Abstract
C16H14N2O2S, orthorhombic, Pca21 (no. 29), a = 11.5382(5) Å, b = 8.2480(5) Å, c = 15.1726(11) Å, V = 1,443.94(15) Å3, Z = 4, Rgt (F) = 0.0379, wRref (F 2) = 0.0990, T = 173 K.
1 Source of material
All reagents were sourced from commercial suppliers and used as received without additional purification. The synthesis of 3-methyl-1-p-tolyl-5-pyrazolone followed the method outlined by Jensen, 4 resulting in an 80.5 % yield and a melting point of 402–403 K o prepare the compound, 5 g of 3-methyl-1-p-tolyl-5-pyrazolone was dissolved in 100 mL of dioxane with gentle heating, after which 3 g of Ca(OH)2 was added. Next, 5 mL of 2-thiophenecarbonyl chloride was added dropwise while stirring over a period of 4 min. The mixture was then refluxed gently for 1 h, cooled, and poured into 420 mL of chilled 2.5 M HCl while stirring. A small amount of an ice-salt mixture was added, and stirring continued vigorously for another 30 min. The reaction mixture was then placed in a refrigerator until crystallization occurred. The resulting product was filtered, yielding yellow crystals with an 80.5 % yield at room temperature.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.18 × 0.14 × 0.12 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.23 mm−1 |
| Diffractometer, scan mode: θ max, completeness: |
Xcalibur, ω scan 29.4°, 100 % |
| N(hkl)measured, N(hkl)unique, R int: | 10820, 3977, 0.024 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2813 |
| N(param)refined: | 196 |
| Programs: | Rigaku, 1 SHELX 2 , 3 |
2 Experimental details
The H atom bonded to N2 was located in a difference Fourier map and refined freely. Other H atoms were placed in calculated positions, with C–H = 0.93 for phenyl and thiophene, 0.96 for methyl H atoms, and refined as riding, with U iso(H) = 1.2 U eq(C) for phenyl H and 1.5 U eq(C) for methyl H.
3 Discussion
Acylpyrazolones represent a fascinating group of β-diketone compounds that are commonly utilized for solvent extraction of metal ions, as materials for lasers, and as NMR shift reagents. 5 , 6 , 7 The metal complexes formed with these compounds have shown catalytic properties, biological activity, and improved luminescence. 8 Furthermore, they hold promise as antifungal agrochemicals, as well as for use in medicine for antiviral, antipyretic analgesic, and anti-inflammatory purposes. 9 , 10 Although there have been many studies on acylpyrazolones, only a limited number of 4-heterocyclic acylpyrazolone compounds have been reported so far. 11 , 12 Moreover, research has been restricted to those acylpyrazolones with alkyl or aryl substituents at the 4-positions. Only a few studies have involved heterocyclic substituents at the 4-positions. 13 In order to expand this field, the title compound was synthesized, and its crystal structure is reported herein.
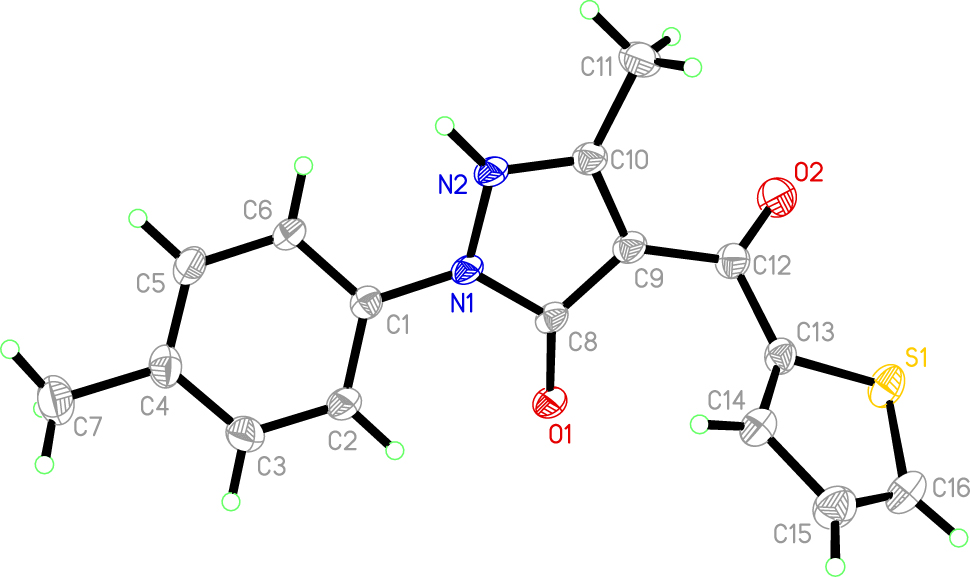
The structure of the title compound is shown in Figure. The molecule is distinctly non-planar. Moreover, the two C=O functionalities are oriented trans to each other not permitting an intramolecular hydrogen bond. The active hydrogen atom is attached to the nitrogen atom N2 and involved in an N–H⋯O hydrogen bond of N⋯O = 2.685 Å to the pyrazolone oxygen of a neighboring molecule. The p-tolylphenyl ring is slightly twisted by 17.11(6)° with respect to the pyrazolone ring, whereas the p-tolylphenyl and pyrazolone rings make dihedral angles of 52.06(5)° and 56.39(8)°, respectively, with the thenoyl ring. Atom O2 has a partial anionic character, as shown by the lengthening of the C=O bond [1.212(2) Å] relative to that normally found for carbonyl groups. All geometric parameters are in the expected ranges. 14 , 15
Acknowledgments
The authors thank the Science Foundation of Hebei Minzu Normal University (DR 2023001) and Research on the Key Technologies for Collaborative Development of Clean Energy Development and Ecological Protection in Chengde (No. 202305B100) support.
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2015.Search in Google Scholar
2. Sheldrick, G. M. SHELXL2018. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Jensen, B. S. The Synthesis of 1-Phenyl-3-Methyhl-4-Acyl-Pyrazolones-5. Acta Chem. Scand. 1959, 13, 1668–1670; https://doi.org/10.3891/acta.chem.scand.13-1668.Search in Google Scholar
5. Marchetti, F.; Pettinari, C.; Di Nicola, C.; Tombesi, A.; Pettinari, R. Coordination Chemistry of Pyrazolone-Based Ligands and Applications of Their Metal Complexes. Coord. Chem. Rev. 2019, 401, 213069; https://doi.org/10.1016/j.ccr.2019.213069.Search in Google Scholar
6. Wang, X. H.; Jia, D. Z.; Liang, Y. J.; Yan, S. L.; Ding, Y.; Chen, L. M.; Shi, Z.; Zeng, M. S.; Liu, G. F.; Fu, L. W. Lgf–YL-9 Induces Apoptosis in Human Epidermoid Carcinoma KB Cells and Multidrug Resistant KBv200 Cells via Reactive Oxygen Species-independent Mitochondrial Pathway. Cancer Lett. 2007, 249, 256–270; https://doi.org/10.1016/j.canlet.2006.09.008.Search in Google Scholar PubMed
7. El-Gaby, M. S.; Atalla, A. A.; Gaber, A. M.; Al-Wahab, K. A. Studies on Aminopyrazoles: Antibacterial Activity of Some Novel Pyrazol. Farmaco 2000, 55, 596–602; https://doi.org/10.1016/s0014-827x(00)00079-3.Search in Google Scholar PubMed
8. Timerbaev, A. R.; Rudnev, A. V.; Semenova, O.; Hartinger, C. G.; Keppler, B. K. Comparative Binding of Antitumor Indazolium [trans-Tetrachlorobi s(1H-indazole)Ruthenate(III)] to Serum Transport Proteins Assayed by Capillary Zone Electrophoresis. Anal. Biochem. 2005, 341, 326–333; https://doi.org/10.1016/j.ab.2005.03.020.Search in Google Scholar PubMed
9. Mukherjee, R. Coordination Chemistry with Pyrazole-Based Chelating Ligands: Molecular Structural Aspects. Coord. Chem. Rev. 2000, 203, 151–158; https://doi.org/10.1016/s0010-8545(99)00144-7.Search in Google Scholar
10. Raman, N.; Selvaganapathy, M. Pyrazolone Incorporating Amino Acid Metallointercalators as Effective DNA Targets: Synthesis and In Vitro Biocidal Evaluation. Inorg. Chem. Commun. 2013, 37, 114–120; https://doi.org/10.1016/j.inoche.2013.09.028.Search in Google Scholar
11. Sun, R. X.; Li, J. Z.; Zhang, H. Q.; Pang, X. Z.; Fu, Y. S.; Cui, J. Z. Synthesis, Crystal Structure, Characterization and DNA Binding Studies of Novel Copper (II) Complex Based on Amino Acid Schiff Base. Curr. Org. Chem. 2012, 16, 2868–2878; https://doi.org/10.2174/138527212804546912.Search in Google Scholar
12. Wu, Q.; Huang, Z.-Y.; Liu, Y.-X.; Zhang, H.-Q.; Jin, T.-Y.; Xue, Y.-N.; Liu, C. Crystal Structure of 1-(p-Tolylphenyl)-4-(2-furoyl)-3-methyl-1H-pyrazol-5-Ol, C16H14N2O3. Z. Kristallogr. New Cryst. Struct. 2024, 239, 327–329; https://doi.org/10.1515/ncrs-2024-0001.Search in Google Scholar
13. Wu, Q.; Zhang, W.-Y.; Zhang, Z.-M.; Zhang, H.-Q.; Jin, T.-Y. Crystal Structure of 1-(4-Chlorophenyl)-4-(2-furoyl)-3-phenyl-1H-pyrazol-5-ol, C20H13ClN2O. Z. Kristallogr. New Cryst. Struct. 2024, 239, 565–567; https://doi.org/10.1515/ncrs-2024-0097.Search in Google Scholar
14. Ding, Y.-J.; Zhao, C.-X.; Pei, C.-Yu.; Wen, G.-X. Synthesis, Properties and Crystal Structure of a Novel Ni(II) Complex Derived from a 4-Heterocyclic Acylpyrazolone. Z. Naturforsch. B. 2012, 67, 204–208; https://doi.org/10.5560/znb.2012.67b0204.Search in Google Scholar
15. Holzer, W.; Mereiter, K.; Plagens, B. 4-Acyl-5-methyl-2-phenylpyrazolones: NMR and X-Ray Structure Investigations. Heterocycles 1999, 50, 799; https://doi.org/10.3987/com-98-s(h)74.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

