Abstract
C22H20F3NO2, monoclinic, P21/c (no. 14), a = 18.2286(3) Å, b = 15.0613(3) Å, c = 6.4904(1) Å, β = 94.082(2)°, V = 1777.40(5) Å3, Z = 4, Rgt(F) = 0.0388, wRref(F2) = 0.1082, T = 293 K.
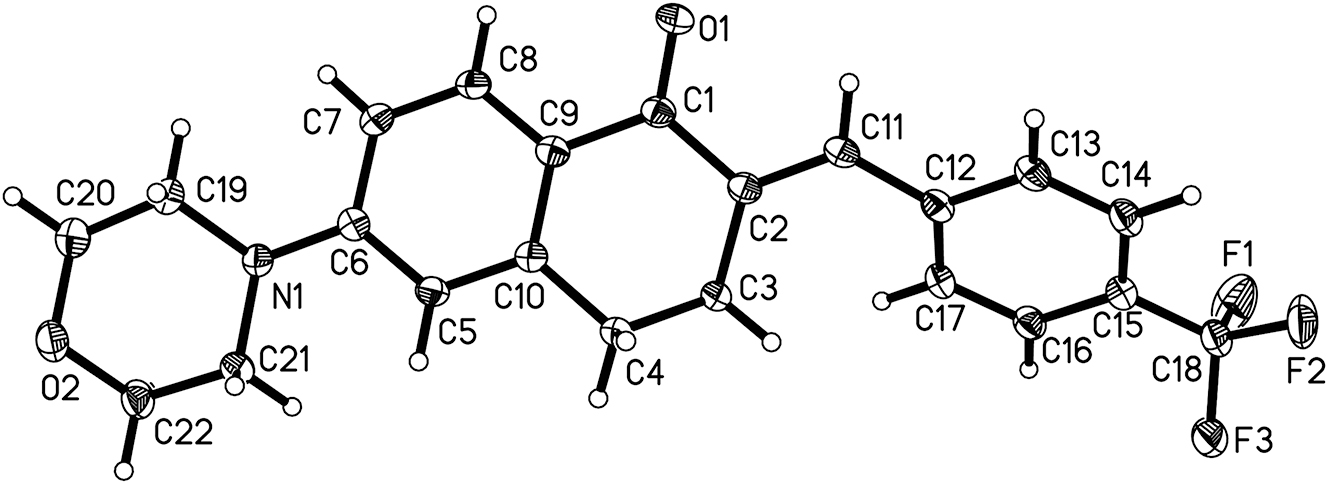
The crystal structure is shown in Figure 1. Displacement ellipsoids are drawn at the 50 % probability level.
Table 1 contains details on crystal structure and measurement conditions. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif- file attached to this article.
Data collection and handling.
| Crystal: | Clear light colourless block |
| Size: | 0.15 × 0.12 × 0.10 mm |
| Wavelength: | CuKα radiation (1.54178 Å) |
| μ: | 0.96 mm−1 |
| Diffractometer, scan mode: | Rigaku, φ and ω scans |
| θmax, completeness: | 74.4°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 9161, 3516, 0.021 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), Iobs > 2σ(Iobs)(Iobs), 3,304 |
| N(param)refined: | 254 |
| Programs: | Rigaku, 1 SHELX 2 , 3 |
1 Source of material
According to the synthesis method in reference 4 , 5 , 6 , morphine (27.86 g, 0.32 mol) and potassium carbonate (55.4 g, 0.40 mol) were weighed into a 500 mL round-bottled flask and N,N-dimethylformamide (25.0 mL) was added to react at 313 K for 12 h. Then 6-fluoro-3,4-dihydronaphthalen-1(2H)-one (6.56 g, 0.04 mol) was added to the system at 393 K reflux reaction. Thin-Layer Chromatography (TLC, Dichloromethane:Methyl alcohol = 15:1, v:v) monitored the reaction process, and the reaction was stopped after 6 h. After the reaction system was restored to room temperature, solid-liquid separation was separated and the filter cake was washed white with dichloromethane (20.0 mL). The solvent was spun off and then separated by column chromatography with dichloromethane:methyl alcohol (30:1, v:v) as eluent to obtain the intermediate 6-morpholino-3,4-dihydronaphthalen-1 (2H)-one. The intermediate (2.31 g, 0.01 mol) and 4-(trifluoromethyl)benzaldehyde (3.48 g, 0.02 mol) were dissolved in methanol (20.0 mL) and followed by 25 % sodium hydroxide solution (10.0 mL) as catalyst at 293 K for 24 h. Then it was washed and precipitated with petroleum ether and ultrasonic with acetone for 15 min. Dichloromethane (5.0 mL) and methanol alcohol (5.0 mL) were added for recrystallization at room temperature to obtain transparent crystal of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one.
2 Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C–H) = 0.97 Å (methylene), Uiso(H) = 1.2 Ueq(C), and d(C–H) = 0.93 Å (aromatic), Uiso(H) = 1.2 Ueq(C).
3 Comment
3,4–Dihydronaphthalen-1(2H)-one derivatives have been reported in many articles and are considered as a new kind anti-inflammatory, anti-tumor modifier 7 , 8 and have also been shown to be active ingredients for the prevention of Alzheimer’s disease and dementia. 9 The study found that there is an active pocket on the right side of it, 10 and small molecule drugs with different effects can be obtained by splicing different active functional groups. The introduction of benzene improves catalytic activity 11 and the electron-withdrawing group has been shown to have excellent anti-inflammatory and anti-tumor effects. 12 At the same time, nitrogen-containing heterocyclic compounds also have good biological activity and are the main pharmacophore of small molecule compounds. Morpholine fragments play a key role in the treatment of malignant tumors. 13 Therefore, we introduced morpholine and electron-absorbing group trifluoromethyl in the synthesis to further improve the activity of 3,4-dihydronaphthalen-1(2H)-one derivatives. The intermediate was obtained by nucleophilic substitution reaction with morpholine and 6-fluoro-3,4-dihydronaphthalen-1(2H)-one, and then the target compound was obtained by Claisen–Schmidt reaction with 4-(trifluoromethyl)benzaldehyde.
Single crystal structure analysis revealed that the title compound is monoclinic with only one drug molecule in the asymmetric unit (cf. Figure 1) and is consistent with previously reported compounds. 14 3,4-Dihydronaphthalone is the main pharmacophore, followed by the introduction of morpholine at C6 position and 4-(trifluoromethyl)benzaldehyde at C2 position to form α,β-unsaturated ketone. In this molecule, the bond lengths of C(1)=O(1), C(1)–C(2), C(2)–C(3) and C(3)–C(4) located on the parent nucleus are 1.2246(15) Å, 1.5030(17) Å, 1.5033(16) Å, 1.5225(16) Å. The dihedral angle between these two planes is 14.69(3)°. At the same time, double bonds can be introduced at the C2 position through Claisen–Schmidt reaction, which allows the parent nucleus to be coplanar with p-trifluorobenzyl group. The bond length between C(2)=C(11) is 1.3459(17) Å, and the torsion angle of O(1)=C(1)–C(2)=C(11) is about 1.56(18)°. It is worth noting that the bond lengths of C(18)–F(1), C(18)–F(2), C(18)–F(3) are essentially the same. They are 1.3351(17) Å, 1.3326(16) Å, 1.3365(16) Å, respectively. 15 , 16 , 17 In addition, C(19)–N(1) and C(21)–N(1) on morphin has a bond length of about 1.4726(16) Å and 1.4675(16) Å. The morphin ring adopts a “chair” configuration. 16
Acknowledgments
This work was supported by Shandong Laboratory Program (No. SYS202205), Shandong Provincial Natural Science Foundation (Nos. ZR2022MH159 and ZR2023MH190) and Shandong Province Science and Technology-based Small and Medium-sized Enterprises Innovation Capacity Enhancement Project (No. 2023TSGC0870).
References
1. Rigaku OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Chen, Y.; Wang, J. P.; Wang, M. D.; Yu, W. X.; Cui, Y. T.; Gao, H. X.; Hou, G. G.; Ren, Y. Crystal Structure of (E)-2-(4-(1H-imidazole-1-yl) Benzylidene)-7-Fluoro-3,4-Dihydronaphthalen-1(2h)-One, C20H15FN2O. Z. Kristallogr. NCS. 2025, 240 (1), 19–21; https://doi.org/10.1515/ncrs-2024-0294.Search in Google Scholar
5. Li, X. W.; Miao, Y. H.; Ding, Y. X.; Hou, G. G. Crystal Structure of 9-Fluoro-4-(6-Methoxypyridin-2-Yl)-5,6-Dihydrobenzo [(h)]quinazolin-2-Amine, C18H15FN4O. Z. Kristallogr. NCS. 2025, 240 (1), 37–39; https://doi.org/10.1515/ncrs-2024-0330.Search in Google Scholar
6. Li, Y. L.; Meng, Q. G.; Hou, G. G.; Geng, Z. K. Crystal Structure of 2-((2-Fluoro-4-(trifluoromethyl)phenyl)(hydroxy)methyl)-7-Methoxy-3,4-Dihydronaphthalen-1((2h))-One, C19H16F4O3. Z. Kristallogr. NCS. 2023, 238, 1157–1159; https://doi.org/10.1515/ncrs-2023-0373.Search in Google Scholar
7. Barlow, J. W.; Zhang, T.; Woods, O.; Adam, J.; John, J. W. Novel Mast Cell-Stabilising Amine Derivatives of 3,4-Dihydronaphthalen-1(2h)-One and 6,7,8,9-tetrahydro-5H-Benzo[7]annulen-5-One. Med. Chem. 2011, 7 (3), 213–223; https://doi.org/10.2174/157340611795564222.Search in Google Scholar PubMed
8. Luan, M. Z.; Zhang, X. F.; Yang, Y.; Meng, Q. G.; Hou, G. G. Anti-inflammatory Activity of Fluorine-Substituted Benzo[h] Quinazoline-2-Amine Derivatives as NF-κB Inhibitors. Bioorg. Chem. 2023, 132, 106360; https://doi.org/10.1016/j.bioorg.2023.106360.Search in Google Scholar PubMed
9. Ding, Y.; Ko, M. H.; Pehar, M.; Kotch, F.; Peters, N. R.; Luo, Y.; Salamat, S. M.; Puglielli, L. Biochemical Inhibition of the Acetyltransferases ATase1 and ATase2 Reduces (β)-Secretase (BACE1) Levels and A(β) Generation. J. Biol. Chem. 2012, 287 (11), 8424–8433; https://doi.org/10.1074/jbc.m111.310136.Search in Google Scholar PubMed PubMed Central
10. Li, W. X.; Yu, L.; Chi, J. B.; Wang, J. P.; Liu, Y. J.; Wang, C. H.; Zhang, M.; Hou, G. G. Discovery of Anti-inflammatory Agents from 3,4-Dihydronaphthalene-1(2h)-One Derivatives by Inhibiting NLRP3 Inflammasome Activation. Eur. J. Med. Chem. 2024, 268, 116284; https://doi.org/10.1016/j.ejmech.2024.116284.Search in Google Scholar PubMed
11. Gao, C. L.; Hou, G. G.; Liu, J.; Ru, T.; Xu, Y. Z.; Zhao, S. Y.; Ye, H.; Zhang, L. Y.; Chen, K. X.; Guo, Y. W.; Pang, T.; Li, X. W. Synthesis and Target Identification of Benzoxepane Derivatives as Potential Anti-neuroinflammatory Agents for Ischemic Stroke. Angew. Chem., Int. Ed. 2020, 59, 2429–2439; https://doi.org/10.1002/anie.201912489.Search in Google Scholar PubMed
12. Sun, Y.; Zhou, Y. Q.; Liu, Y. K.; Zhang, H. Q.; Hou, G. G.; Meng, Q. G.; Hou, Y. Potential Anti-neuroinflammatory NF-κB Inhibitors Based on 3,4-Dihydronaphthalen-1(2h)-One Derivative. J. Enzym. Inhib. Med. Chem. 2020, 35, 1631–1640; https://doi.org/10.1080/14756366.2020.1804899.Search in Google Scholar PubMed PubMed Central
13. Luo, W. J.; Liu, Y. Q.; Qin, H.; Zhao, Z. Y.; Wang, S. Q.; He, W. M.; Tang, S. S.; Peng, J. M. Nitrogen-containing Heterocyclic Drug Products Approved by the FDA in 2023: Synthesis and Biological Activity. Eur. J. Med. Chem. 2024, 279, 116838; https://doi.org/10.1016/j.ejmech.2024.116838.Search in Google Scholar PubMed
14. Xia, D. L.; Wang, J. P.; Yu, W. X.; Wang, M. D.; Gao, H. X.; Cui, Y. T.; Hou, G. G. Crystal Structure of (E)-6,8-Dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5. Z. Kristallogr. NCS. 2024, 239, 1133–1136; https://doi.org/10.1515/ncrs-2024-0329.Search in Google Scholar
15. Yu, L.; Meng, Q. G.; Hou, G. G.; Liu, Y. J.; Geng, Z. K. Crystal Structure of 2-Amino-4-(2-fluoro-3-(trifluoromethyl)phenyl)-9-methoxy-1,4,5,6-tetrahydrobenzo[h]quinazolin-3-ium chloride, C20H18ClF4N3O. Z. Kristallogr. NCS. 2023, 238 (6), 1161–1163; https://doi.org/10.1515/ncrs-2023-0374.Search in Google Scholar
16. Luo, H. L.; Li, W. X.; Bai, X. Y.; Meng, Q. G.; Hou, Y. Crystal Structure of (E)-7-Fluoro-2-(4-morpholinobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C21H20FNO2. Z. Kristallogr. NCS. 2023, 238 (3), 495–497; https://doi.org/10.1515/ncrs-2023-0053.Search in Google Scholar
17. Lu, Y.; Wang, J.- P.; Wang, M.- D.; Wen-Xiao, Y.; Cui, Y.- T.; Gao, H.- X.; Liu, Y.- J.; Hou, G.-G. Crystal structure of (E)-6-(4-ethylpiperazin-1-yl)-2-(3-fluorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one, C23H25FN2O. Z. Kristallogr. - New Cryst. Struct. 2024, 239, 515; https://doi.org/10.1515/ncrs-2024–0066.10.1515/ncrs-2024-0066Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

