Abstract
C30H52BBrCuN6, monoclinic, P21/n (no. 14), a = 9.4265(3) Å, b = 17.4945(5) Å, c = 20.2269(5) Å, β = 99.926(3)°, V = 3285.73(17) Å3, Z = 4, R gt(F) = 0.0387, wR ref(F 2) = 0.1055, T = 184 K.
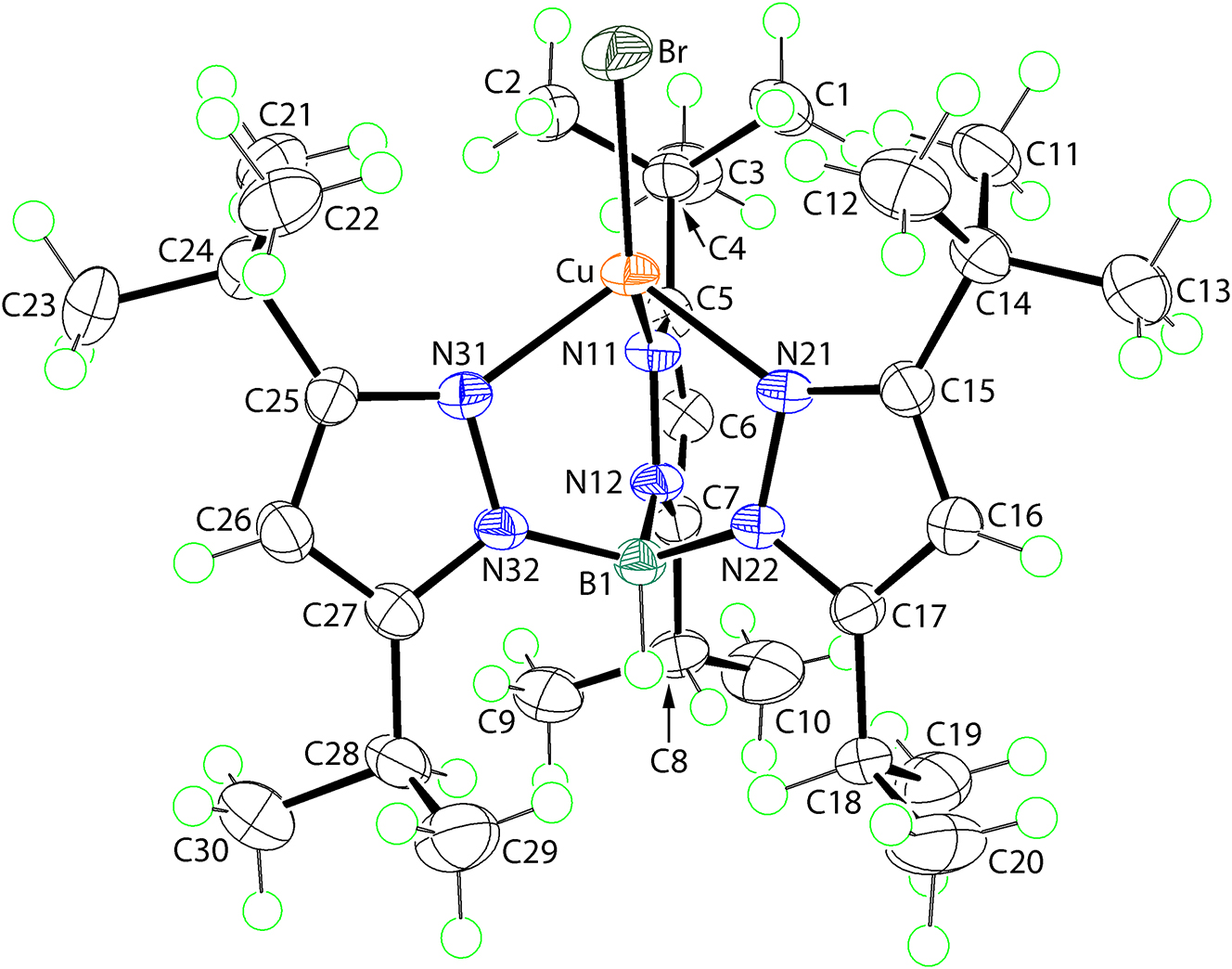
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red prism |
| Size: | 0.27 × 0.16 × 0.06 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.91 mm−1 |
| Diffractometer, scan mode: | Rigaku XtaLAB P200, φ and ω scan |
| θ max, completeness: | 27.5°, 100 % |
| N(hkl)measured , N(hkl)unique, R int: | 28794, 7526, 0.066 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 6,316 |
| N(param)refined: | 367 |
| Programs: | CrysAlisPRO, 1 IL MILIONE, 2 SHELX, 3 WinGx 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | U iso*/U eq | |
|---|---|---|---|---|
| Cu | 0.71437 (3) | 0.26216 (2) | 0.79844 (2) | 0.01843 (8) |
| Br | 0.74312 (3) | 0.25603 (2) | 0.91409 (2) | 0.03550 (9) |
| N11 | 0.83958 (17) | 0.26421 (9) | 0.73195 (8) | 0.0184 (3) |
| N12 | 0.76670 (18) | 0.26602 (9) | 0.66713 (8) | 0.0177 (3) |
| N21 | 0.59343 (17) | 0.17396 (9) | 0.74948 (7) | 0.0182 (3) |
| N22 | 0.54517 (16) | 0.19462 (9) | 0.68359 (7) | 0.0174 (3) |
| N31 | 0.59999 (17) | 0.35536 (9) | 0.75527 (8) | 0.0187 (3) |
| N32 | 0.55159 (17) | 0.34082 (9) | 0.68818 (7) | 0.0176 (3) |
| C1 | 1.0822 (3) | 0.18600 (13) | 0.83124 (12) | 0.0328 (5) |
| H1A | 1.157305 | 0.184768 | 0.871306 | 0.049* |
| H1B | 1.092839 | 0.141586 | 0.802967 | 0.049* |
| H1C | 0.987271 | 0.184658 | 0.844720 | 0.049* |
| C2 | 1.0824 (2) | 0.32889 (13) | 0.83631 (11) | 0.0304 (5) |
| H2A | 1.155017 | 0.325744 | 0.877195 | 0.046* |
| H2B | 0.986046 | 0.329368 | 0.848350 | 0.046* |
| H2C | 1.096907 | 0.375890 | 0.811970 | 0.046* |
| C3 | 1.2465 (2) | 0.26155 (15) | 0.77104 (13) | 0.0358 (6) |
| H3A | 1.320996 | 0.260104 | 0.811329 | 0.054* |
| H3B | 1.256515 | 0.308591 | 0.745942 | 0.054* |
| H3C | 1.257359 | 0.217194 | 0.742742 | 0.054* |
| C4 | 1.0968 (2) | 0.25960 (11) | 0.79163 (11) | 0.0220 (4) |
| C5 | 0.9813 (2) | 0.26162 (10) | 0.72933 (10) | 0.0187 (4) |
| C6 | 0.9983 (2) | 0.26088 (11) | 0.66252 (10) | 0.0216 (4) |
| H6 | 1.086807 | 0.258555 | 0.646160 | 0.026* |
| C7 | 0.8614 (2) | 0.26418 (11) | 0.62417 (10) | 0.0193 (4) |
| C8 | 0.8165 (2) | 0.26999 (12) | 0.54946 (10) | 0.0230 (4) |
| H8 | 0.717645 | 0.247792 | 0.537401 | 0.028* |
| C9 | 0.8090 (3) | 0.35395 (14) | 0.52819 (11) | 0.0414 (6) |
| H9A | 0.774280 | 0.357488 | 0.479751 | 0.062* |
| H9B | 0.905103 | 0.376812 | 0.538982 | 0.062* |
| H9C | 0.742759 | 0.381370 | 0.552211 | 0.062* |
| C10 | 0.9160 (3) | 0.22534 (18) | 0.51211 (12) | 0.0445 (6) |
| H10A | 0.884778 | 0.231809 | 0.463659 | 0.067* |
| H10B | 0.912798 | 0.171034 | 0.523627 | 0.067* |
| H10C | 1.014702 | 0.244377 | 0.524933 | 0.067* |
| C11 | 0.7292 (2) | 0.05594 (13) | 0.84985 (11) | 0.0301 (5) |
| H11A | 0.746570 | 0.023995 | 0.890233 | 0.045* |
| H11B | 0.764847 | 0.107780 | 0.861072 | 0.045* |
| H11C | 0.779815 | 0.034198 | 0.815774 | 0.045* |
| C12 | 0.4860 (3) | 0.09357 (15) | 0.87410 (11) | 0.0350 (5) |
| H12A | 0.503322 | 0.062951 | 0.915276 | 0.052* |
| H12B | 0.382690 | 0.094049 | 0.855889 | 0.052* |
| H12C | 0.519407 | 0.146003 | 0.884238 | 0.052* |
| C13 | 0.5158 (3) | −0.02372 (14) | 0.80835 (12) | 0.0432 (6) |
| H13A | 0.535820 | −0.053597 | 0.849892 | 0.065* |
| H13B | 0.566445 | −0.046485 | 0.774736 | 0.065* |
| H13C | 0.411967 | −0.023724 | 0.791367 | 0.065* |
| C14 | 0.5679 (2) | 0.05884 (12) | 0.82252 (10) | 0.0241 (4) |
| C15 | 0.5385 (2) | 0.10408 (11) | 0.75763 (10) | 0.0195 (4) |
| C16 | 0.4538 (2) | 0.08107 (11) | 0.69747 (10) | 0.0224 (4) |
| H16 | 0.401391 | 0.034638 | 0.689555 | 0.027* |
| C17 | 0.4610 (2) | 0.13878 (11) | 0.65168 (9) | 0.0196 (4) |
| C18 | 0.3981 (2) | 0.14069 (11) | 0.57794 (10) | 0.0224 (4) |
| H18 | 0.380371 | 0.195314 | 0.564432 | 0.027* |
| C19 | 0.5040 (3) | 0.10722 (14) | 0.53643 (11) | 0.0359 (5) |
| H19A | 0.465212 | 0.113268 | 0.488565 | 0.054* |
| H19B | 0.518552 | 0.052798 | 0.546932 | 0.054* |
| H19C | 0.596302 | 0.134143 | 0.547183 | 0.054* |
| C20 | 0.2551 (3) | 0.09876 (17) | 0.56390 (13) | 0.0434 (6) |
| H20A | 0.211058 | 0.105991 | 0.516778 | 0.065* |
| H20B | 0.190828 | 0.119087 | 0.592904 | 0.065* |
| H20C | 0.271120 | 0.044123 | 0.572947 | 0.065* |
| C21 | 0.7392 (2) | 0.46084 (14) | 0.86510 (11) | 0.0319 (5) |
| H21A | 0.756482 | 0.486667 | 0.908745 | 0.048* |
| H21B | 0.794015 | 0.486476 | 0.834540 | 0.048* |
| H21C | 0.770051 | 0.407400 | 0.870839 | 0.048* |
| C22 | 0.4894 (3) | 0.42670 (14) | 0.88324 (11) | 0.0332 (5) |
| H22A | 0.504334 | 0.454309 | 0.926039 | 0.050* |
| H22B | 0.519119 | 0.373322 | 0.891071 | 0.050* |
| H22C | 0.387168 | 0.428607 | 0.863029 | 0.050* |
| C23 | 0.5360 (3) | 0.54880 (13) | 0.82703 (13) | 0.0420 (6) |
| H23A | 0.562126 | 0.575349 | 0.870068 | 0.063* |
| H23B | 0.431812 | 0.552736 | 0.811655 | 0.063* |
| H23C | 0.586684 | 0.572282 | 0.793774 | 0.063* |
| C24 | 0.5790 (2) | 0.46391 (12) | 0.83581 (10) | 0.0250 (4) |
| C25 | 0.5483 (2) | 0.42466 (11) | 0.76805 (10) | 0.0203 (4) |
| C26 | 0.4655 (2) | 0.45344 (12) | 0.70944 (10) | 0.0248 (4) |
| H26 | 0.415319 | 0.500761 | 0.704695 | 0.030* |
| C27 | 0.4705 (2) | 0.40018 (11) | 0.65986 (10) | 0.0210 (4) |
| C28 | 0.4004 (2) | 0.40367 (12) | 0.58762 (10) | 0.0263 (5) |
| H28 | 0.466386 | 0.379293 | 0.560039 | 0.032* |
| C29 | 0.2605 (3) | 0.36026 (15) | 0.57644 (12) | 0.0385 (6) |
| H29A | 0.217786 | 0.362000 | 0.528731 | 0.058* |
| H29B | 0.194201 | 0.383530 | 0.602967 | 0.058* |
| H29C | 0.278532 | 0.306974 | 0.590331 | 0.058* |
| C30 | 0.3756 (3) | 0.48740 (15) | 0.56474 (13) | 0.0449 (6) |
| H30A | 0.337693 | 0.488824 | 0.516498 | 0.067* |
| H30B | 0.467091 | 0.515266 | 0.573885 | 0.067* |
| H30C | 0.306342 | 0.511329 | 0.589304 | 0.067* |
| B1 | 0.6000 (2) | 0.26838 (12) | 0.65464 (11) | 0.0174 (4) |
| H1 | 0.558873 | 0.270823 | 0.599318 | 0.021* |
1 Source of material
A solution of K{HB(3-tBu-5-iPrpz)3} (potassium{hydridotris(3-tert-butyl-5-isopropylpyrazol-1-yl)borate}; 373.1 mg, 0.682 mmol) 5 in dichloromethane (20 mL) was added slowly to a solution of CuBr2 (183.4 mg, 0.821 mmol) in methanol (20 mL). After the mixture was stirred for 90 min, the solvent was evaporated under vacuum, and the resulting solid was washed with methanol (15 mL). A brown powder was obtained. Red-brown crystals were obtained by the slow evaporation of a saturated dichloromethane/n-heptane solution of the product held at room temperature and characterised as [Cu(Br){HB(3-tBu-5-iPrpz)3}]·1/2H2O (250.0 mg, 0.379 mmol, 56 %). Anhydrous, single crystals suitable for X-ray crystallography were also obtained by the slow evaporation of a saturated dichloromethane/n-heptane solution of the recrystallised material held at room temperature. Anal. calcd. for C30H52BBrCuN6·1/2H2O (bulk material): C, 54.59; H, 8.09; N, 12.73 %. Found: C, 54.33; H, 8.16; N, 12.77 %. IR (KBr, cm−1): 3348 m ν(O–H), 2965 s ν(C–H), 2932 m ν(C–H), 2868 m ν(C–H), 2566 m ν(B–H), 1533 m ν(C=N). Far–IR (CsI, cm−1): 287 ν(Cu–Cl). UV–Vis [CH2Cl2, λ max, nm (ε, M−1 cm−1)] 325 (1,640), 387 (790), 469 (800), 1048 (100).
2 Experimental details
The B- and C-bound H atoms were geometrically placed (B–H = 1.12 Å and C–H = 0.95–1.00 Å) and refined as riding with U iso(H) = 1.2–1.5U eq(B, C). Owing to poor agreement, two reflections, i.e. (−11 1 2) and (−11 2 6), were omitted from the final cycles of refinement.
3 Discussion
The coordination chemistry of strategically substituted hydrotris(pyrazolyl)borate ligands has proven to be a very productive area of research to control the secondary coordination sphere about the metal centre. 5 , 6 Herein, is reported a bromido copper(II) complex, [Cu(Br){HB(3-tBu-5-iPrpz)3}], (I), where the copper(II) centre is ligated by a highly sterically hindered hydridotris(pyrazolyl)borate ligand; [HB(3-tBu-5-iPrpz)3]– is the hydridotris(3-tert-butyl-5-isopropylpyrazol-1-yl)borate anion. Recently, related structures with the same ligand were reported, i.e. the three-coordinate thallium(I) complex [Tl{HB(3-tBu-5-iPrpz)3}], 7 the four-coordinate chlorido copper(II) complex, [Cu(Cl){HB(3-tBu-5-iPrpz)3}] 8 and the four-coordinate hydroxido copper(II) complex [Cu(OH){HB(3-tBu-5-iPrpz)3}]. 5
The reaction of K{HB(3-tBu-5-iPrpz)3} with CuBr2 led to the title complex, (I), in 58 % yield. The IR spectrum shows the B–H stretching band at 2566 cm−1, which is slightly shifted from the absorption band at 2562 cm−1 for [Tl{HB(3-tBu-5-iPrpz)3}] 7 and signficantly from that at 2469 cm−1 for the uncoordinated anion, K{HB(3-tBu-5-iPrpz)3}. 8 In the far–IR spectrum, (I) shows a characteristic band at 287 cm−1, assignable to Cu–Br stretching. This band was also clearly shifted from that at 359 cm−1 for [Cu(Cl){HB(3-tBu-5-iPrpz)3}], which was assigned to Cu–Cl stretching. 8 The value of the ν(Cu–Cl)/ν(Cu–Br) ratio (= 1.25) is consistent with the calculated reduced mass (1.25). The d-d transition band was observed at 1048 nm (ε = 100 M−1 cm−1), which is almost the same energy to 1009 nm (ε = 50 M−1 cm−1) for [Cu(Cl){HB(3-tBu-5-iPrpz)3}]. 8 The red-shift in the UV–Vis spectra arises from the ground state change from d(x2-y2) to d(z2). 6 , 8
The molecular structure of (I) is shown in the figure (70 % probability ellipsoids) and features a tridentate [HB(3-tBu-5-iPrpz)3]− anion coordinated to copper(II) which is also connected to a bromido ligand. The tridentate ligand coordinates in a fashion so that the t-butyl groups are orientated towards the copper(II) atom. The Cu–N bond lengths are not equivalent with Cu–N11 of 1.9365(17) Å being significantly shorter than those for Cu–N21 and Cu–N31 of 2.0670(16) and 2.0636(16) Å, respectively. With respect to the bromido atom, the N11 atom subtends the widest angle at the copper(II) centre, i.e. 136.45(5)∘, compared with the N21, N31–Cu–Br angles of 114.15(4) and 115.25(4)∘, respectively. It is proposed that this feature of the molecular structure is related to the presence of intramolecular methyl–C–H⃛Br interactions which are less than the sum of the van der Waals radii and are directional. These interactions involve two t-butyl methyl groups derived from two pyrazolyl residues, i.e. containing the coordinating N21 and N31 atoms that form the longer Cu–N bonds [geometric parameters: C11–H11⃛Br: H11b⃛Br = 2.83 Å and angle at H11b = 153°; C12–H12c⃛Br: H12c⃛Br = 2.84 Å and angle at H12c = 151°; C21–H21c⃛Br: H21c⃛Br = 2.81 Å and angle at H21c = 153°; C22–H22b⃛Br: H22b⃛Br = 2.92 Å and angle at H22b = 151°]. It is the presence of the intramolecular methyl–C–H⃛Br interactions that draws the N21- and N31-containing 3-tBu-5-iPrpz residues away from the copper(II) centre, thereby elongating the Cu–N21, N31 bonds and widening the N11–Cu–Br angle.
The four-coordinate geometry about the copper(II) atom exhibits a wide range of angles, spanning 91.61(6)∘, for N11–Cu–N31, to 136.45(5)∘, for N11–Cu–Br. From the equation, 10 τ 4 = [360 – (α + β)/141], where α and β are the two widest angles subtended at the copper(II) centre, an indication of the coordination geometry may be obtained. The value calculated for (I) is 0.77 and compares with 0.85 for a trigonal-pyramidal geometry and 0.64 for a see-saw geometry; a value of 1.00 corresponds to an ideal tetrahedral geometry. 10
The Cu–Br bond length in (I) is 2.3105(3) Å which compares to 2.2891(4) Å in the structure of [Cu(Br){HB(3,5-iPr2pz)3}]. 11 The reduction of the Cu–Br bond length in the all i-propyl molecule reflects the lack of signifcant intramolecular C–H⃛Br contacts described above for (I).
Being surrounded by hydrogen-rich regions and with the bromide atom engaged in four close intramolecular methyl–C–H⃛Br interactions, see above, the molecule in (I) does not form any directional intermolecular interactions in the crystal; a similiar conclusion was made for the isostructural chlorido complex. 8
These observations are confirmed by the calculation of the Hirshfeld surface contacts and two-dimensional fingerprint plots which were conducted with CrystalExplorer 12 using standard protocols. 13 This analysis shows there are only four types of surface contacts in the crystal of (I), namely, and in order of significance, H⃛H (84.5 %) > CH/H⃛C (5.8 %) > Br⃛H/H⃛Br (4.9 %) > N⃛H/H⃛N (4.7 %). Similiar calculations were performed on the isostructural chlorido species. 8 In this crystal, the contribution from H⃛H contacts increased to 85.4 % cf. (I), with small, concomittant decreases in the C⃛H/H⃛C (5.6 %), Cl⃛H/H⃛Cl (4.4 %) and N⃛H/H⃛N (4.5 %) surface contacts. Calculations were also performed on the all i-propyl/bromido complex, [Cu(Br){HB(3,5-iPr2pz)3}]. 11 As expected with the decrease percentage content of hydrogen in the latter complex, there is a decrease in the contribution of H⃛H contacts to the surface, i.e. 80.2 %. This decrease is compensated by increases in the C⃛H/H⃛C (6.9 %), Br⃛H/H⃛Br (6.7 %) and N⃛H/H⃛N (6.2 %) surface contacts.
Acknowledgments
This research was supported by the Joint Usage/Research Centre for Catalysis and the Koyanagi Foundation.
-
Conflict of interest: The authors declare no conflicts of interest.
-
Research funding: This study was financially supported by the Joint Usage/Research Centre for Catalysis (Proposals 22DS0143, 23DS0198 and 24ES0584).
-
Author contribution: All authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
References
1. Rigaku Oxford Diffraction. CrysAlis PRO; Rigaku Corporation: Oxford, UK, 2021.Search in Google Scholar
2. Burla, M. C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G. L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL Milione: a Suite of Computer Programs for Crystal Structure Solution of Proteins. J. Appl. Cryst. 2007, 40, 609–613. https://doi.org/10.1107/S0021889807010941.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/S2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Farrugia, L. J. WinGX and Ortep for Windows: an Update. J. Appl. Cryst. 2012, 45, 849–854. https://doi.org/10.1107/S0021889812029111.Search in Google Scholar
5. Fujisawa, K.; Kobayashi, T.; Fujita, K.; Kitajima, N.; Moro-oka, Y.; Miyashita, Y.; Yamada, Y.; Okamoto, K. Mononuclear Copper(II) Hydroxo Complex: Structural Effect of a 3-position of Tris(pyrazolyl)borates. Bull. Chem. Soc. Jpn. 2000, 73, 1797–1804. https://doi.org/10.1246/bcsj.73.1797.Search in Google Scholar
6. Fujisawa, K.; Shimizu, D.; Tiekink, E. R. T. Crystal Structure of Chlorido {hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato }copper(II).. Z. Kristallogr. – New Cryst. Struct. 2021, 236, 135–138. https://doi.org/10.1515/ncrs-2020–0410.10.1515/ncrs-2020-0410Search in Google Scholar
7. Fujisawa, K.; Shimizu, D.; Tiekink, E. R. T. Crystal Structure of {hydridotris[3-(t-butyl)- 5-isopropylpyrazol-1-yl-κN3]borato}thallium(I). Z. Kristallogr. New Cryst. Struct. 2021, 236, 169–172. https://doi.org/10.1515/ncrs-2020–0405.10.1515/ncrs-2020-0405Search in Google Scholar
8. Fujisawa, K.; Tada, N.; Ishikawa, Y.; Higashimura, H.; Miyashita, Y.; Okamoto, K. The Most Hindered Hydrotris(pyrazolyl)borate Ligand, X-ray Structure of Chlorocopper(II) Complex: [Cu(Cl){HB(3-Ad-5-Pripz)3}] as Compared with [Cu(Cl){HB(3-But-5-Pripz)3}]. Inorg. Chem. Commun. 2004, 7, 209–212; https://doi.org/10.1016/j.inoche.2003.10.030.Search in Google Scholar
9. Imai, S.; Fujisawa, K.; Kobayashi, T.; Shirasawa, N.; Fujii, H.; Yoshimura, T.; Kitajima, N.; Moro-oka, Y. 63Cu NMR Study of Copper(I) Carbonyl Complexes with Various Hydrotris(pyrazolyl)borates: Correlation between 63Cu Chemical Shifts and CO Stretching Vibrations. Inorg. Chem. 1998, 37, 3066–3070. https://doi.org/10.1021/ic970138r.Search in Google Scholar
10. Yang, L.; Powell, D. R.; Houser, R. P. Structural Variation in Copper(I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, τ4. Dalton Trans. 2007, 955–964. https://doi.org/10.1039/B617136B.Search in Google Scholar PubMed
11. Marx, M.; Frauendorf, H.; Spannenberg, A.; Neumann, H.; Beller, M. Revisiting Reduction of CO2 to Oxalate with First-Row Transition Metals: Irreproducibility, Ambiguous Analysis, and Conflicting Reactivity. JACS Au 2022, 2, 731–744. https://doi.org/10.1021/jacsau.2c00005.Search in Google Scholar PubMed PubMed Central
12. Spackman, P. R.; Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Jayatilaka, D.; Spackman, M. A. CrystalExplorer: a Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. https://doi.org/10.1107/S1600576721002910.Search in Google Scholar PubMed PubMed Central
13. Tan, S. L.; Jotani, M. M.; Tiekink, E. R. T. Utilizing Hirshfeld Surface Calculations, Non-covalent Interaction (NCI) Plots and the Calculation of Interaction Energies in the Analysis of Molecular Packing. Acta Crystallogr. 2019, E75, 308–318. https://doi.org/10.1107/S2056989019001129.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

