Abstract
C75H725CuF6N2O4P3, monoclinic, P21/n (no. 62), 10.9629(7) Å, 33.452(2) Å, 19.7777(13) Å, β = 101.149(2)°, V = 7,116.2(8) Å3, Z = 4, Rgt(F) = 0.0623, wR(F2) = 0.1632, T = 170 K.
1 Source of materials
The title compound was prepared according to the following procedure: In a dried Schlenk tube, [Cu(MeCN)4]+PF6− (373 mg, 1 mmol MeCN = acetonitrile) and 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (xantphos, 579 mg, 1 mmol) were dissolved in 20 mL of dry DCM at room temperature. The solution was stirred under reflux overnight. After cooling to room temperature, 2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline (phenanthroline, 416 mg, 1 mmol) dissolved in a minimal amount of DCM was added. The mixture was then heated to reflux for another 3 h. After cooling to room temperature, n-hexane was added to precipitate the product, which was filtered and washed with n-hexane. The resulting solid was further purified by recrystallization from a DCM/n-hexane mixture. 1 , 2 , 3 , 4
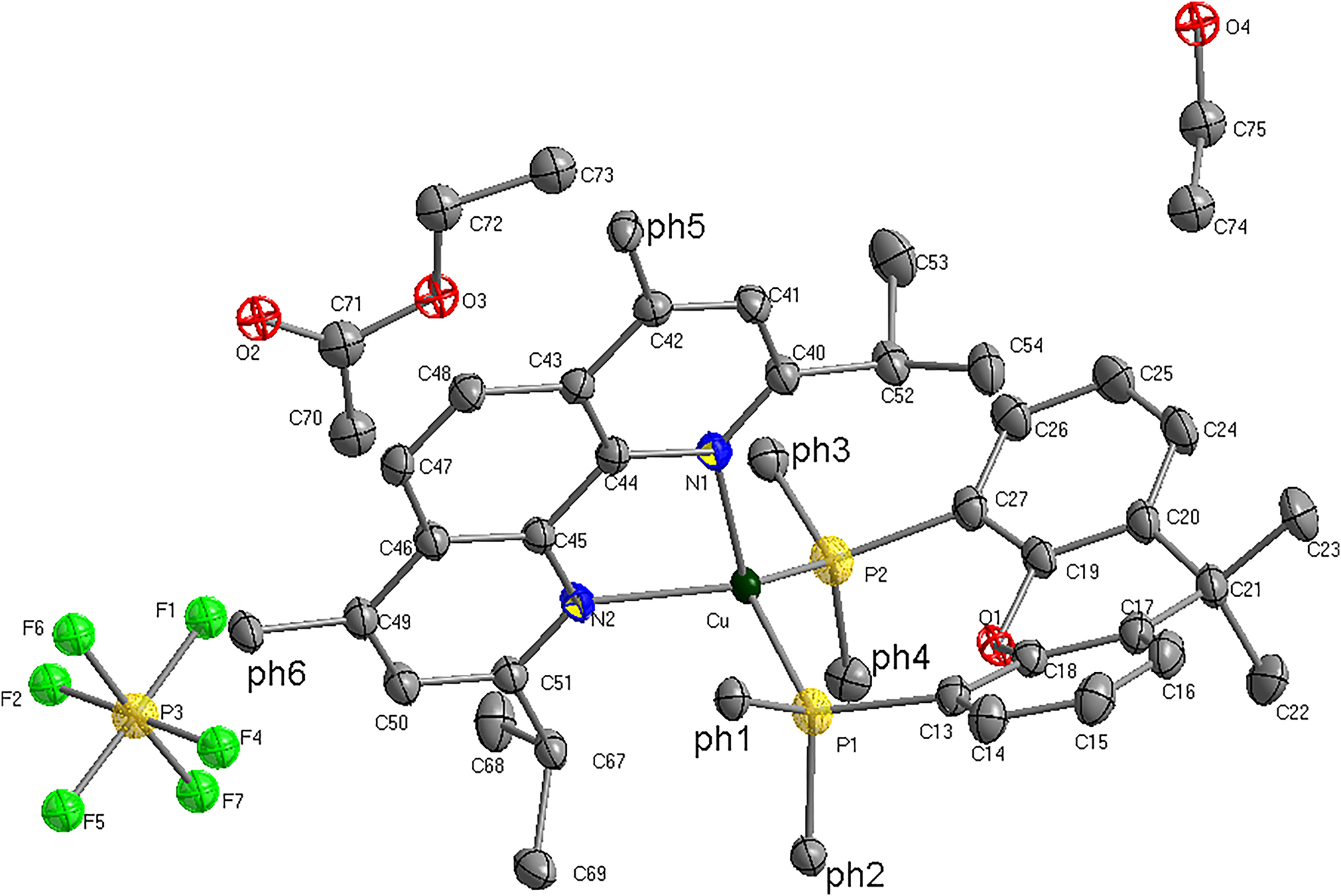
Then 15 mg of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6− was added to 5 mL of EtOAc, stirred for 5 min, and filtered. The clear solution was allowed to evaporate slowly at room temperature, yielding crystals after several days.
2 Experimental details
Single-crystal X-ray diffraction measurements were carried out on Bruker D8 Venture with graphite monochromated Mo Kα radiation (λ = 0.71073 Å) at low temperature. After absorption correction, the crystal structure was solved using the Olex2 software 5 and the programs SHELXT 6 program and refined with SHELXL 7 and the molecular graphics were drawn by using DIAMOND software. 8 All hydrogens were generated geometrically (C–H bond fixed at 0.96 Å), assigned isotropic thermal parameters, and allowed to ride on their parent carbon atoms before the final cycle of refinement.
There are solvent accessible VOIDS of 69 Å3 in the unit cell and the volume of the void is reasonable. The entire EA molecule is located within a single pore and there are severely disordered solvent molecules which may be EA or water molecules that cannot be crystallographically located residing in the void(s).
3 Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1.
4 Comment
Photoredox catalysis, driven by visible light, is rapidly developing as an important strategy for organic synthesis and is very attractive as a clean and sustainable technology. However, the most widely used visible light catalysts are mainly based on precious metal complexes such as ruthenium and iridium. Although these precious metal complexes have good stability and catalytic activity, their low abundance and high cost limit large-scale industrial applications.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.15 × 0.09 × 0.06 mm |
| Wavelength: μ: |
Ga Kα radiation (1.34139 Å) 2.42 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Bruker D8, φ and ω scans 57.0°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 49725, 14517, 0.051 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 10149 |
| N(param)refined: | 857 |
| Programs: | SHELX, 5 , 6 Diamond, 7 Olex2 8 |
Cu(I) phthalocyanine derivatives have excellent excited state and redox properties, 9 and the McMillin team has conducted in-depth studies on them, synthesizing [Cu(dmp)2]+(dmp = 2,9-dimethyl-1,10 phthalocyanine), 10 proving that Cu(I) photosensitizers have long excited state lifetimes and excellent photoluminescence performance, which is conducive to constructing efficient photocatalytic systems. With the development of photocatalytic organic reactions, Cu-based photocatalysis gradually replace expensive Ir- and Ru-based photosensitizers in some photocatalytic reactions. 11 , 12 Although these relatively rare transition metals are the subject of an increasingly large body of work, the potential of copper, an earth-abundant transition metal, to serve as a photocatalyst under visible-light irradiation has been still in its infancy.
The crystal structure reported of the title compound is a novel skeleton copper base of P/N hybrid type photosensitizer, with 1,10-phenanthroline and xantphos as ligand. Cu-based visible-light photoredox catalysis indeed falls across the new phase of fast progress. 13 It is believed that more and more organic reactions catalyzed by Cu-based photosensitizers under visible light will be studied and reported by scientists.
There is one crystallographically independent Cu centre, one 2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline (phenanthroline), one 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (xantphos) ligand, 1.5 solvent molecules and one hexafluorophosphate ion in the asymmetric unit. Each copper(II) is four-coordinated and is coordinated by two nitrogen atoms from phenanthroline ligands and two phosphorus atoms from xantphos ligands (Cu(1)–N(1) 2.114(2) Å and Cu(1)–N(2) 2.102(2) Å, Cu(1)–P(1) 2.2992(9) Å, Cu(1)–P(2) 2.2585(9) Å). All Cu centres display distorted tetrahedral coordination geometries with the N–Cu–N angles ranging 79.50(9)°, the P–Cu–P angles 118.99(3)° and the N–Cu–P angles from 101.59(7) to 126.28(7)°.
Abundant intramolecular hydrogen bonds are found in the compound, three carbon atoms of phenanthroline ligands C62, C50 and C68, respectively donate one hydrogen atom to F4 atom and C62 donates one hydrogen atom to F6 atom. Furthermore, the guest acetones molecules are not in coordination, but are involved in intramolecular hydrogen bonds with guest acetone molecules (C70–H70B⋯F1 2.795(5) Å). Obviously, the extensive hydrogen bonds make a considerable contribution to stabilization of the crystal structure.
There are three intermolecular C4–H4⋯F1, C5–H5⋯F7, C65–H65⋯F2#1 (symmetry code: #1:x + l, y, z) hydrogen bonding interactions involving the xantphos carbon atoms (C4 and C5), phenanthroline C65 atom and the corresponding fluorine atom in neighboring hexafluorophosphate ion.
Acknowledgments
We are grateful to the Jinhua Science and Technology Bureau (No. 2020-1-003a and 2023-4-55) and Domestic University–Industry Cooperation Project for Higher Education Visiting Engineers of Zhejiang Province in 2024 (Grant No. FG2024018) for financial support.
References
1. Mejia, E.; Luo, S. P.; Karnahl, M.; Friedrich, A.; Tschierlei, S.; Surkus, A.; Junge, H.; Gladiali, S.; Lochbrunner, S.; Beller, M. A Noble–Metal–Free System for Photocatalytic Hydrogen Production from Water. Chem. Eur. J. 2013, 19, 15972–15978; https://doi.org/10.1002/chem.201302091.Search in Google Scholar PubMed
2. Bao, H. Y.; Zhou, B. W.; Luo, S. P.; Xu, Z.; Jin, H. W.; Liu, Y. K. P/N Heteroleptic Cu(I)–Photosensitizer–Catalyzed Deoxygenative Radical Alkylation of Aromatic Alkynes with Alkyl Aldehydes Using Dipropylamine as a Traceless Linker Agent. ACS Catal. 2020, 10 (14), 7563–7572; https://doi.org/10.1021/acscatal.0c02454.Search in Google Scholar
3. Zheng, L. M.; Xue, H.; Zhou, B. W.; Luo, S. P.; Jin, H. W.; Liu, Y. K. Single Cu(I)–Photosensitizer Enabling Combination of EnergyTransfer and Photoredox Catalysis for the Synthesis of Benzo[b] fluorenols from 1,6-Enynes. Org. Lett. 2021, 23, 4478–4482; https://doi.org/10.1021/acs.orglett.1c01427.Search in Google Scholar PubMed
4. Lyu, X. L.; Huang, S. S.; Song, H. J.; Liu, Y. X.; Wang, Q. M. Visible–Light–Induced Copper–Catalyzed Decarboxylative Coupling of Redox–Active Esters with N–Heteroarenes. Org. Lett. 2019, 21, 5728–5732; https://doi.org/10.1021/acs.orglett.9b02105.Search in Google Scholar PubMed
5. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
6. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
7. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
8. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
9. Mara, M. W.; Bowman, D. N.; Buyukcakir, O.; Shelby, M. L.; Haldrup, K.; Huang, J.; Harpham, M. R.; Stickrath, A. B.; Zhang, X.; Stoddart, J. F.; Coskun, A.; Jakubikova, E.; Chen, L. X. Electron Injection from Copper Diimine Sensitizers into TiO2: Structural Effects and their Implications for Solar Energy Conversion Devices. J. Am. Chem. Soc. 2015, 137 (30), 9670–9684; https://doi.org/10.1021/jacs.5b04612.Search in Google Scholar PubMed
10. Breddels, P. A.; BerdowskiI, P. A. M.; Blasse, G.; McMillin, D. R. Luminescence of Some CuI Complexes. J. Chem. Soc. 1982, 78 (3), 595–601; https://doi.org/10.1039/f29827800595.Search in Google Scholar
11. Michelet, B.; Deldaele, C.; Kajouj, S.; Moucheron, C.; Evano, G. A General Copper Catalyst for Photoredox Transformations of Organic Halides. Org. Lett. 2017, 19 (13), 3576–3579; https://doi.org/10.1021/acs.orglett.7b01518.Search in Google Scholar PubMed
12. Wang, C.; Guo, M. Z.; Qir, p.; Shang, Q. Y.; Liu, Q.; Wang, S.; Zhao, L.; Wang, R.; Xu, Z. Visible–Light–Driven, Copper–Catalyzed Decarboxylative C(sp3)H Alkylation of Glycine and Peptides. Angew. Chem., Int. Ed. 2018, 57, 15841–15846; https://doi.org/10.1002/anie.201809400.Search in Google Scholar PubMed
13. Hossain, A.; Bhattacharyya, A.; Reiser, O. Copper’s Rapid Scent in Visible-Light Photoredox Catalysis. Science 2019, 364, 450; https://doi.org/10.1126/science.aav9713.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 5,5′-bis(2,4,6-trinitrophenyl)-2,2′-bi(1,3,4-oxadiazole), C16H4N10O14
- Crystal structure of catena-poly[(μ3-4,4′-oxydibenzoato- κ5 O,O′: O″,O‴:O‴)-bis(2,4,6-tri(3-pyridine)-1,3,5-triazine-κ1 N)cadmium(II)], C50H32CdN12O5
- The crystal structure of 1,4-diazepane-1,4-diium potassium trinitrate, C5H14KN5O9
- The crystal structure of benzyl 2,2,5,5-tetramethylthiazolidine-4-carboxylate, C15H21NO2S
- Crystal structure of 2-hydroxyethyl-triphenylphosphonium tetracyanidoborate, C24H20BN4OP
- The crystal structure of 1-methyl-3-(N-methylnitrous amide–N-methylene) imidazolidine-2,4,5-trione
- Crystal structure of N-((3-cyano-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(2,2,2-trifluoroacetyl)-1H-pyrazol-5-yl)carbamoyl)-2,6-difluorobenzamide, C20H7Cl2F8N5O3S
- Crystal structure of 5-(2,2-difluoropropyl)-5-methylbenzo[4,5]imidazo[2,1-a] isoquinolin-6(5H)-one, C20H18F2N2O
- The crystal structure of N′,N″-[1,2-bis(4-chlorophenyl)ethane-1,2-diylidene]bis(furan-2- carbohydrazide), C24H16Cl2N4O4
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromoantimony(III), [C25H21BrP]+[SbBr4]−
- Crystal structure of [(4-bromobenzyl)triphenylphosphonium] tetrabromidoindium(III), [C25H21BrP]+[InBr4]−
- The crystal structure of 4-carboxy-2-oxobutan-1-aminium chloride, C5H10ClNO3
- Crystal structure of (4-(4-chlorophenyl)-1H-pyrrole-3-carbonyl)ferrocene, C21H16ClFeNO
- The crystal structure of dichlorido(η6-p-cymene)(triphenylarsine)ruthenium(II), C28H29AsCl2Ru
- Crystal structure of (Z)-2-hydroxy-N′-(1-(o-tolyl)ethylidene)benzohydrazide, C16H16N2O2
- The crystal structure of 10-(1-bromoethyl)-14-(bromomethyl)dibenzo[a, c]acridine, C24H17NBr2
- Synthesis and crystal structure of 6-methoxy-7-[(4-methoxyphenyl)methoxy]-2H-1-benzopyran-2-one, C18H16O5
- Synthesis and crystal structure of ethyl 4-((4-trifluoromethylbenzyl)amino)benzo, C17H16F3NO2
- The crystal structure of (Z)-2-(tert-butyl)-6-(7-(tert-butyl)-5-methylbenzo[d][1,3]oxathiol-2-ylidene)-4-methylcyclohexa-2,4-dien-1-one, C23H28O2S
- The crystal structure of (R)-2-aminobutanamide hydrochloride, C4H11ClN2O
- Crystal structure of bromido[hydridotris(3-tert-butyl-5-isopropylpyrazolyl)borato-κ3 N,N′,N″]copper(II), C30H52BBrCuN6
- Crystal structure of chlorido{hydridotris[3-mesityl-5-methyl-1H-pyrazol-1-yl-κN3]borato}-copper(II) dichloromethane monosolvate
- Crystal structure of 4-[3,5-bis(propan-2-yl)-1H-pyrazol-4-yl]pyridine, C14H19N3
- Crystal structure of ((4-(4-bromophenyl)-1H-pyrrol-3-yl)methyl)ferrocene, C21H16BrFeNO
- Crystal structure of [(4-chlorobenzyl)triphenylphosphonium] dichloridocopper(I), {[C25H21ClP]+[CuCl2]−}n
- The crystal structure of {Cu(2,9-diisopropyl-4,7-diphenyl-1,10-phenanthroline)[4,5-bis(diphenylphosphino)-9,9-dimethylxanthene]}+ PF6−·1.5(EtOAC)
- Crystal structure of 3,5-bis(t-butyl)-1H-pyrazol-4-amine, C11H21N3
- Crystal structure of [(2,4-dichlorobenzyl)triphenylphosphonium] trichloridocopper(II), [C25H20Cl2P]+[CuCl3]−
- The crystal structure of dipotassium sulfide, K2S
- Crystal structure of (4-(4-methoxyphenyl)-1H-pyrrole-3-carbonyl)ferrocene, C22H19FeNO2
- Crystal structure of (E)-6-(4-methylpiperazin-1-yl)-2-(4-(trifluoromethyl)benzylidene)-3, 4-dihydronaphthalen-1(2H)-one, C23H23F3N2O
- Crystal structure of (E)-6-morpholino-2-(4-(trifluoromethyl)benzylidene)-3,4-dihydronaphthalen-1(2H)-one, C22H20F3NO2
- Crystal structure of Ce9Ir37Ge25
- The crystal structure of ethyl 6-(2-nitrophenyl)imidazo[2,1-b]thiazole-3-carboxylate, C14H11N3O4S
- Crystal structure of (4-(4-isopropylphenyl)-1H-pyrrol-3-yl)(ferrocenyl)methanone, C24H23FeNO
- Crystal structure of bis(methylammonium) tetrathiotungstate(VI), (CH3NH3)2[WS4]
- Crystal structure of 6,11-dihydro-12H-benzo[e]indeno[1,2-b]oxepin-12-one, C17H12O2
- Crystal structure of 3-[(4-phenylpiperidin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4- oxadiazole-2-thione, C18H19N3OS2
- Crystal structure of N-isopropyl-1,8-naphthalimide C15H13NO2
- TiNiSi-type EuPdBi
- Crystal structure of 1-(p-tolylphenyl)-4-(2-thienoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O2S
- The crystal structure of 3-(3-carboxypropyl)-2-nitro-1H-pyrrole 1-oxide, C7H9N3O5
- The crystal structure of tetraaqua-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k2O:N)-tetrakis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)acetato-k1N)trizinc(II) hexahydrate C36H52N18O32Zn3
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 4-hydroxy-3,5-dimethoxybenzoate monohydrate, C25H30FN3O9
- Crystal structure of bis(DL-1-carboxy-2-(1H-indol-3-yl)ethan-1-aminium) oxalate — acetic acid (1/2)
- Crystal structure of methyl (E)-4-((4-methylphenyl)sulfonamido)but-2-enoate, C12H15NO4S
- The crystal structure of actarit, C10H11NO3
- The crystal structure of bicyclol, C19H18O9
- The crystal structure of topiroxostat, C13H8N6
- Crystal structure of 2,2-dichloro-N-methyl-N-(4-p-tolylthiazol-2-yl)acetamide, C13H12Cl2N2OS
- Crystal structure of 4-(trifluoromethyl)-7-coumarinyl trifluoromethanesulfonate C11H4F6O5S
- Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6)-((Z)-N,N′-bis(2-(dimethylamino)phenyl)carbamimidato-κ1N)potassium(I)
- Crystal structure of (Z)-2-(5-((4-(dimethylamino)naphthalen-1-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid, C18H16N2O3S2
- Crystal structure of (4-fluorobenzyl)triphenylphosphonium bromide, C25H21BrFP
- The crystal structure of dichlorido-[6-(pyridin-2-yl)phenanthridine-κ2N, N′]zinc(II)-chloroform (1/1), C19H13N2ZnCl5
- Crystal structure of (E)-(3-(2,4-dichlorophenyl)acryloyl)ferrocene, C19H14Cl2FeO
- The crystal structure of (E)-7-chloro-1-cyclopropyl-6-fluoro-3-((2-hydroxybenzylidene)amino)quinolin-4(1H)-one, C19H14ClFN2O2
- Crystal structure of 2-bromo-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13BrFNO4S2
- Crystal structure of 2-chloro-11-(((fluoromethyl)sulfonyl)methyl)-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepine 5,5-dioxide, C16H13ClFNO4S2
- Crystal structure of 5-(2,2-difluoropropyl)-5-methyl-6-oxo-5,6-dihydrobenzo[4,5]imidazo[2,1-a]isoquinoline-3-carbonitrile, C20H15F2N3O

