strategy, various highly fluorescent dyes can be developed toward the application in display device fabrication. 14 , 15 , 16
In the title crystal structure, the asymmetric unit contanins two crystallographically independent molecules. Both the bond lengths and the angles are in the expected ranges. The coumarin part (C1/C2/C3/C4/C5/C6/C7/C8/C9/O1/O2, C12/C13/C14/C15/C16/C17/C18/C19/C20/O7/O6) is planar with the root mean square error (RMSD) in distance estimated to be 0.020 Å. The trifluoromethyl (C10/F1/F2/F3 and C22/F7/F8/F9) attached to C3/C14 is the rigid framework of coumarin. And therefore, both the two trifluoromethyl are ordered in the crystal lattice, which is different from those attached to S1/S2. Due to the twisted character of sulfonate, the two trifluoromethyl (C11/F10/F11/F12 and C21/F4/F5/F6) are prone to be disordered. The bond lengths of S1–O3/S2–O8 are determined to be 1.574 and 1.569 Å, respectively, having the character of C–O single bonds. Therefore, the O3/O8 are also coplanar with the corresponding coumarin ring and donate their electrons to the coumarin. Vice versa, the C1–O2/C12–O6 are typical C–O double with the bond lengths 1.199 and 1.192 Å, which facilitates the electron withdrawing from the coumarin. Totally, the electron donor (O3/O8) and the electron acceptor (carbonyl group and trifluoromethyl), together with the π system of coumarin, jointly configured the intramolecular electron “push-pull” effect in a fluorescent dye system. By the construction of intramolecular electron “push-pull” system, specifically optical photophysical properties can be effectively developed. 17 , 18 , 19 , 20 The adjacent parallel molecules are packed by strong π⋯π interaction. The distance between the two coumarin planes (C1/C2/C3/C4/C5/C6/C7/C8/C9/O1/O2i1, C12/C13/C14/C15/C16/C17/C18/C19/C20/O7/O6i1, i1: x, y, z) is determined to be 3.543 Å and the parallel shift is 0.849 Å, corresponding to the parallel-displaced geometry. 21 Typical intermolecular C–H⋯O interactions are also established. Between the adjacent nonparallel molecules, six hydrogen bonds are found (C8–H8⋯O6i2, C19–H19⋯O2i3, C17–H17⋯O10i4, C6–H6⋯O9i2, C8–H8⋯O5i2, and C13–H13⋯O4i2, i2: −x, −y, 1−z; i3: −1−x, 1−y, 2−z; i4: 1−x, 1−y, 1−z) with the C⋯O contacts ranging from 3.262 to 3.689 Å, C–H⋯O angle ranging from 121.7 to 159.2°. The distances of C⋯O are well inside the interval of 3.0–4.0 Å and quoted by Desiraju. 22 And C–H⋯O angles are also in agreement with the above mentioned survey. 22 , 23 In addition, the fluoride atoms are also involved in the hydrogen bond construction. The typical H⋯F contacts include (C6–H6⋯F4i5, C2–H2⋯F12i6, C20–H20⋯F11i3, i5: −x, 1−y, 1−z; i6: 1−x, 1−y, 2−z). The H⋯F distances range from 3.2.841 to 2.939 Å. The hydrogen bond configured in the crystal lattice is significant to modify the emission behavior of the fluorescent dyes. 24 , 25 , 26 , 27 , 28 Therefore, reasonable design various inter- and/or intra-molecular hydrogen bonds into the crystal lattice is an effective way to control the ratio between the radiative and nonradiative channels. 29 , 30 Weaker intramolecular interactions is easy to generate highly emissive crystals. Stronger interactions can open more nonradiative channels in the excited states. 31 , 32 , 33 , 34
The driving force that packing the dye molecule parallel to each other along the axis c is based on the stronger π⋯π interaction. However, the parallel molecules are cohesive by the hydrogen bonds. The weaker intramolecular interactions derived from H–O/H–F contacts determine the highly emissive character of the title compound in crystal state. 35 With the establishing of the T-shaped π⋯π interaction between the parallel coumarin ring, a tight regular packing can be avoided and thus avoiding significant fluorescence quench. 36 , 37 , 38 , 39 , 40 Once the nonradiative channel of excited dye molecules is inhibited to a large extent, the proportion of emission channel will become prominent. 41 , 42 , 43 , 44 To the title compound, the molecular packing model, together with its intramolecular electron “push-pull” system, constructed the unique fluorescence emitting property in solid and crystal state. 45 , 46 , 47 In conclusion, the molecules are staggered layer by layer along with the axis a due to the π⋯π interaction. The hydrogen bonds based on H⋯O and H⋯F joint the layers side by side.
Acknowledgments
X. Z. Qin appreciates the finical supporting by the Basic Research and Applied Basic Research Project of Zhengzhou Science and Technology Bureau (No. zkz202204), Open Laboratory Project of Zhengzhou University of Technology (2024). The authors gratefully thanks Prof. Xiaochuan Li (HenanNormal University) for his assistance with the lab facilities supportation/chemical purification, single crystal X-ray diffraction data acquisition and providing his expertise on crystal structure solving.
References
1. Otsuka, Y.; Sasaki, A.; Teshima, T.; Yamada, K.; Yamamoto, T. Syntheses of D–Glucose Derivatives Emitting Blue Fluorescence Through Pd–Catalyzed C–N Coupling. Org. Lett. 2016, 18, 1338–1341; https://doi.org/10.1021/acs.orglett.6b00280. https://doi.org/10.1021/acs.orglett.6b00280.Search in Google Scholar PubMed
2. Gandioso, A.; Palau, M.; Bresoli-Obach, R.; Galindo, A.; Rovira, A.; Bosch, M.; Nonell, S.; Marchan, V. High Photostability in Nonconventional Coumarins with Far–Red/NIR Emission Through Azetidinyl Substitution. J. Org. Chem. 2018, 83, 11519–11531; https://doi.org/10.1021/acs.joc.8b01422. https://doi.org/10.1021/acs.joc.8b01422.Search in Google Scholar PubMed
3. Oxford Diffraction Ltd. CrysAlisPRO: Abingdon, Oxfordshire, England, 2006.Search in Google Scholar
4. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Sheldrick, G. M. A Short History of SHELX. Acta Crystallor. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
6. Sheldrick, G. M. SHELXTL – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
7. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
8. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
9. Zheng, X.; Liu, X.; Liu, L.; Li, X.; Jiang, S.; Niu, C.; Xie, P.; Liu, G.; Cao, Z.; Ren, Y.; Qin, Y.; Wang, J. Multi-Stimuli-Induced Mechanical Bending and Reversible Fluorescence Switching in a Single Organic Crystal. Angew. Chem., Int. Ed. 2022, 61, e202113073; https://doi.org/10.1002/anie.202113073.Search in Google Scholar PubMed
10. Li, X.; Han, Y.; Sun, S.; Shan, D.; Ma, X.; He, G.; Mergu, N.; Park, J.-S.; Kim, C.-H.; Son, Y.-A. A Diaminomaleonitrile-Appended BODIPY Chemosensor for the Selective Detection of Cu2+ via Oxidative Cyclization and Imaging in SiHa Cells and Zebrafish. Spectrochim. Acta, Part A 2020, 233, 118179; https://doi.org/10.1016/j.saa.2020.118179.Search in Google Scholar PubMed
11. Li, B.; Li, J.; Xu, B. S.; Zhao, L. L.; Yi, S. Z. Recent Advances in Smart Sensing Based on Excited-State Intramolecular Proton-Transfer (ESIPT)-inspired Emitters. Asian J. Org. Chem. 2025, 14, e202400572; https://doi.org/10.1002/ajoc.202400572.Search in Google Scholar
12. Li, X.; Liu, X.; Li, F. Configuration of Super-fast Cu2+-Responsive Chemosensor by Attaching Diaminomaleonitrile to BODIPY Scaffold for High-Contrast Fluorescence Imaging of Living Cells. Spectrochim. Acta, Part A 2024, 304, 123377; https://doi.org/10.1016/j.saa.2023.123377.Search in Google Scholar PubMed
13. Xie, P.; Zhou, Y.; Li, X.; Liu, X.; Liu, L.; Cao, Z.; Wang, J.; Zheng, X. Strong Dual-State Emission of Unsymmetrical and Symmetrical Thiazolothiazole-Bridged Imidazolium Salts. Chin. Chem. Lett. 2023, 34, 107582; https://doi.org/10.1016/j.cclet.2022.06.005.Search in Google Scholar
14. Li, X.; Han, Y.; Kim, M.; Son, Y. A. A BODIPY-Based Highly Emissive Dye with Thiophene-Based Branch Harvesting the Light. Mol. Cryst. Liq. Cryst. 2018, 662, 157–164; https://doi.org/10.1080/15421406.2018.1467613.Search in Google Scholar
15. Liu, Y.; Li, X.; Min, K. S.; Son, Y. A. Highly Fluorescent Response of 4-(2,5-Dimethylthiophen-3-Yl)-2-Hydroxyphenylbenzothiazole Toward BF3.Et2O and Zn2+. Mol. Cryst. Liq. Cryst. 2018, 662, 132–138; https://doi.org/10.1080/15421406.2018.1466531.Search in Google Scholar
16. Li, X.; Han, Y.; Min, K.; Son, Y. A. Configuration of White Light Emission by Courmarin and Naphthalimide. Mol. Cryst. Liq. Cryst. 2018, 660, 10–16; https://doi.org/10.1080/15421406.2018.1452861.Search in Google Scholar
17. Li, X.; Li, F.; Ji, G. A Fluorescent Turn-On Sensor Toward Multiple Heavy Metal Ions Based on Meso-Anisole Modified BODIPY Scaffold. J. Fluoresc. 2023, 33, 631–637; https://doi.org/10.1007/s10895-022-03110-1.Search in Google Scholar PubMed
18. Li, Y.; Liu, K.; Zhang, W.; Wang, Y.; Wang, B.; Wang, Y.; Li, X. Two 3D Ln(III)-MOFs Based on Phosphineoxide Ligand: Synthesis, Structure Luminescent and Photocatalytic Properties. J. Fluoresc. 2023, 33, 2119–2129; https://doi.org/10.1007/s10895-023-03218-y.Search in Google Scholar PubMed
19. Cao, Z.; Yang, F.; Wu, D.; Wu, L.; Liu, L.; Liu, G.; Li, X.; Zheng, X.; Zheng, X.; Qu, D. Supramolecular Aggregates Constructed by Pillar[5]arene-Based Host–Guest Interaction with Aggregation-Induced Emission. Polym. Chem. 2023, 14, 1318–1322; https://doi.org/10.1039/d3py00026e.Search in Google Scholar
20. Li, X.; Yao, C.; Jiang, W. Emission and Energy Transfer Investigation of Non-conjugated Total Carbon Configuration Between BODIPY and Naphthalimide. J. Chem. Sci. 2023, 135, 65; https://doi.org/10.1007/s12039-023-02181-2.Search in Google Scholar
21. Wang, J.; Gu, X.; Zhang, P.; Huang, X.; Zheng, X.; Chen, M.; Feng, H.; Kwok, R. T. K.; Lam, J. W. Y.; Tang, B. Z. Ionization and Anion-π+ Interaction: a New Strategy for Structural Design of Aggregation-Induced Emission Luminogens. J. Am. Chem. Soc. 2017, 139, 16974–16979; https://doi.org/10.1021/jacs.7b10150.Search in Google Scholar PubMed
22. Desiraju, G. R. The C–H⋯O Hydrogen Bond in Crystals: What is it? Acc. Chem. Res. 1991, 24, 290–296; https://doi.org/10.1021/ar00010a002.Search in Google Scholar
23. Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem., Int. Ed. 2002, 41, 48–76; https://doi.org/10.1002/1521-3773(20020104)41:1<48::aid-anie48>3.0.co;2-u.10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-USearch in Google Scholar
24. Li, X.; Qian, Q.; Jiang, W. Photo-Induced Fluorochromism of a Star-Shaped Photochromic Dye with 2,4-Dimethylthiazole Attaching to Triangle Terthiophene. J. Fluoresc. 2023, 33, 1907–1915; https://doi.org/10.1007/s10895-023-03196-1.Search in Google Scholar
25. Ji, G.; Hou, Q.; Jiang, W.; Li, X. Investigating the Properties of Double Triangle Terthiophene Configured Dumbbell-Like Photochromic Dye with Ethyne and 1,3-butadiene Bridge. J. Fluoresc. 2023, 33, 1495–1503; https://doi.org/10.1007/s10895-023-03171-w.Search in Google Scholar
26. Li, X.; Zou, Y.; Heo, G.; Son, Y. A. Emission Shift of an Imidazole Bridged Diethylaminocoumarin and Diphenyl. Mol. Cryst. Liq. Cryst. 2020, 704, 48–56; https://doi.org/10.1080/15421406.2020.1741801.Search in Google Scholar
27. Padghan, S. D.; Hu, J. W.; Wang, L. C.; Hsu, Y. H.; Chen, K. Y. Intermolecular C–H⋯O Hydrogen Bond–Induced H-Aggregates for Rapid, Real-Time Detection of Cyanide. Dyes Pigm. 2024, 235, 112567; https://doi.org/10.1016/j.dyepig.2024.112567.Search in Google Scholar
28. Li, X.; Tian, G.; Shao, D.; Xu, Y.; Wang, Y.; Ji, G.; Ryu, J.; Son, Y. A. A BODIPY Based Emission Signal Turn-on Probe Toward Multiple Heavy Metals. Mol. Cryst. Liq. Cryst. 2020, 706, 38–46; https://doi.org/10.1080/15421406.2020.1743436.Search in Google Scholar
29. Liu, Y.; Li, X.; Min, K. S.; Son, Y. A. Emission Behavior of Naphthalimide-Coumarin Cassette. Mol. Cryst. Liq. Cryst. 2018, 662, 139–146; https://doi.org/10.1080/15421406.2018.1466533.Search in Google Scholar
30. Kong, Y.; Liu, X.; Jiang, W.; Li, X. Photochromic Properties of Triangle Terthiophene- and Triphenylamine-configured Diarylethene Type Dye with Propeller-Like Conformation. Chem. Pap. 2025, 79, 2401–2409. https://doi.org/10.1007/s11696–025–03934–8.10.1007/s11696-025-03934-8Search in Google Scholar
31. Janiak, C. A Critical Account on π–π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc. Dalton. Trans. 2000, 21, 3885–3896; https://doi.org/10.1039/b003010o.Search in Google Scholar
32. Li, X.; Liao, M.; Sun, J.; Heo, G.; Son, Y. A. Thiophene Modulated BODIPY Dye as a Light Harvester. Mol. Cryst. Liq. Cryst. 2019, 679, 127–136; https://doi.org/10.1080/15421406.2019.1597557.Search in Google Scholar
33. Li, X.; Guo, X.; Chen, Y.; Cui, T.; Xing, L. Double 3-Ethyl-2,4-dimethylpyrrole Configured Fluorescent Dye with Fluorine-Boron as the Bridge. J. Fluoresc. 2021, 31, 1797–1803; https://doi.org/10.1007/s10895-021-02819-9.Search in Google Scholar PubMed
34. Liu, Y.; Li, X.; Kim, H.; Son, Y. A. Investigation of Fluorescent Optical Properties of Fluorine-boron Cored Dye. Mol. Cryst. Liq. Cryst. 2018, 677, 27–33; https://doi.org/10.1080/15421406.2019.1597508.Search in Google Scholar
35. Ji, G.; Hou, Q.; Zhang, J.; Li, X. Investigation of Triangle Terthiophene and Hydroxyphenylbenzothiazole Configured Fluorescent dye with a Triple Bond Bridge. J. Fluoresc. 2023, 33, 153–159; https://doi.org/10.1007/s10895-022-03049-3.Search in Google Scholar PubMed
36. Li, X.; Cai, Q.; Zhang, J.; Kim, H.; Son, Y. A. An “Electron Lock” Toward the Photochromic Activity of Phenylacetylene Appended Bisthienylethene. Mol. Cryst. Liq. Cryst. 2020, 706, 141–149; https://doi.org/10.1080/15421406.2020.1743450.Search in Google Scholar
37. Li, X.; Zhou, Q.; Heo, G.; Son, Y. A. 2,4–Dimethylpyrrole Configured Fluorine-Boron Complexes. Mol. Cryst. Liq. Cryst. 2018, 677, 34–41; https://doi.org/10.1080/15421406.2019.1597509.Search in Google Scholar
38. He, W.; Li, X.; Kim, H.; Son, Y. A. Shifting the Emission of Proton Transfer Fluorescence with Fluorine-Boron as the Rotation Lock. Mol. Cryst. Liq. Cryst. 2020, 704, 41–47; https://doi.org/10.1080/15421406.2020.1741800.Search in Google Scholar
39. Li, X.; Han, Y.; Kim, M. J.; Son, Y. A. Reversed Photochromism Reactivity of Malononitrile Attached Bisthienylthene. Mol. Cryst. Liq. Cryst. 2018, 662, 147–156; https://doi.org/10.1080/15421406.2018.1466534.Search in Google Scholar
40. Li, X.; Wang, Y.; Jia, C.; Kim, H.; Son, Y. A. Photochromic Reactivity Induced by Electron Distribution: Active or Inactive. Mol. Cryst. Liq. Cryst. 2019, 689, 83–91; https://doi.org/10.1080/15421406.2019.1597556.Search in Google Scholar
41. Ji, G.; Hou, Q.; Jiang, W.; Li, X. Investigating the Properties of Triangle Terthiophene and Triphenylamine Configured Propeller-Like Photochromic Dye with Ethyne Bridge. J. Fluoresc. 2025, 35, 933–941. https://doi.org/10.1007/s10895–023–03557-w.10.1007/s10895-023-03557-wSearch in Google Scholar PubMed
42. Li, X.; Liu, X. A Sensitive Probe of Meso-cyanophenyl Substituted BODIPY Derivative as Fluorescent Chemosensor for the Detection of Multiple Heavy Metal Ions. J. Fluoresc. 2025, 35, 1089–1098. https://doi.org/10.1007/s10895–024–03581–4.10.1007/s10895-024-03581-4Search in Google Scholar PubMed
43. Zheng, X.; Wang, G.; Liu, L.; Li, X.; Xie, P.; Fan, Y.; Cao, Z.; Niu, C.; Tian, D.; Xie, L. Hydrogen Bonding–Induced Multicolor and Thermochromic Emissions of Triphenylamines. Chem. -Eur. J. 2025, https://doi.org/10.1002/chem.202500643, In press.Search in Google Scholar PubMed
44. Qin, X.; Li, H.; Wang, Y.; Li, Y.; LI, X. Conjugated Iminodibenzyl Dyes Incorporating Phenolic Hydroxyl Group and Strong Electron Donating or Accepting Groups for Facilitating ESIPT and Proton Transfer in Six‑ or Seven‑Membered Cycles. J. Fluoresc. 2025, https://doi.org/10.1007/s10895-025-04285-z, In press.Search in Google Scholar PubMed
45. Liu, Y.; Li, X.; Sun, S.; Ji, G.; Son, Y.A. Crystal Structure of 2,7-Diiodo-1,3,6,8-tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N4. Z. Kristallogr. -New Cryst. Struct. 2020, 235, 371–372; https://doi.org/10.1515/ncrs-2019-0678.Search in Google Scholar
46. He, W.; Liu, Y.; Sun, S.; Ji, G.; Li, X. Crystal Structure of 2-bromo- 1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl) methylene)hydrazine), C14H15B2BrF4N4. Z. Kristallogr. -New Cryst. Struct. 2021, 236, 949–952; https://doi.org/10.1515/ncrs-2021-0163.Search in Google Scholar
47. Liu, Y.; Sun, S.; Ji, G.; Li, X.; Son, Y. A. Crystal structure of 2-phenylethynyl- 1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl) methylene)hydrazine), C22H20B2F4N4. Z. Kristallogr. -New Cryst. Struct 2021, 236, 749–752; https://doi.org/10.1515/ncrs-2021-0074.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

